Abstract
Herpesvirus infections cause considerable morbidity and mortality through lifelong recurrent cycles of lytic and latent infection in several tissues, including the human nervous system. Acyclovir (ACV) and its prodrug, the current antivirals of choice for herpes simplex virus (HSV) and, to some extent, varicella zoster virus (VZV) infections are nucleoside analogues that inhibit viral DNA replication. Rising viral resistance and the need for more effective second-line drugs have motivated searches for additional antiviral agents, particularly non-nucleoside based agents. We evaluated the antiviral activity of five compounds with predicted lysosomotropic activity using conventional and human induced pluripotent stem cell-derived neuronal (iPSC-neurons) cultures. Their potency and toxicity were compared with ACV and the lysosomotropic agents chloroquine and bafilomycin A1. Out of five compounds tested, micromolar concentrations of 30N12, 16F19, and 4F17 showed antiviral activity comparable to ACV (50μM) during lytic herpes simplex virus type 1 (HSV-1) infections, reduced viral DNA copy number, and reduced selected HSV-1 protein levels. These compounds also inhibited the reactivation of ‘quiescent’ HSV-1 infection established in iPSC-neurons, but did not inhibit viral entry into host cells. The same compounds had greater potency than ACV against lytic VZV infection; they also inhibited replication of human cytomegalovirus. The anti-herpetic effects of these non-nucleoside agents merit further evaluation in vivo.
Keywords: Antiviral, Human Cytomegalovirus, Varicella Zoster Virus, HSV, Herpes Simplex Virus Type 1, Induced Pluripotent Stem Cells
1. Introduction
Herpesvirus infections affect the vast majority of adults world-wide, with rates exceeding 95% for human cytomegalovirus (HCMV) and varicella zoster virus (VZV), and up to 60% for herpes simplex virus type I (HSV-1) (Cannon et al., 2010) (Xu et al., 2006). These viruses can infect an array of human tissues including the peripheral and/or central nervous system (CNS), causing severe CNS disease (Schmutzhard, 2001) (Steiner et al., 2007). Currently, the list of FDA approved antiviral drugs is limited, particularly for HCMV or VZV (Strasfeld and Chou, 2010), moreover, the available drugs can produce serious adverse effects. Acyclovir (ACV) or its prodrug valacyclovir (VACV) are the most widely used agents for HSV-1 and VZV. Both drugs are processed to nucleoside analogues that are selectively activated in virus-infected cells and then block viral DNA replication. Resistance to ACV can develop from mutations in the viral thymidine kinase and/or DNA polymerase (Burrel et al., 2013), with incidence rates of 0.3–7.1% (Stránská et al., 2005) (Malvy et al., 2005). Though some new antiherpetic drugs target the viral helicase and primase, viral resistance to these agents has also been reported (Kleymann et al., 2002) (Sukla et al., 2010). In addition, nephrotoxicity can occur following prolonged ACV treatment (Izzedine et al., 2005). Moreover, ACV is not clinically effective against HCMV. There is thus a compelling need to search for additional drugs to treat herpes virus infections.
Here, we report on novel anti-herpetic compounds that are based on non-nucleoside agents initially proposed to have lysosomotropic activities. Our studies were motivated by earlier reports indicating the efficacy of lysosomotropic drugs like chloroquine (CQ) and bafilomycin A1 (BFLA) in inhibiting HSV-1 infections (Harley et al., 2001). Lysosomotropic agents increase intracellular pH and presumably inhibit viral packaging and maturation through the trans-Golgi network, although their precise mechanisms of action against herpesviruses remain unclear (Nieland et al., 2004). In the present study, the in vitro efficacy of selected agents for HSV-1, VZV, and CMV infections was tested. We focused particularly on HSV-1, including analyses of lytic infection, as well as a model of ‘quiescent’ infection in human iPSC-derived neuronal cells that mimics several aspects of HSV-1 latent infections (D’Aiuto et al., 2014). We report on three promising agents that appear to have a broader spectrum of activity compared with ACV.
2. Materials and methods
2.1. Drugs
Compounds 30N12, 16F19, 4F17 (95% purity), 16D20, and 17G7 (90% purity) were purchased from ChemBridge. CQ (≥98% purity), phosphonoacetic acid (PAA; ≥98% purity), and BFLA (≥90% purity) were purchased from Sigma. ACV (≥98% purity) was purchased from Spectrum Chemical Mfg Corp. The chemical structure of the compounds is depicted in Fig. 1.
Figure 1.
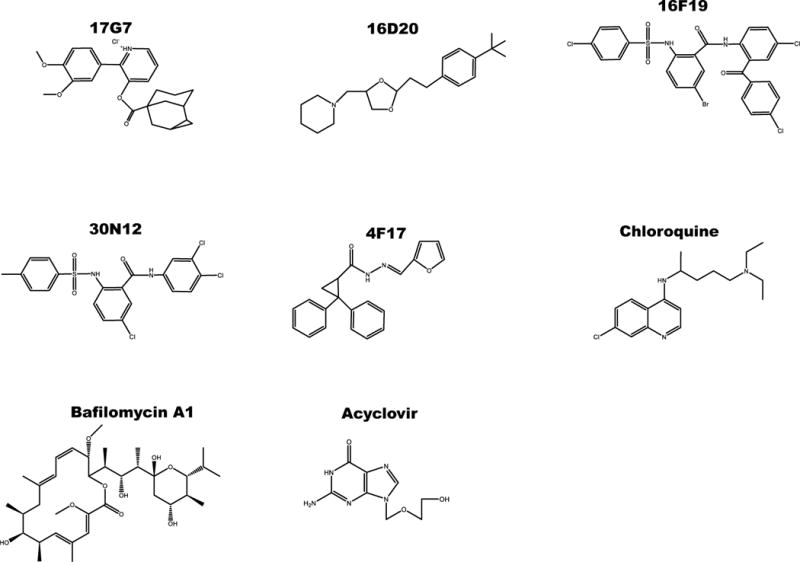
Structure of compounds tested against herpes viruses.
2.2. Cell lines and viral constructs
Vero cells were maintained in Eagle’s minimum essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS; HyClone) and 5% antibiotic/antimycotic (HyClone). VZV permissive human retinal pigmented epithelial cells (ARPE-19) were maintained in Dulbecco’s modified Eagle medium/F12 (DMEM/12) supplemented with 10% FBS (HyClone) and 5% antibiotic/antimycotic (HyClone). Human foreskin fibroblasts (HFF; ATCC, CRL-2088) were maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% FBS (HyClone). Human iPSC lines 73-56010-02 and HFF1-S were differentiated into functional neurons (iPSC-neurons) as described (D’Aiuto et al., 2012). All cells were grown at 37°C, 5% CO2, and 100% humidity.
The HSV-1 strain was based on the KOS virus (VR-1493; ATCC). This recombinant virus expresses the enhanced green fluorescent protein (EGFP) from the ICP0 promoter, and the monomeric red fluorescent protein (RFP) from the glycoprotein C promoter (Ramachandran et al., 2008). The VZV construct, derived from an infectious Bacterial Artificial Chromosome of the whole VZV genome of the Parent of Oka strain, was engineered to express the luciferase reporter as a fusion to the VZV ORF9 protein, as described recently (Bayer et al., 2015). The human cytomegalovirus (HCMV) recombinant virus, derived from the Towne strain, was engineered to express the luciferase under the control of the viral pp28 gene promoter (He et al., 2011).
2.3. Viral infections
To test the effects of the compounds during acute HSV-1 infection, cell-free virus was adsorbed onto cells for 2 hours (h) at a multiplicity of infection (MOI) of 1.0 for Vero cells and 0.3 for iPSC-neurons. The inocula were removed, cells were washed with PBS, and medium was exchanged. Compounds 30N12, 16F19, 4F17, 16D20, and 17G7 (Nieland et al., 2004) were added 2h post infection (hpi) at the concentrations indicated. Parallel cultures were pretreated for 24h with CQ (50 μM) or BFLA (0.1 μM; (Joubert et al., 2009). As positive controls, cultures were pretreated with ACV (50 μM; (D’Aiuto et al., 2014) for 24h or pretreated with PAA, a HSV-1 DNA polymerase inhibitor (300 μg/ml; (Blaho et al., 1993) for 1h prior to the infection.
We tested effects on HSV-1 attachment to the host cell membrane and host cell entry using a published protocol (Krepstakies et al., 2012; Supplementary Figure S6).
To test the effect of the compounds on the HSV-1 reactivation, quiescence was established using (E)-5-(2-bromovinyl)-2′-deoxyuridine+interferon alpha (5BVdU+IFN-α; 30 μM, 125 U/ml, respectively) for 7 days as reported (D’Aiuto et al., 2014). After this period, the quiescence-inducers were withdrawn, and iPSC-neurons were cultured for 48h in the presence or absence of the test compounds. HSV-1 infection was then reactivated by treatment with sodium butyrate (NaB; 5 mM) for 5 days (D’Aiuto et al., 2014). The number of cells expressing EGFP and RFP was determined by flow cytometry (FC) on Fortessa FACS analyzer (Becton Dickinson).
Inhibition of VZV was assessed on ARPE-19 cells, inoculated with 1,000 PFU/well of a cell-associated high titer VZV stock for 2h at 37°C, in which >70% of cells were infected and infectious. Small molecules were added in the same manner as just detailed, and luciferase activities were assayed 48hpi.
Inhibition of HCMV was evaluated by cell-free virus infection adsorbed on HFF cells for 90 min at MOI of 1.0, at which point, small molecule inhibitors were added in a manner as just detailed. Ganciclovir (5 μM) was used as control in all experiments. Luciferase activities were assayed 72hpi.
2.4. qPCR assays for viral DNA
The EGFP sequence from the genetically engineered HSV-1 was utilized as the target for copy number analysis. Copy number qPCR was performed as previously reported (Kaufman et al., 2005), employing an EGFP plasmid standard curve, using 5 ng total DNA with biological and technical triplicates, along with corresponding non-template controls. The probe master mix had final concentrations of 18 μM EGFP forward primer (5′-ccacatgaagcagcacgactt-3′), 18 μM EGFP reverse primer (5′-ggtgcgctcctggacgta-3′), and 5 μM probe (5′-6FAM-ttcaagtccgccatgcccgaa-TAMRA-3′). The reaction conditions were 95°C for 3 min followed by 45 cycles of 95°C for 15 sec and 55°C for 30 sec, and finished with 72°C for 30 sec. All qPCRs were conducted on a 7900HT Fast Real-Time PCR System (Applied Biosystems) and analyzed with manufacturer’s software SDS 2.4.
2.5. Cytotoxicity assay
The viability of iPSC-neurons following exposure for 24h at different concentrations of compounds 30N12, 16F19, and 4F17 was assayed by FC using the LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Life Technologies). The cytotoxicity analysis was also performed in ARPE-19 cells as described for the iPSC-neurons, but the cells were incubated with the drugs for 48h instead of 24h. The drug-induced cytotoxicity in HFF cells used for HCMV infection was estimated using CellTiter-Glo Assay Kit following incubation with the drugs for 72h.
2.6. Statistical analyses
Statistical analyses were conducted using the open source software R statistical package version 3.0.1, IBM SPSS v21, GraphPad Prism v5.02, and SigmaPlot. The drug concentration that reduced the number of fluorescent cells in cultures infected with HSV-1 or VZV by 50% (IC50) was estimated using the drc package in R (http://cran.r-project.org/web/packages/drc/drc.pdf; version 2.3–96); briefly, model fit was first assessed by comparison to an ANOVA model using an F-test. Then, a Box-Cox transformation to fit a 4 parameter, log logistic model provided the best fit to the data (results not shown). The IC50 for HCMV was calculated from the dose response curve constructed by using SigmaPlot software. Statistical significance was calculated by first testing for equality of variance using Levine’s test, where two-tailed Student’s t-tests (where variances were not significantly different at α<0.05) or Welch’s t-tests (where variances were significantly different at α<0.05) were conducted. Data used represent three independent experiments.
3. RESULTS
3.1. Antiviral activity of new drugs on HSV-1 infection
3.1.1. Vero cells
Vero (green monkey kidney epithelial) cells were infected with HSV-1 at MOI of 1 for 24h in the presence or absence of the test compounds, as well as CQ, BFLA, and ACV (Figure 2a). FC analysis indicated that at 50 μM, compounds 30N12 and 16F19 displayed a 90-, 73-fold reduction of EGFP+ cells, respectively, compared with a 22-fold reduction at the same concentration of ACV. Compound 4F17 (50 μM) produced a more modest 2.7-fold decrease, while 16D20 and 17G7 did not reduce HSV-1 infection substantially. The presence of infectious particles in supernatant of treated infected cultures was analyzed by a conventional plaque assay using Vero cells. Consistent with the FC analysis, infectious particles were undetectable in the supernatants of the cultures incubated with 30N12, 16F19, 4F17, CQ, BFLA, or ACV (Figure 2b), while infectious particles were detected in the supernatants from cultures treated with compounds 16D20 and 17G7. Thus, at 50 μM concentrations, 30N12 and 16F19 showed greater potency than ACV.
Figure 2. Comparison of inhibitory effects of test compounds on lytic HSV-1 infection.
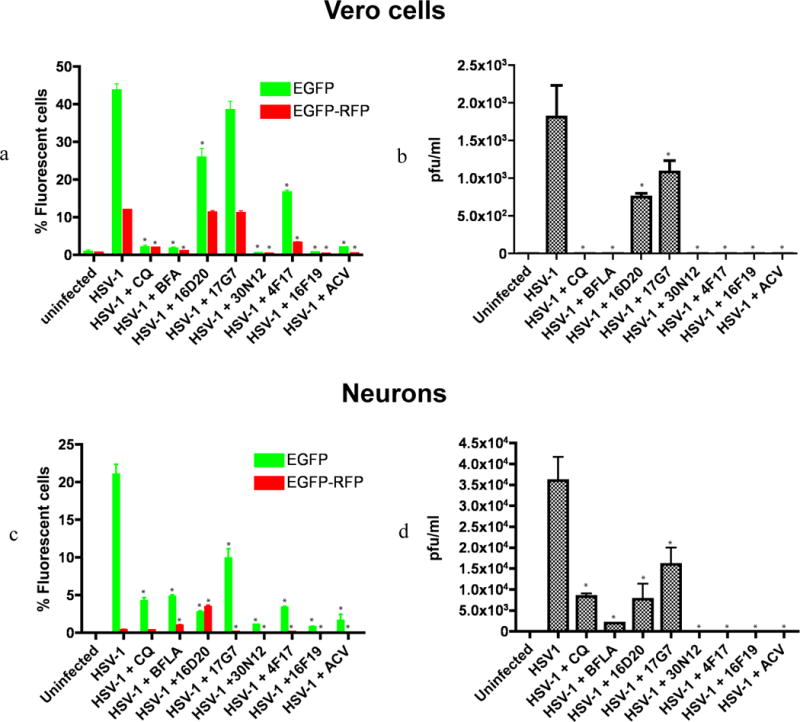
(a) Flow cytometry (FC) analysis of uninfected and HSV-1 infected Vero cells in the presence or absence of test compounds using HSV-1 expressing EGFP from an IE gene promoter and RFP from a late gene promoter. The fractions of cells showing fluorescence of the appropriate color are indicated. *p≤0.0019. (b) Plaque assays of virus produced in the supernatant of HSV-1 infected Vero cells titrated on Vero cells, shown as plaque forming units per milliliter (PFU/ml). *p≤0.0017. (c) FC analysis of uninfected and HSV-1 infected iPSC-neurons in the presence or absence of test compounds. *p≤0.026. (d) Plaque assays of virus produced in the supernatant of HSV-1 infected iPSC-neurons titrated on Vero cells, shown as plaque forming units per milliliter (PFU/ml). *p≤0.045. The data represent an average of three independent experiments. CQ: chloroquine (50 μM); BFLA: bafilomycin A1 (0.1 μM); ACV: acyclovir (50 μM). Test compounds were used at a concentration of 50 μM. pfu: plaque forming units. Error bars represent standard deviations.
For both viral attachment and entry, no statistically significant reduction in viral DNA copy number was observed in cells treated with the test compounds compared to the untreated HSV-1 group (Supplementary Figure S6).
3.1.2. iPSC-neurons
In view of the toxic effects of HSV-1 on neuronal cells, we employed iPSC derived neurons (iPSC-neurons) to test the antiviral effect of the aforementioned compounds. iPSC-neurons were infected with the HSV-1 construct at MOI of 0.3 and assayed at 24hpi. FC analysis indicated that compounds 30N12 and 16F19 reduced fluorescent cells approximately 20-fold (approximately 1% of EGFP+ cells in drug-treated cultures, versus over 20% in the control, drug-free cultures), similar to the inhibition caused by ACV, CQ or BFLA (Figure 2c; all drugs at 50 μM). Compound 17G7 resulted in a modest 2-fold reduction of EGFP+ cells (9.9% EGFP; Figure 2c). Unlike ACV, CQ, or BFLA, however, supernatants from cell cultures treated with 4F17, 30N12, or 16F19 produced no detectable infectious virus (Figure 2d). The patterns of effects produced by 4F17, 30N12, or 16F19 were moderately correlated in Vero cells and iPSC-neurons, motivating further analyses of these drugs in iPSC-neurons (r=0.72, 95% CI=0.031–0.945, p=0.044; Supplementary Figure S3).
3.2. IC50 estimation
The concentration of 30N12, 16F19, and 4F17 that inhibited HSV-1 infection by 50% (IC50) in iPSC-neurons was 9.27 μM, 0.42 μM, and 41.35 μM, respectively (Figure 3a). The IC50 of ACV in iPSC-neurons is 0.27 μM (Supplementary Figure S4).
Figure 3. Antiviral efficacy and cytotoxicity of 30N12, 16F19, and 4F17 against HSV-1.
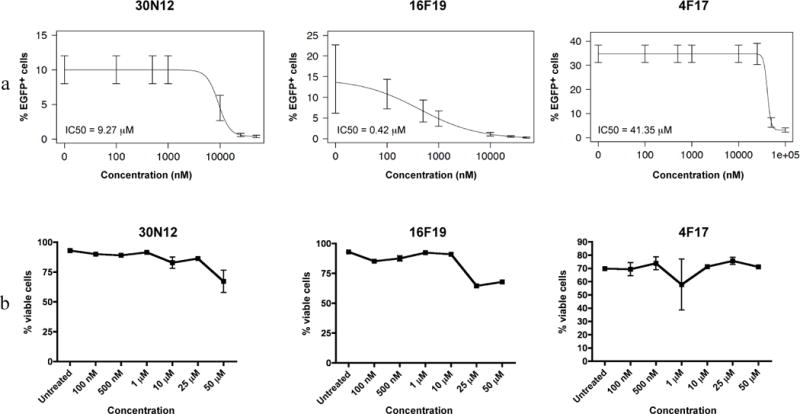
(a) Efficacy of varying drugs concentrations against HSV-1 lytic infection; FC performed 24hpi. (b) Neurotoxicity of 30N12, 16F19, and 4F17 without HSV-1 infection was assessed by FC using fixable viability dye. The data represent an average of three independent experiments. Error bars represent standard deviations.
3.3. Cellular Toxicity
At concentrations up to 50 μM, 30N12, 16F19, or 4F17 caused less than 50% reduction in iPSC-neuron viability, precluding estimation of their therapeutic index (Figure 3b). At higher concentrations, compound 30N12 showed mild cytotoxicity at 100 μM and 500 μM (viable cells: 74.5 ± 4.95% and 67.1 ± 0.808%, respectively). Compound 16F19 produced relatively mild cytotoxicity at 100 μM (viable cells: 75.7 ± 2.05%), but substantially higher effects at 500 μM (viable cells: 38.6 ± 0.723%). Compound 4F17 did not show significant cytotoxicity at either 100 μM or 500 μM (viable cells: 97.1 ±1.43% and 95.4 ± 0.289%, respectively; Supplementary Figure S5).
3.4. Effects on HSV-1 DNA copy number in iPSC-neurons
Next, we analyzed HSV-1 copy number using quantitative PCR (qPCR) to investigate whether these compounds alter HSV-1 DNA replication. HSV-1 DNA copy number was estimated in acutely infected iPSC-neurons 24hpi, using the EGFP sequence in the HSV-1 viral construct as a probe. In 5 ng of total DNA, the number of copies of viral DNA were as follows: HSV-1 infected, untreated iPSC-neurons (3,461,970), 30N12 (1,664), 16F19 (48,530), 4F17 (561,665), PAA (924), and ACV (483; Figure 4). Fluorescence in situ hybridization (FISH) analyses were consistent with the qPCR results: similar to ACV or PAA, a substantial reduction in viral DNA copy number was observed in infected cells treated with 30N12, or 16F19, and to a lesser extent with 4F17, (Supplementary Figure S1). Further, markedly reduced expression of the HSV-1 immediate early genes ICP0 and ICP4 was observed in separate immunocytochemistry experiments when infected cells were treated with ACV, PAA, 30N12, 16F19 or 4F17 (Supplementary Figure S2).
Figure 4. qPCR assays for HSV-1 genome copy number in iPSC-neurons.
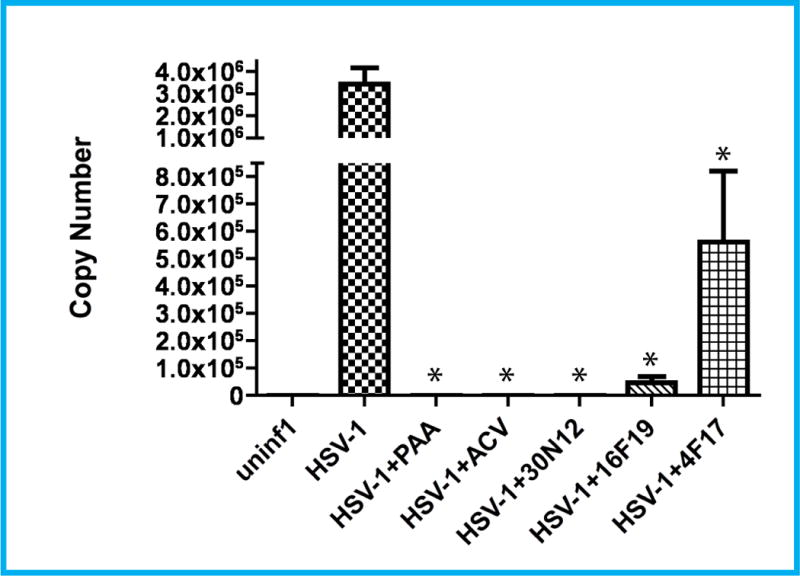
Cells were infected with HSV-1 for 24h in the presence or absence of test compounds (all at 50 μM). HSV-1 copy number calculated in 5 ng total DNA. ACV: acyclovir, PAA: phosphonoacetic acid. *p≤0.015. Error bars represent standard deviations.
3.5. Effects on reactivation of HSV-1 quiescent infection
Based on protocols for rodent models of HSV-1 latent infection, we have proposed a model of quiescent HSV-1 infection in iPSC-neurons (D’Aiuto et al., 2014). Treatment with 5BVdU+IFN-α causes a sustained reduction in the percentage of EGFP+ cells and EFGP+-RFP+ cells in HSV-1 infected iPSC-neurons; furthermore, HSV-1 can be reactivated subsequently by incubation with sodium butyrate (NaB, 5mM), a histone deacetylase inhibitor for 5 days (D’Aiuto et al., 2014). The pattern of infection, denoted “quiescent infection,” mimics several features of rodent latent infection models. In the present experiments, quiescent infection was induced with 5BVdU+IFN-α, the drugs were withdrawn and cells were incubated for 48h in the presence of the vehicle (DMSO) or drugs. Subsequently, the infection was reactivated with NaB (5 days). FC analysis showed a 16-fold increase of EGFP+ cells and 850-fold increase of EGFP+-RFP+ cells in vehicle treated cells (Figure 5a). Following incubation with 30N12, 16F19, 4F17, or ACV for 48h, a more modest reactivation was indicated by lower levels of EGFP+ cells (8-, 6-, 6-, and 2-fold, respectively) or EGFP+-RFP+ cells (201-, 97-, 26-, and 17-fold, respectively; Figure 5a). Compared to vehicle, treatment with ACV or test compounds also lowered the mean intensity of fluorescence (MFI; Figure 5b).
Figure 5. Effects of test compounds on reactivation of quiescent HSV-1 infection in iPSC-neurons.
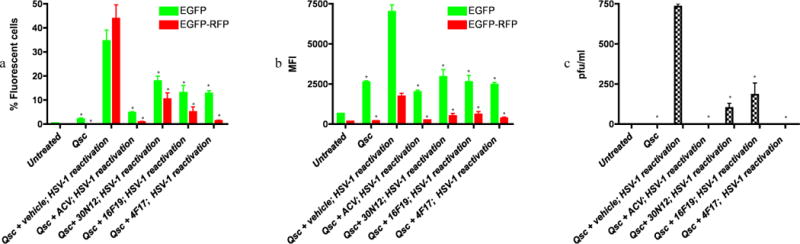
FC was performed after reactivation of quiescently infected iPSC-neuronal cultures 48h as described. (a) percentage of fluorescent cells. *p≤0.027. (b) mean fluorescence intensity (MFI). *p≤0.016. (c) Plaque assays of virus produced in the supernatant of reactivated cells titrated on Vero cells, shown as plaque forming units per milliliter (PFU/ml). *p≤0.002. The data represent an average of three independent experiments. 5BVdU: (E)-5-(2-bromovinyl)-2′-deoxyuridine, IFN-α: interferon alpha, NaB: sodium butyrate, vehicle: DMSO. Error bars represent standard deviations.
“Qsc”: Quiescent HSV-1 infection established by infecting iPSC-neurons with HSV-1 and then culturing in neurobasal medium containing 5BVdU+IFN-α for 7 days (See methods).
“Qsc+vehicle or compounds (ACV, 4F17, 16F19, or 30N12); HSV-1 reactivation”: 5BVdU+IFN-α were withdrawn and cells were cultured in the presence of vehicle or compounds for 48h; HSV-1 was then reactivated by treatment with NaB for 5 days.
Consistent with these results, the supernatant culture medium from neuronal cultures treated with 5BVdU+IFN-α, followed by ACV or 4F17 had no detectable infectious viral particles, while treatment with 30N12 and 16F19 resulted in a 7.3- and 4-fold decrease in infectious virion production as compared to treatment with vehicle for 48h (Figure 5c). Taken together, these results indicate that treatment with ACV or test compounds reduce HSV-1 reactivation.
3.6. Effects on VZV and HCMV infection
In the VZV permissive ARP19 line, infection with the VZV construct was monitored using luciferase activity. PAA effectively inhibited VZV infection, while ACV treatment caused a more modest 1.8 fold reduction at 50 μM. Incubation with 30N12, 16F19, and 4F17 resulted in 60.3-, 548.2-, and 2.7-fold reduction in luciferase activity, respectively, at 50 μM (Figure 6a). At these concentrations, none of the drugs were cytotoxic for ARPE-19 cells (Figure 6b). The IC50 values for 30N12 and 16F19 were estimated to be 11.52 μM and 2.43 μM (Figure 6c). At the highest concentration tested, 4F17 and ACV produced less than 50% inhibition.
Figure 6. Analysis of VZV infection.
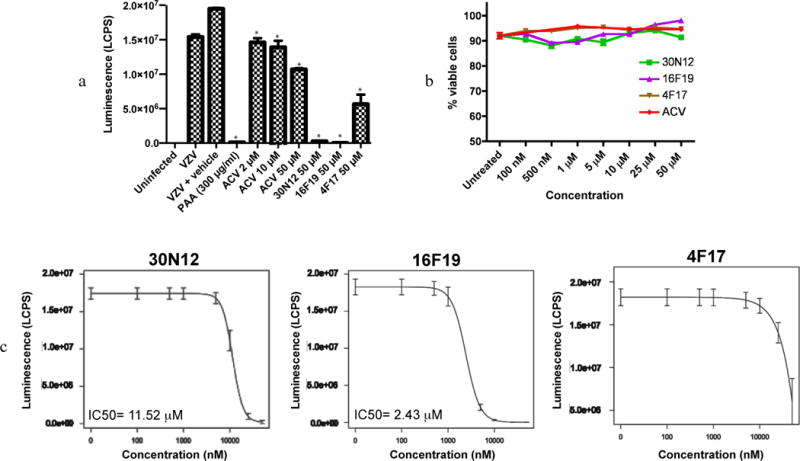
(a) ARPE-19 cell lysates were assayed for luciferase activity following infection with VZV-ORF63fluc in the presence or absence of drugs for 48hpi. LCPS: luciferase counts per second. *p≤0.013. (b) Cytotoxicity of the compounds in the absence of VZV in ARPE-19 cells. (c) Efficacy of 30N12, 16F19, and 4F17 at different concentrations; with the luciferase assay performed at 48hpi. The data represent an average of three independent experiments. Error bars represent standard deviations.
In permissive HFF cells infected with HCMV (MOI of 1), the IC50 and the concentration that reduced the HFF cell viability by 50% (CC50) for 4F17 were estimated at 8.85 μM and 123.68 μM, respectively (selectivity index: 13.98; Figure 7). IC50 and CC50 were estimated for compounds 30N12 (10.4 μM, 37.8 μM; selectivity index: 3.63) and 16F19 (4.1 μM, 12.3 μM; selectivity index: 3). However, both compounds exhibited a low selectivity index.
Figure 7. Anti-HCMV activity of 4F17.
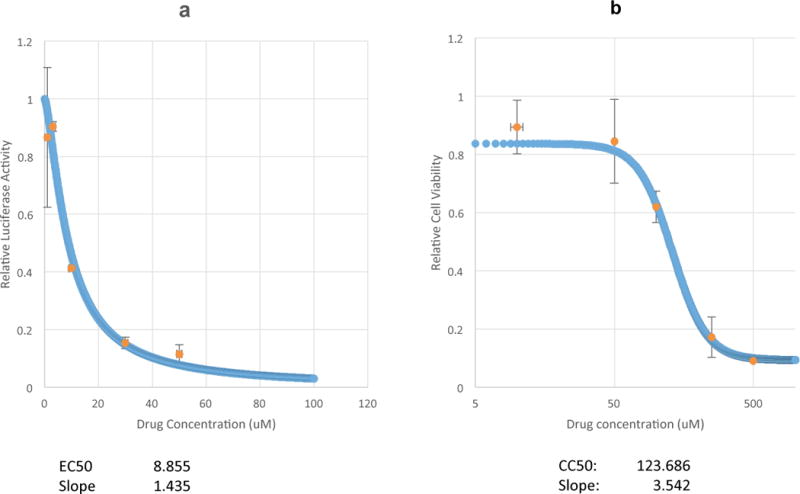
(a) Dose response of HCMV inhibition based on luciferase expression. (b) Cellular toxicity in non-infected HFF cells as determined by Cell-titer GLo assay. The data represent an average of three independent experiments. Error bars represent standard deviations.
4. Discussion
We assessed the potency and cytotoxicity of five novel non-nucleoside drugs for inhibiting infections with HSV-1, VZV, and CMV. Of these, compounds 30N12, 16F19, and 4F17 inhibited lytic HSV-1 with potencies similar to ACV, CQ, and BFLA. Like ACV, these compounds also inhibited viral reactivation in iPSC-neurons with prior quiescent HSV-1 infection. Furthermore, they were efficacious against VZV and compound 4F17 was also effective in vitro for HCMV infection. To our knowledge, these antiviral effects have not been reported before.
The mechanism of action of these drugs is unknown. The present investigations were motivated by the reported efficacy of lysosomotropic agents like CQ and BFLA against lytic HSV-1 infection (Harley et al., 2001). It was hypothesized that the antiviral activity of CQ and BFLA is due to increase in the pH of the trans-Golgi network (TGN) and endosomes–organelles that are critical for HSV-1 egress (Johnson and Baines, 2011). As the test compounds were previously reported to interfere with Golgi-to-cell surface traffic (Nieland et al., 2004), we predicted that they would inhibit productive HSV-1 infection. Productive infection was indeed reduced by 30N12, 16F19, and 4F17. In addition, these agents also reduced the proportion of IE-expressed EGFP+ cells, with concomitant reduction in MFI. Further, FISH analyses also indicated a substantial reduction in viral DNA load (Supplementary Figure S1). Several recent reports have strongly implied that, at least for HSV-1, viral entry may utilize the endosomes in some cell types (Nicola et al., 2005) (Döhner et al., 2002) (Harkness et al., 2014). It is unlikely these lysosomotropic compounds may affect HSV-1 entry in Vero cells and neurons, as all the drugs were added to cell cultures 2h after HSV-1 infection, when most of the nucleocapsids are expected to be transported to the nuclear membrane; further, viral entry was previously reported to be pH-independent in neurons (Nicola et al., 2005). These compounds do not appear to affect viral attachment and entry (Supplementary Figure S6). Taken together, these results indicate that 30N12, 16F19, and 4F17 exert their antiviral effects by influencing one or more stages of the early phase of the HSV-1 lytic cycle and late stages of infection. Although our data clearly indicate that 30N12, 16F19, and 4F17 efficiently block events at or before DNA replication, the exact stage of the block remains to be determined. While our analyses suggest that these compounds reduce IE gene expression consistent with earlier reports, the repression may be a secondary effect of multiple rounds of replication (Park et al., 2013; Pierce et al., 2005; Zambrano et al., 2008).
The patterns of the effects on HSV-1 infection may differ between the compounds. For example, the IC50 for 1619 is 10- to 100-fold lower than the other compounds, the slope of the dose response curve is more gradual, and the values are more variable. This suggests a different mechanism of action compared with 30N12 and 4F17. Indeed, 16F19 may have multiple targets in Vero cells, although it is not possible to identify them at present.
The spectrum of antiviral activity of these compounds is also noteworthy. 30N12, 16F19, and 4F17 inhibited lytic VZV infection with greater potency than ACV (Figure 6a). These compounds also effectively inhibited HCMV infection; however, 30N12 and 16F19 showed a low selectivity index. It is not known whether the mechanisms of action against VZV and HCMV are similar to their effects against HSV-1 infection. It is likely to be different at least for 16F19, as the IC50 curve for this compound is typical, in contrast to a more complex effect against HSV-1 (Figure 6).
In summary, we identified three non-nucleoside compounds with potent antiviral potency comparable to that of ACV against HSV-1. Consistent with prior reports of relatively low efficacy of ACV against VZV vis a vis HSV-1 infection (Iwayama et al., 1998), the test compounds showed a higher potency than ACV against VZV, at concentrations that produced relatively low cellular toxicity. In addition, HCMV infection was inhibited effectively. The selective activities for individual herpesviruses suggest the agents have different mechanisms of activity, or influence cellular processes that are more important for one herpesvirus over another. Further studies are needed to investigate the underlying mechanism/s accounting for the antiviral activity of these compounds.
Supplementary Material
Highlights.
We analyzed the antiherpetic properties of novel lysosomotropic agents on herpesviruses infection.
We identified three compounds, 30N12, 16F19, and 4F17, that efficiently inhibit HSV-1, HCMV, and VZV infection.
Compounds reduce HSV-1 reactivation from quiescently infected human neurons derived from induced pluripotent stem cells.
Acknowledgments
The authors acknowledge the following colleagues for the critical reading of the manuscript and technical support: Joel Wood, Chowdari Kodavali, Peter Dimitrion, Benjamin Treat, and Rebecca Lipski. This study was funded by the Stanley Medical Research Institute (SMRI) grant ID 07R-1712 to VLN and NIH 5R01AI093701 to RAB. PRK acknowledges support from EY08098, Research to Prevent Blindness Inc. and the Eye & Ear Foundation of Pittsburgh. The funding source had no involvement in the collection, analysis, and interpretation of the data; the conduct of this research; nor the preparation of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayer A, Delorme-Axford E, Sleigher C, Frey TK, Trobaugh DW, Klimstra WB, Emert-Sedlak LA, Smithgall TE, Kinchington PR, Vadia S, Seveau S, Boyle JP, Coyne CB, Sadovsky Y. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am J Obstet Gynecol. 2015;212:71.e71–78. doi: 10.1016/j.ajog.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho JA, Mitchell C, Roizman B. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J Virol. 1993;67:3891–3900. doi: 10.1128/jvi.67.7.3891-3900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrel S, Aime C, Hermet L, Ait-Arkoub Z, Agut H, Boutolleau D. Surveillance of herpes simplex virus resistance to antivirals: a 4-year survey. Antiviral Res. 2013;100:365–372. doi: 10.1016/j.antiviral.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- D’Aiuto L, Di Maio R, Heath B, Raimondi G, Milosevic J, Watson AM, Bamne M, Parks WT, Yang L, Lin B, Miki T, Mich-Basso JD, Arav-Boger R, Sibille E, Sabunciyan S, Yolken R, Nimgaonkar V. Human induced pluripotent stem cell-derived models to investigate human cytomegalovirus infection in neural cells. PLoS One. 2012;7:e49700. doi: 10.1371/journal.pone.0049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aiuto L, Prasad KM, Upton CH, Viggiano L, Milosevic J, Raimondi G, McClain L, Chowdari K, Tischfield J, Sheldon M, Moore JC, Yolken RH, Kinchington PR, Nimgaonkar VL. Persistent Infection by HSV-1 Is Associated With Changes in Functional Architecture of iPSC-Derived Neurons and Brain Activation Patterns Underlying Working Memory Performance. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhner K, Wolfstein A, Prank U, Echeverri C, Dujardin D, Vallee R, Sodeik B. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol Biol Cell. 2002;13:2795–2809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness JM, Kader M, DeLuca NA. Transcription of the herpes simplex virus 1 genome during productive and quiescent infection of neuronal and nonneuronal cells. J Virol. 2014;88:6847–6861. doi: 10.1128/JVI.00516-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CA, Dasgupta A, Wilson DW. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J Virol. 2001;75:1236–1251. doi: 10.1128/JVI.75.3.1236-1251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Sandford G, Hayward GS, Burns WH, Posner GH, Forman M, Arav-Boger R. Recombinant luciferase-expressing human cytomegalovirus (CMV) for evaluation of CMV inhibitors. Virol J. 2011;8:40. doi: 10.1186/1743-422X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwayama S, Ono N, Ohmura Y, Suzuki K, Aoki M, Nakazawa H, Oikawa M, Kato T, Okunishi M, Nishiyama Y, Yamanishi K. Antiherpesvirus activities of (1′S,2′R)-9-[[1′,2′-bis(hydroxymethyl)cycloprop-1′-yl]methyl]guanine (A-5021) in cell culture. Antimicrob Agents Chemother. 1998;42:1666–1670. doi: 10.1128/aac.42.7.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45:804–817. doi: 10.1053/j.ajkd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- Joubert PE, Meiffren G, Grégoire IP, Pontini G, Richetta C, Flacher M, Azocar O, Vidalain PO, Vidal M, Lotteau V, Codogno P, Rabourdin-Combe C, Faure M. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleymann G, Fischer R, Betz UA, Hendrix M, Bender W, Schneider U, Handke G, Eckenberg P, Hewlett G, Pevzner V, Baumeister J, Weber O, Henninger K, Keldenich J, Jensen A, Kolb J, Bach U, Popp A, Mäben J, Frappa I, Haebich D, Lockhoff O, Rübsamen-Waigmann H. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nat Med. 2002;8:392–398. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- Krepstakies M, Lucifora J, Nagel CH, Zeisel MB, Holstermann B, Hohenberg H, Kowalski I, Gutsmann T, Baumert TF, Brandenburg K, Hauber J, Protzer U. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J Infect Dis. 2012;205:1654–1664. doi: 10.1093/infdis/jis273. [DOI] [PubMed] [Google Scholar]
- Malvy D, Treilhaud M, Bouée S, Crochard A, Vallée D, El Hasnaoui A, Aymard M, Group RS. A retrospective, case-control study of acyclovir resistance in herpes simplex virus. Clin Infect Dis. 2005;41:320–326. doi: 10.1086/431585. [DOI] [PubMed] [Google Scholar]
- Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79:7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieland TJ, Feng Y, Brown JX, Chuang TD, Buckett PD, Wang J, Xie XS, McGraw TE, Kirchhausen T, Wessling-Resnick M. Chemical genetic screening identifies sulfonamides that raise organellar pH and interfere with membrane traffic. Traffic. 2004;5:478–492. doi: 10.1111/j.1398-9219.2004.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PJ, Antoine TE, Farooq AV, Valyi-Nagy T, Shukla D. An investigative peptide-acyclovir combination to control herpes simplex virus type 1 ocular infection. Invest Ophthalmol Vis Sci. 2013;54:6373–6381. doi: 10.1167/iovs.13-12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AT, DeSalvo J, Foster TP, Kosinski A, Weller SK, Halford WP. Beta interferon and gamma interferon synergize to block viral DNA and virion synthesis in herpes simplex virus-infected cells. J Gen Virol. 2005;86:2421–2432. doi: 10.1099/vir.0.80979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Knickelbein JE, Ferko C, Hendricks RL, Kinchington PR. Development and pathogenic evaluation of recombinant herpes simplex virus type 1 expressing two fluorescent reporter genes from different lytic promoters. Virology. 2008;378:254–264. doi: 10.1016/j.virol.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutzhard E. Viral infections of the CNS with special emphasis on herpes simplex infections. J Neurol. 2001;248:469–477. doi: 10.1007/s004150170155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- Stránská R, Schuurman R, Nienhuis E, Goedegebuure IW, Polman M, Weel JF, Wertheim-Van Dillen PM, Berkhout RJ, van Loon AM. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J Clin Virol. 2005;32:7–18. doi: 10.1016/j.jcv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Strasfeld L, Chou S. Antiviral drug resistance: mechanisms and clinical implications. Infect Dis Clin North Am. 2010;24:809–833. doi: 10.1016/j.idc.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Sukla S, Biswas S, Birkmann A, Lischka P, Zimmermann H, Field HJ. Mismatch primer-based PCR reveals that helicase-primase inhibitor resistance mutations pre-exist in herpes simplex virus type 1 clinical isolates and are not induced during incubation with the inhibitor. J Antimicrob Chemother. 2010;65:1347–1352. doi: 10.1093/jac/dkq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Zambrano A, Solis L, Salvadores N, Cortés M, Lerchundi R, Otth C. Neuronal cytoskeletal dynamic modification and neurodegeneration induced by infection with herpes simplex virus type 1. J Alzheimers Dis. 2008;14:259–269. doi: 10.3233/jad-2008-14301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


