Abstract
Stroke survivors without cerebellar involvement retain the ability to adapt to the split-belt treadmill, however it has been suggested that their rate of adaptation may be slowed compared to those who are neurologically intact. Depending on limb placement, the split-belt treadmill can be configured to either exaggerate baseline asymmetry, or reduce it, which may affect the behavior of adaptation or de-adaptation. The objectives of this study were to characterize the rate and magnitude of locomotor (de)adaptation in chronic stroke survivors compared to healthy matched subjects, and to evaluate whether exaggeration or reduction of baseline asymmetry impact the responses. Seventeen stroke survivors and healthy subjects completed 10 minutes of split-belt treadmill walking, then 5 minutes of tied-belt walking. Stroke survivors completed this once with each leg on the fast belt. Magnitude and rate of (de)adaptation were evaluated for step length and limb phase asymmetry. There were no differences between the groups with the exception of the reduced step length asymmetry configuration, in which case there was a significantly reduced magnitude (p=<0.000) and rate (p=0.011) of adaptation when compared to controls. There was a similar trend observed during post-adaptation for the exaggerated asymmetry group. The rate and magnitude of locomotor (de)adaptation is similar between chronic stroke survivors and neurologically intact controls, except when the adaptation or de-adaptation response would take the stroke survivors away from a symmetric step length pattern. This suggests that there may be some benefit to symmetry that is recognized by the system.
Keywords: Stroke, Locomotion, Adaptation
Introduction
Each year, approximately 795,000 people in the United States suffer a stroke, and stroke is the leading cause of serious long-term disability (Lloyd-Jones et al., 2010). Walking deficits, such as slow speed and temporal and spatial asymmetries, are often observed following stroke and have a profound impact on functional independence (Bohannon, 1987; Olney et al., 1994; Schmid et al., 2007). Motor adaptation has been a suggested method through which such abnormal movement patterns can be improved after stroke (Abdollahi et al., 2014; Reisman et al., 2010).
Martin et al. defines motor adaptation as a process of adjusting an already well-learned motor skill over a period of trial-and-error practice while being exposed to a novel and perturbing environment (Martin et al., 1996). Upon introduction of the novel environment, there are motor errors made because of a mismatch between what the nervous system expects and the newly perturbed movement. However, with practice in the novel environment, feed-forward adjustments are used to change the existing motor command to meet the demands of the novel environment. This process is called adaptation. When the perturbing environment is removed, the nervous system continues to use the newly adapted pattern, which is no longer appropriate for the given demands. This again causes a mismatch between what is expected and what occurs (after-effects), so an additional series of trial-and-error practice is required to return the movement to its original state. This process is called de-adaptation (Reisman et al., 2010).
One method that has been used to create a novel and perturbing locomotor environment is the split-belt treadmill. This unique treadmill has two independently controlled belts: one under each foot. It can therefore be configured such that one belt, and therefore one foot, is forced to travel double the speed of the other. This perturbing locomotor environment has been shown to induce locomotor adaptation and de-adaptation in both healthy subjects and stroke survivors who are free from cerebellar dysfunction (Reisman et al., 2005; Reisman et al., 2007; Reisman et al., 2009; Reisman et al., 2010). This demonstrates that the basic capacity for locomotor adaptation remains intact after stroke.
While the basic capacity of locomotor adaptation appears to be intact post-stroke, the speed or magnitude of these adaptations may be altered. Previous studies have found that the rate of adaptation to the split-belt treadmill varies with age and between spatial and temporal variables (Musselman et al., 2011; Reisman et al., 2005; Vasudevan et al., 2009), and recent studies suggest that locomotor adaptation may be slowed after stroke (Savin et al., 2012). Understanding differences in the behavior of adaptation and de-adaptation after stroke compared to those who are neurologically intact could provide insight into how stroke impacts the feed-forward control mechanisms that are used to modify gait on an ongoing basis.
It has been previously reported that there is no difference in the behavior of locomotor adaptation and de-adaptation when there is a comparison made between conditions where the paretic limb is placed on the slow belt and the paretic limb is placed on the fast belt (Reisman et al., 2007). This comparison, however, may not be the most relevant, due variations in the behavior of spatiotemporal gait asymmetries after stroke. It is known that most survivors of stroke walk with spatial and temporal asymmetries, however, it is important to note that these asymmetries are not in the same “direction” from person to person, or from variable to variable (Malone and Bastian, 2014; Reisman et al., 2007). For example, one stroke survivor can have a longer paretic step, and the next can have a longer non-paretic step. We know that the split-belt treadmill can be configured to either exaggerate the baseline step length asymmetry or reduce it, depending on which limb is placed on the slow belt (Reisman et al., 2007). Thus, given the variation in step length asymmetry “direction”, for one person, the paretic leg on the slow belt configuration would exaggerate the baseline step length asymmetry, and for the other, it would reduce it. Asymmetries of temporal variables follow similar patterns. These differences are important because it is possible that the error signals during early adaptation are interpreted differently if the participants' asymmetry is exaggerated versus reduced.
The purpose of this study is therefore, two fold. First, we aim to compare the rate and magnitude of adaptation and de-adaptation in subjects who have had a stroke to those who are neurologically intact. Second, we aim to examine the effect of exaggerating or reducing a stroke survivor's asymmetry on the rate and magnitude of adaptation and de-adaptation. We hypothesized that stroke survivors would demonstrate a larger magnitude and faster rate of adaptation when the split-belt is configured to exaggerate the baseline asymmetry compared to when it is configured to reduce baseline asymmetry. Additionally, we hypothesized that there would be a larger magnitude and faster rate of de-adaptation when the after-effects result in an exaggeration of baseline asymmetry.
Methods
Participants
Participants with chronic stroke and age- and gender-matched neurologically intact participants were recruited and all participants signed an informed consent approved by the University of Delaware Human Subjects Review Board. To be included, participants with stroke must have sustained a single stroke at least 6 months prior to study participation and be able to walk independently at a treadmill speed > 0.2 m/s with no more than light touch on the handrail. The participants who were neurologically intact must have been free from any neurological dysfunction and any musculoskeletal problem that impacted ambulation, and have an age ± 5 years of their stroke participant counterpart. Exclusion criteria for both groups included uncontrolled blood pressure or diabetes, cardiovascular or arthritic dysfunction exacerbated by exercise, and active cancer.
Instrumentation and Procedures
The participants with stroke completed the split-belt walking protocol on two separate days, with at least one week separating the two data collections. One data collection was completed with the paretic leg on the fast belt (HemiFast), and the other with the paretic leg on the slow belt (HemiSlow), the order of which was randomized.
For each participant, the speeds used during the split-belt walking portion were set in a 2:1 ratio. For the participants with stroke, the fast treadmill belt speed was chosen when: (1) the participant declined further increase in speed (2) the researcher determined that it was unsafe to further increase the speed. Fifty percent of this speed was used as the slow belt speed during split-belt treadmill walking. Participants who were neurologically intact walked at the same speeds as their stroke participant counterparts, and the limb that was placed on the fast belt was randomized. Figure 1 represents the experimental protocol.
Figure 1. Experimental Protocol.
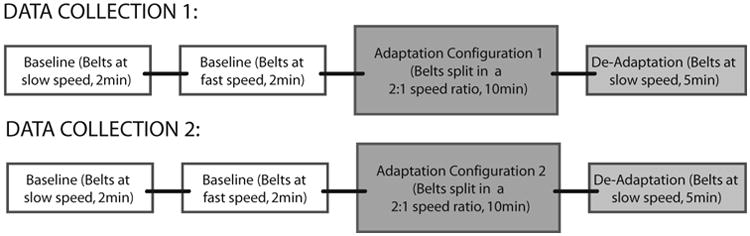
All participants walked on a split-belt treadmill instrumented with two independent 6 degree of freedom force platforms (AMTI, Watertown, MA) from which ground reaction force data were continuously collected at 2000Hz. Kinematic data were continuously collected using an 8-camera Vicon Motion Capture System (Vicon MX, Los Angeles, CA) at 100Hz. Retro-reflective markers (14-mm diameter) were placed on rigid shells over the pelvis, bilateral thigh, shank, and foot segments. Single markers were placed on the medial and lateral iliac crests, greater trochanters, knee joint line, and malleoli. During walking, all participants rested fingertips on an instrumented handrail which provided real time quantitative data for vertical force exerted by the participant. To keep handrail use consistent, vertical forces were monitored and verbal cues were provided if a change in force was observed.
All participants wore a safety harness around their chest for fall prevention. Participants with stroke were permitted to have standing or sitting rests during testing, but they did not dismount from the treadmill.
Data Analysis
All data were processed using Visual 3D (C-Motion, Inc., Germantown, MD). The gait events of foot strike and lift off were determined for each limb individually using an automatic algorithm in Visual 3D, after which they were visually checked for accuracy.
Dependent variables
Previous studies have found that spatial and temporal variables respond differently to split-belt walking (Malone and Bastian, 2010; Malone et al., 2011; Malone et al., 2012a; Tyrell et al., 2014). We therefore evaluated a spatial (step length) and temporal (limb phasing) variable in this study. Step length and limb phasing were calculated for each leg continuously throughout the protocol. Step length was calculated as the sagittal distance between the right and left heel markers at foot strike. Step asymmetry was calculated as follows:
Limb phase was a measure of the time lag between contralateral peak flexion and ipsilateral peak extension, and was calculated as described in our previous work (Tyrell et al., 2014). In order to evaluate the specific characteristics of adaptation in our participants, we calculated the following measures for each variable (Malone et al., 2011):
Magnitude of initial perturbation was calculated as the absolute value of the average of the first 30 strides of adaptation symmetry data, minus the average of baseline. This value was a measure of how much a subject's walking pattern was perturbed by the split-belt treadmill, before adaptation occurred.
Magnitude of adaptation was calculated as the average of the final 30 strides of split-belt walking, minus the average of baseline. This value was a measure of how well a subject was able to resolve the early asymmetry caused by the split-belt treadmill by the end of adaptation. A value of 0 would signify that a subject had completely resolved the initial perturbation, and was able to resume their baseline symmetry pattern despite the continued 2:1 belt speed discrepancy.
Rate of adaptation was a measure of the time required to resolve the initial perturbation, and achieve a stable pattern in gait symmetry despite the continued 2:1 belt speed discrepancy (Huang et al., 2011). Rate of adaptation was determined by creating bins of 10 strides (symmetry data minus the average of baseline) for each subject during the adaptation period. The contents of the first 10 bins were then compared statistically between the neurologically intact group and the stroke conditions.
Magnitude of initial after-effect was calculated as the absolute value of the average of the first 10 strides of de-adaptation symmetry data, minus the average of baseline. Magnitude of de-adaptation was analogous to magnitude of adaptation, but was calculated as the average of the final 20 strides of the de-adaptation period, minus the average of baseline.
Rate of de-adaptation was analogous to rate of adaptation, and was calculated similarly.
Conditions and subjects
As mentioned above, previous studies of split-belt treadmill walking in stroke survivors have reported rate and magnitude data during adaptation and de-adaptation based solely on whether the hemiparetic limb is on the slow belt (HemiSlow), or on the fast belt (HemiFast). In an effort to test our hypotheses, as well as put our findings in the context of this previously published work, we have chosen to present our data according to the conditions of exaggerated and reduced baseline asymmetry, as well as HemiFast and HemiSlow.
Seventeen participants with stroke and seventeen participants who were neurologically intact participated in this study. Table 1 contains participant information. Table 2 contains information about how the subjects' data were re-grouped based on baseline asymmetry and symmetry of initial after-effects.
Table 1. Participant Demographics and Speeds.
| Subject # | Age | Sex | Months since stroke | Hemiparetic side | Speeds (m/s) |
|---|---|---|---|---|---|
| 1 | 67 | M | 30 | L | 0.5 / 1.0 |
| 2 | 52 | M | 119 | L | 0.5 / 1.0 |
| 6 | 62 | M | 49 | R | 0.3 / 0.6 |
| 9 | 67 | M | 114 | R | 0.3 / 0.6 |
| 13 | 48 | M | 100 | R | 0.5 / 1.0 |
| 14 | 62 | M | 82 | R | 0.3 / 0.6 |
| 53 | 72 | M | 80 | R | 0.2 / 0.4 |
| 67 | 78 | M | 37 | R | 0.3 / 0.6 |
| 71 | 46 | F | 30 | L | 0.3 / 0.6 |
| 87 | 74 | M | 65 | R | 0.3 / 0.6 |
| 110 | 66 | F | 21 | R | 0.3 / 0.6 |
| 115 | 70 | M | 25 | R | 0.5 / 1.0 |
| 116 | 59 | M | 12 | L | 0.2 / 0.4 |
| 136 | 58 | M | 20 | R | 0.4 / 0.8 |
| 142 | 70 | F | 16 | L | 0.2 / 0.4 |
| 155 | 54 | M | 5 | L | 0.3 / 0.6 |
| 171 | 60 | M | 6 | L | 0.4 / 0.8 |
Table 2.
Participant Groups Based on Baseline Asymmetry.
| Subject # | Subjects in Exaggerated Step Length Baseline Asymmetry condition | Subjects in Reduced Step Length Baseline Asymmetry condition | Subjects in Exaggerated Limb Phase Baseline Asymmetry condition | Subjects in Reduced Limb Phase Baseline Asymmetry condition |
|---|---|---|---|---|
| 1 | Sub1HF (s) | Sub1HS | Sub1HS | Sub1HF |
| 2 | Sub2HS (u) | Sub2HF | Sub2HS | Sub2HF |
| 6 | Sub6HS (o) | Sub6HF | Sub6HS | Sub6HF |
| 9 | Sub9HS (u) | Sub9HF | Sub9HS | Sub9HF |
| 13 | Sub13HS (u) | Sub13HF | Sub13HS | Sub13HF |
| 14 | Sub14HS (s) | Sub14HF | Sub14HF | Sub14HS |
| 53 | Sub53HS (u) | Sub53HF | Sub53HS | Sub53HF |
| 67 | Sub67HF (o) | Sub67HS | Sub67HF | Sub67HS |
| 71 | Sub71HS (s) | Sub71HF | Sub71HS | Sub71HF |
| 87 | Sub87HF (o) | Sub87HS | Sub87HF | Sub87HS |
| 110 | Sub110HS (u) | Sub110HF | Sub110HS | Sub110HF |
| 115 | Sub115HF (u) | Sub115HS | Sub115HS | Sub115HF |
| 116 | Sub116HS (s) | Sub116HF | Sub116HF | Sub116HS |
| 136 | Sub136HF (s) | Sub136HS | Sub136HF | Sub136HS |
| 142 | Sub142HS (o) | Sub142HF | Sub142HS | Sub142HF |
| 155 | Sub155HS (s) | Sub155HF | Sub155HF | Sub155HS |
| 171 | Sub171HF (u) | Sub171HS | Sub171HF | Sub171HS |
HF=HemiFast and HS=HemiSlow.
(s)=symmetrical initial after-effect
(o)=overshot perfect symmetry at initial after-effect
(u)=undershot perfect symmetry at initial after-effect
Statistical Analysis
Normality of the data distribution was confirmed using the Kolmogorov-Smirnov test for normality. All statistical testing was completed using SPSS v19. The magnitude of initial perturbation, magnitude of initial after-effect, magnitude of adaptation, and magnitude of de-adaptation were compared between groups and conditions. Stroke conditions were compared to each other using a paired t-test, and each stroke condition was compared to the neurologically intact group using independent samples t-tests. A Bonferonni correction was used to account for the repeated comparisons to the neurologically intact group, so the level of statistical significance was determined to be p<0.025. To evaluate rate of (de)adaptation between groups, a repeated measures ANOVA was used to compare the contents of the first 10 bins of symmetry data (100 strides) during adaptation and the first 8 bins of de-adaptation data (80 strides), (within-subjects factor of bin number, and a between subjects factor of group). In these analyses a group × bin interaction would indicate that the rate of change differed between the two groups.
Results
Figure 2 illustrates step length symmetry data for a representative participant who is neurologically intact, and a stroke survivor in each condition. In all cases, the participant is most perturbed during early adaptation and becomes progressively less so with increased exposure. Note that the participant who is neurologically intact has baseline data averaging near the value of 0 (zero indicates perfect symmetry), and the participant with stroke has a baseline with an average greater than 0, signifying a longer paretic step at baseline. Late in the adaptation period in both the neurologically intact participant and in the stroke participant in the exaggerated asymmetry (HemiSlow) condition, the initial asymmetry has been resolved with a return to baseline (a)symmetry, where as the participant with stroke in the reduced asymmetry condition (HemiFast) demonstrates a substantial difference between the symmetry in late adaptation when compared to baseline. Limb phase data followed a similar pattern.
Figure 2. Representative Subjects for Step Length.
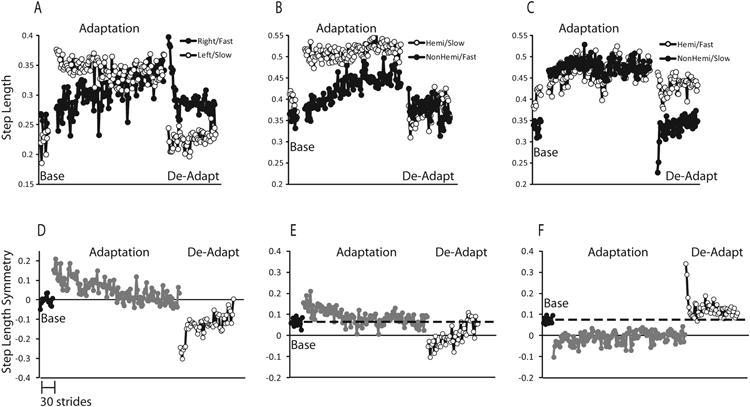
Individual limb data during the baseline, adaptation, and de-adaptation periods from a representative participant who is neurologically intact (A), a stroke participant exposed to the exaggerated/HemiSlow condition (B), and the reduced aymmetry/HemiFast condition (C). Symmetry data during the baseline, adaptation, and de-adaptation periods from a representative participant who is neurologically intact (D), a representative stroke participant in the exaggerated/HemiSlow condition (E) and in the reduced asymmetry/HemiFast condition (F). Each data point represents the average over 3 strides of data. In E and F, the dashed line represents baseline data. It should be noted that although the HemiSlow condition resulted in exaggerated asymmetry for this subject for another subject the opposite may have been true.
Magnitude of initial perturbation
There were no differences in the magnitude of initial perturbation for either variable between any group/condition, indicating that all groups/conditions were initially perturbed similarly by the split-belts (Figure 3).
Figure 3.
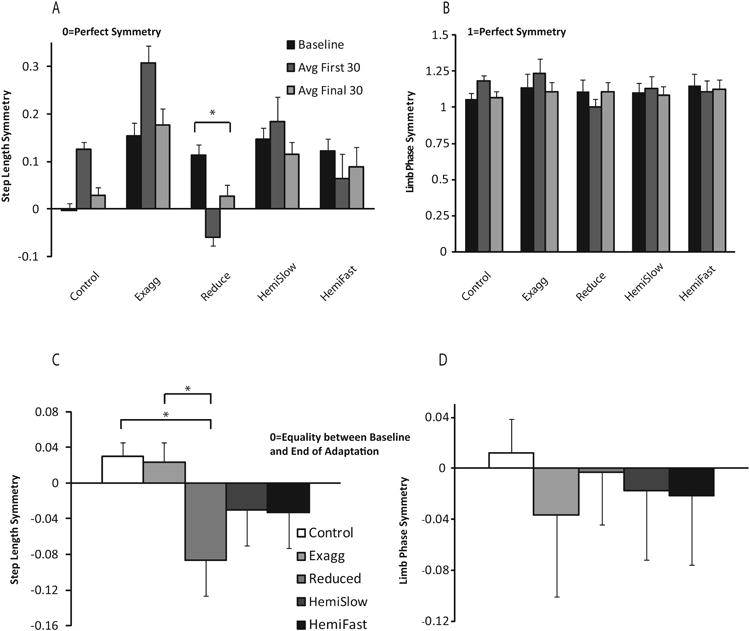
Step length symmetry (A) and limb phase symmetry (B) averaged across phases. Error bars represent standard error. Magnitude of step length (C) and limb phase (D) adaptation. Complete return to baseline is represented by the value of 0, positive values represent an average symmetry value higher than baseline at the end of adaptation, whereas negative values represent an average symmetry value lower than baseline at the end of adaptation. Neurologically intact group data is represented by white bars and each stroke condition is represented by a different shade of grey. Error bars represent standard error.
Magnitude of adaptation
The magnitude of adaptation is reduced after stroke when the initial split-belt perturbation is configured to reduce baseline step length asymmetry, as compared to neurologically intact control subjects (Figure 3). Figure 3a and b represents the average of baseline, the average of the first 30 strides, and the average of the final 30 strides of adaptation for the control subjects, and then for our group of 17 stroke subjects, grouped as described in the Data Analysis section. We compared the average of the last 30 strides of adaptation to the average of baseline in each group. By the end of the adaptation period, symmetry has returned to baseline in all cases except for the reduced asymmetry condition for step length (Figure 3a, p=0.001). In this case, at the end of adaptation the stroke participants remain more symmetric than at baseline.
Figure 3c and d presents the data for step length and limb phase magnitude of adaptation for each group and condition. A value of 0 for this variable demonstrates a complete return to baseline after 10 minutes of exposure. For step length symmetry, the large negative values in the reduced asymmetry condition indicate that, on average, in this condition participants did not adapt back to baseline to the same extent as in the exaggerated asymmetry condition (Figure 3c, p=0.001) or the neurologically intact group (Figure 3c, p<0.000). For the remaining step length conditions, and for all limb phase conditions, there were no statistical differences between conditions or groups (Figure 3d).
To further examine the differences in the magnitude of asymmetry in the reduced asymmetry condition, we analyzed the behavior of the exaggerated baseline asymmetry and reduced baseline asymmetry conditions as seen in Figure 4. Figure 4a shows clearly that for step length, the reduced asymmetry condition remains near perfect symmetry (symmetry = 0) through the end of the adaptation period, as compared to the exaggerated asymmetry condition, in which the subjects adapt back toward their baseline. Figure 4b shows parallel data for limb phase, but in this case, both conditions adapt back toward their respective baselines by the end of the adaptation period.
Figure 4.
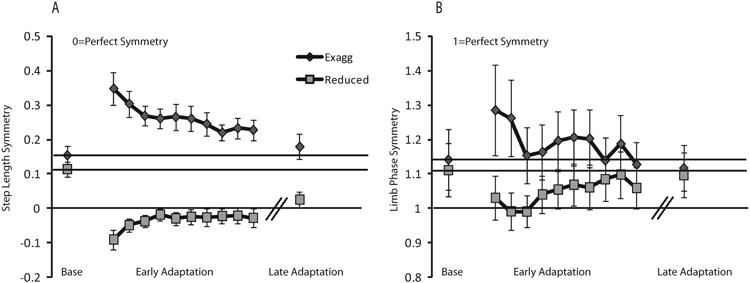
Step length adaptation asymmetry (A) and limb phase adaptation asymmetry (B) at baseline (Base), early adaptation, and late adaptation averaged over subjects in each condition. Baseline data is averaged over the entire baseline period. Each data point in the Early adaptation period represents an average of 10 strides. Late adaptation data is averaged over the last 20 strides during adaptation. Error bars represent standard error. Black lines represent baseline for each individual group.
Rate of adaptation
The rate of adaptation is slowed after stroke when the initial split-belt perturbation is configured to reduce baseline step length asymmetry, as compared to neurologically intact control subjects (Figure 5a). This is visually evident in Figure 5a as the slope of change between bins 2 and 5 is less steep for this group when compared to the neurologically intact group. This is reflected by a bin × group interaction (Figure 5a, p=0.011) and is consistent with the magnitude data showing that in this group subjects adapted more slowly and to a lesser magnitude. There were no differences between the exaggerated, HemiSlow or HemiFast condition and the neurologically intact group (Figure 5c). For limb phase, there were no group × bin interactions suggesting a similar rate of adaptation across groups and conditions (Figure 5b, d).
Figure 5. Asymmetry of Adaptation over Strides.
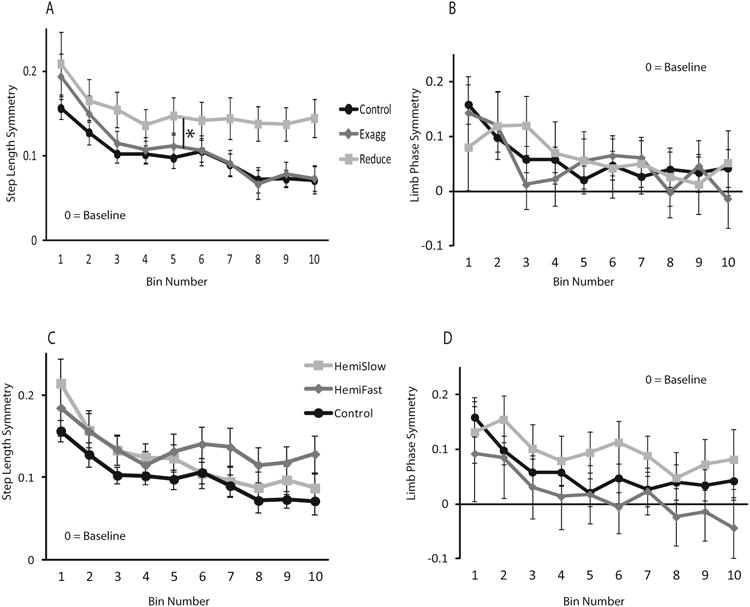
A: Step length asymmetry exaggerated and reduced conditions and neurologically intact group, B: limb phase asymmetry exaggerated and reduced conditions and neurologically intact group, C: Step length asymmetry HemiSlow and HemiFast conditions and neurologically intact group, D: limb phase asymmetry HemiSlow and HemiFast conditions and neurologically intact group. In order to compare across groups, negative adaptation data for the reduced condition was transformed across the x-axis before figures and calculations were completed. Each data point represents an average of 10 consecutive strides. Zero equals baseline. Error bars represent standard error. * indicates a significant difference between the reduced asymmetry group and the neurologically intact group.
Magnitude of initial after-effect
There were no differences in the magnitude of initial after-effect for either variable between any group or condition, indicating that all groups/conditions showed similar after-effects at the start of the de-adaptation phase.
Magnitude and rate of de-adaptation
There were no differences in magnitude of de-adaptation for either variable between any group or condition. Figure 6 presents the binned de-adaptation symmetry data for step length (a) and limb phase (b) for each group/condition. There were no group × bin interactions, suggesting a similar rate of de-adaptation across groups This includes the comparison between the HemiSlow and the control groups depicted in 6c. Although visually it appears as if the HemiSlow subjects have a slower rate of de-adaptation, the p-value for this comparison was 0.363.
Figure 6. Asymmetry of De-Adaptation over Strides.
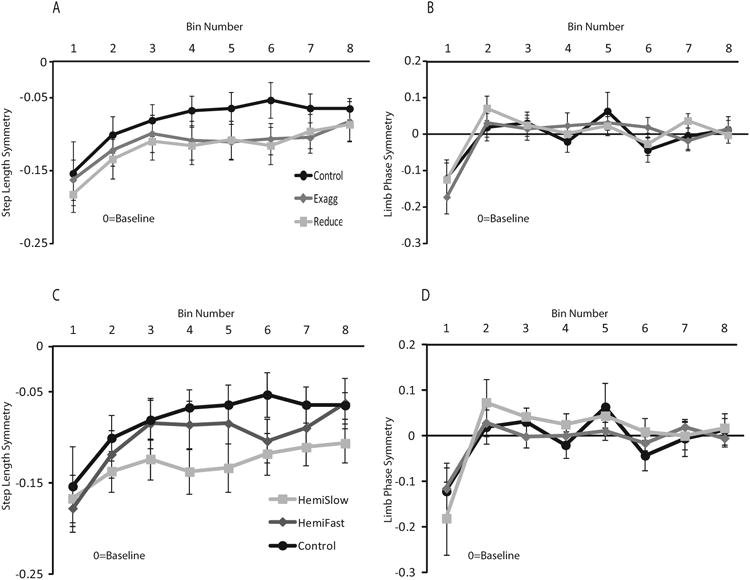
A: Step length asymmetry exaggerated and reduced conditions and neurologically intact group, B: limb phase asymmetry exaggerated and reduced conditions and neurologically intact group, C: Step length asymmetry HemiSlow and HemiFast conditions and neurologically intact group, D: limb phase asymmetry HemiSlow and HemiFast conditions and neurologically intact group. In order to compare across groups, positive de-adaptation data for the exaggerated condition data were transformed across the x-axis before figures and calculations were completed. Each data point represents an average of 10 consecutive strides. Zero equals baseline. Error bars represent standard error.
Discussion
Previous studies of split-belt locomotor adaptation found that the cerebral damage from stroke did not impair the capacity to adapt inter-limb coordination during locomotion (Reisman et al., 2007; Reisman et al., 2009). However, recent evidence suggests that the rate of locomotor adaptation may be altered after stroke (Savin et al., 2012). In the present study, when the stroke survivor data was grouped according to which leg was on the fast belt, as has been done previously, we found no differences in magnitude or rate of adaptation or de-adaptation in stroke survivors compared to neurologically intact controls, which is consistent with previous findings (Reisman et al., 2007; Reisman et al., 2009).
However, we hypothesized that differences in the magnitude and rate of adaptation and de-adaptation between stroke survivors and controls may be present if we grouped the stroke survivors' data based on whether their baseline asymmetries were exaggerated or reduced by the initial split-belt perturbation. When the data was regrouped, we found that stroke survivors adapt more slowly and to a lesser extent when the initial split-belt perturbation reduced their step length asymmetries, as compared to neurologically intact control subjects. Additionally, these subjects remain closer to perfect symmetry through the adaptation phase despite having had the same time of exposure to the split-belt perturbation, when compared to all other subjects and groups. In contrast, there were no differences, in the magnitude or rate of adaptation or de-adaptation when initial split-belt perturbation exaggerated their baseline asymmetries, as compared to neurologically intact controls subjects. Finally, there were no differences in these measures when the temporal variable of limb phasing was evaluated, regardless of how the data was grouped.
Because persons post-stroke typically walk with spatial and temporal asymmetry at baseline, the initial perturbation imposed by the split-belt treadmill can be configured to push their walking pattern further away from perfect spatial and temporal symmetry (exaggerated asymmetry condition), or to push their pattern closer to perfect symmetry (reduced asymmetry condition). In contrast, participants who are neurologically intact typically walk with a spatially and temporally symmetric gait pattern at baseline (Reisman et al., 2007), so regardless of whether their right or their left leg is paired with the faster moving belt, the initial perturbation imposed by the split-belt treadmill pushes their pattern away from perfect symmetry (equivalent to the exaggerated asymmetry condition for the stroke survivors). Our results indicate that when participants are perturbed in a direction that takes their gait pattern away from perfect symmetry (neurologically intact group and exaggerated asymmetry condition), participants who are neurologically intact and those post-stroke have a similar magnitude and rate of adaptation. However, if participants with stroke are perturbed in a manner that pushes them closer to perfect symmetry, there is a significantly reduced magnitude and rate of step length adaptation. Thus, it appears that when locomotor adaptation would move the walking pattern away from the initially imposed spatial symmetry, the speed is slowed and the rate is reduced. Similarly, if, at the time of initial after-effect, symmetry is achieved by our stroke subjects, there is a tendency to stay closer to perfect symmetry without substantial de-adaptation. This suggests that after stroke, the nervous and musculoskeletal systems may, in fact, still recognize and prefer a more symmetrical spatial gait pattern.
One possible explanation for this finding is that there is some advantage or preference for spatially symmetric walking. Previous studies have shown that step length asymmetry has negative functional consequences after stroke. Step length asymmetry is associated with reduced forward propulsion, slow walking speed and reduced dynamic balance (Balasubramanian et al., 2007; Bowden et al., 2006; Patterson et al., 2010), as well as an increased energy cost of walking (Awad et al., 2014; Mattes et al., 2000). Other evidence has shown that muscle activation in one leg directly facilitates out-of-phase muscle activation in the contralateral leg during cycling, thereby facilitating smooth reciprocal and symmetric timing of leg movement (Kautz et al., 2002; Ting et al., 2000). Thus, the nervous system seems to be configured to favor symmetric timing and activation of the lower extremity during the reciprocal movements of locomotion, which may therefore reinforce the maintenance of the spatial symmetry that the induced symmetry condition produces.
We did not find differences in the magnitude or rate of adaptation or de-adaptation between conditions or groups for our temporal variable. This finding is not unexpected. A number of recent studies have shown that in neurologically intact subjects spatial and temporal variables adapt at different rates and respond differently to conscious control and manipulations in practice structure during split-belt treadmill walking (Malone and Bastian, 2010; Malone et al., 2012a). Moreover, temporal parameter adaptation has been shown to adapt at twice the rate of step length and be much more difficult to manipulate through conscious efforts compared to spatial parameter adaptation (Malone and Bastian, 2010; Malone et al., 2012a). Together these results have led to the suggestion that the temporal and spatial aspects of gait may have separate neural control, and that the temporal aspects of gait may be less influenced by cerebral control (Malone et al., 2012b). Thus, the cerebral damage resulting from stroke may be more likely to affect the adaptation of spatial, rather than the temporal aspects of gait. Moreover, when healthy adults are exposed to conditions that prevent spatial parameter adaptation during split-belt walking, they are unable to prevent the temporal adaptation from proceeding normally (Malone et al., 2012a). This may explain why the stroke survivors were able to maintain the induced spatial symmetry but adapted the temporal parameter similarly across conditions.
We found that the rate and magnitude of adaptation is reduced after stroke when the initial perturbation induced by the split-belt treadmill is configured to reduce asymmetry. If we accept the explanation that this is because the system recognizes a pattern of improved spatial symmetry, it follows that the exaggerated asymmetry group would have a reduced magnitude and slower rate of de-adaptation because we expect that the initial after-effect would result in near perfect symmetry. Our results, however, did not show a difference in magnitude or rate of de-adaptation between the two conditions. We suspect that this may be because in many participants, the initial after-effect in the exaggerated condition was actually beyond perfect symmetry (overshot perfect symmetry) or was between perfect symmetry and baseline (undershot perfect symmetry). Recent studies have confirmed that after-effects do not necessarily result in perfect symmetry (Malone and Bastian, 2014). Upon a more detailed analysis of the initial after-effect for step length in the exaggerated asymmetry group, we observed this same effect. Figure 7 represents the exaggerated asymmetry group's de-adaptation for step length, broken down into these sub-groups: those who showed symmetrical initial after-effect (n=6), those who undershot perfect symmetry (n=7), and those who overshot perfect symmetry (n=4). Interestingly, in parallel to what was observed during adaptation, the group that showed symmetrical step length during initial after-effect remained near perfect symmetry throughout the de-adaptation period and did not de-adapt back to baseline. Similarly, the group that overshot perfect symmetry very rapidly de-adapted to perfect symmetry and remained there throughout the de-adaptation period, rather than further de-adapting to baseline. In contrast, the group that undershot perfect symmetry de-adapted back to their asymmetrical baseline and remained there throughout the de-adaptation period. This supports the idea that when adaptation or de-adaptation would move the participant with stroke away from symmetry, the rate of change is slowed and reduced.
Figure 7.
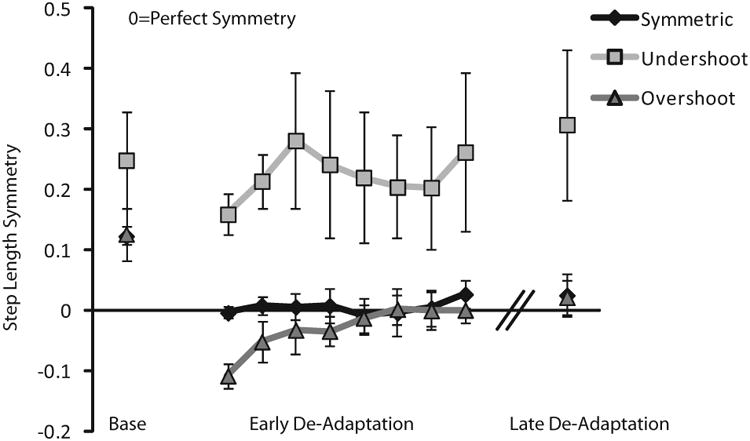
Step length de-adaptation asymmetry at baseline (Base), early de-adaptation, and late de-adaptation for the exaggerated asymmetry condition. Error bars represent standard error.
Limitations
We chose to have the neurologically intact control subjects walk at speeds that matched their stroke subject counterparts. In many cases, this resulted in control subjects walking at speeds slower than their typical self-selected speed, and could therefore result in a perturbation too small to induce locomotor adaptation or de-adaptation. In order to evaluate this potential issue, we used statistical analysis to compare the magnitude of initial perturbation imposed by the treadmill, and the magnitude of the initial after-effect across groups. Because no statistical significance was found, we know that all groups were adapting or de-adapting to perturbations of similar magnitude with respect to their individual baselines, despite the individual gait speeds that were chosen for each subject.
Conclusions and clinical implications
This study expands previous work describing the capacity for locomotor adaptation after stroke (Reisman et al., 2007; Reisman et al., 2009; Savin et al., 2012). Here, we present evidence that the rate and magnitude of locomotor adaptation is similar between chronic stroke survivors and neurologically intact controls, except when the act of adaptation (or de-adaptation) would take the stroke survivors away from a symmetric step length pattern. This suggests that the nervous and musculoskeletal systems may, in fact, recognize and prefer a more symmetrical spatial gait pattern after a stroke, despite tendency for baseline asymmetry. Therefore, our results support the use of therapeutic interventions that promote spatial symmetry during walking, because even the nervous system damaged by stroke may embrace this improved pattern.
Acknowledgments
External Support: NIH S10 RR022396
NIH K01 HD050582-01A1
NIH R01 HD078330-01A1
Footnotes
Conflict of Interest Statement: There are no conflicts of interest to report with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdollahi F, Case Lazarro ED, Listenberger M, Kenyon RV, Kovic M, Bogey RA, Hedeker D, Jovanovic BD, Patton JL. Error augmentation enhancing arm recovery in individuals with chronic stroke: a randomized crossover design. Neurorehabil Neural Repair. 2014;28:120–8. doi: 10.1177/1545968313498649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking Speed and Step Length Asymmetry Modify the Energy Cost of Walking After Stroke. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314552528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–9. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Gait performance of hemiparetic stroke patients: selected variables. Arch Phys Med Rehabil. 1987;68:777–81. [PubMed] [Google Scholar]
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37:872–6. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron. 2011;70:787–801. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz SA, Brown DA, Van der Loos HF, Zajac FE. Mutability of bifunctional thigh muscle activity in pedaling due to contralateral leg force generation. J Neurophysiol. 2002;88:1308–17. doi: 10.1152/jn.2002.88.3.1308. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103:1954–62. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Vasudevan EV, Bastian AJ. Motor adaptation training for faster relearning. J Neurosci. 2011;31:15136–43. doi: 10.1523/JNEUROSCI.1367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ, Torres-Oviedo G. How does the motor system correct for errors in time and space during locomotor adaptation? J Neurophysiol. 2012a;108:672–83. doi: 10.1152/jn.00391.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Vasudevan EV, Bastian AJ. Motor adaptation training for faster relearning. J Neurosci. 2012b;31:15136–43. doi: 10.1523/JNEUROSCI.1367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Spatial and temporal asymmetries in gait predict split-belt adaptation behavior in stroke. Neurorehabil Neural Repair. 2014;28:230–40. doi: 10.1177/1545968313505912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119(Pt 4):1199–211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- Mattes SJ, Martin PE, Royer TD. Walking symmetry and energy cost in persons with unilateral transtibial amputations: matching prosthetic and intact limb inertial properties. Arch Phys Med Rehabil. 2000;81:561–8. doi: 10.1016/s0003-9993(00)90035-2. [DOI] [PubMed] [Google Scholar]
- Musselman KE, Patrick SK, Vasudevan EV, Bastian AJ, Yang JF. Unique characteristics of motor adaptation during walking in young children. J Neurophysiol. 2011 doi: 10.1152/jn.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther. 1994;74:872–85. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31:241–6. doi: 10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–15. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–72. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23:735–44. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys Ther. 2010;90:187–95. doi: 10.2522/ptj.20090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin DN, Tseng SC, Morton SM. Bilateral adaptation during locomotion following a unilaterally applied resistance to swing in nondisabled adults. J Neurophysiol. 2010;104:3600–11. doi: 10.1152/jn.00633.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin DN, Tseng SC, Whitall J, Morton SM. Poststroke Hemiparesis Impairs the Rate but not Magnitude of Adaptation of Spatial and Temporal Locomotor Features. Neurorehabil Neural Repair. 2012 doi: 10.1177/1545968311434552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, Wu SS. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- Ting LH, Kautz SA, Brown DA, Zajac FE. Contralateral movement and extensor force generation alter flexion phase muscle coordination in pedaling. J Neurophysiol. 2000;83:3351–65. doi: 10.1152/jn.2000.83.6.3351. [DOI] [PubMed] [Google Scholar]
- Tyrell CM, Helm E, Reisman DS. Learning the spatial features of a locomotor task is slowed after stroke. J Neurophysiol. 2014;112:480–9. doi: 10.1152/jn.00486.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan EV, TO G, YA J, Bastian AJ. Development of motor learning from childhood to adulthood; Society for Neuroscience Annual Meeting; Chicago IL. 2009. [Google Scholar]


