Abstract
BACKGROUND
MYCN amplification is a key molecular hallmark of high-risk neuroblastoma. Previously considered an “undruggable” target, MYCN transcription can be disrupted by inhibiting the bromodomain and extraterminal (BET) domain family of proteins that epigenetically regulates MYCN transcription. JQ1 is a potent small molecule BET inhibitor that has been shown to induce cell cycle arrest and to initiate apoptosis in neuroblastoma. Here, we sought to validate the anti-tumorigenic effects of JQ1 in neuroblastoma and to evaluate whether blocking N-myc expression with JQ1 promotes neural differentiation.
METHODS
We determined the effects of JQ1 treatment on human neuroblastoma in vitro cell growth in both monolayer and sphere-forming conditions. Subcutaneous neuroblastoma xenografts were used for in vivo study. Western blotting and immunohistochemistry were performed to evaluate for neural differentiation and stem cell markers.
RESULTS
JQ1 treatment blocked neuroblastoma cell growth in both monolayer and sphere-forming conditions; JQ1 also attenuated neuroblastoma xenograft growth. Neurofilament expression was enhanced with JQ1 treatment, indicating that JQ1 induces neuronal differentiation. Sphere forming conditions resulted in increased expression of stem cell markers; these were reversed with JQ1 treatment.
CONCLUSIONS
BET inhibition attenuates neuroblastoma progression and promotes neural differentiation, providing insight into clinical applications of BET inhibitors in the treatment of patients with neuroblastoma.
Keywords: JQ1, Neuroblastoma, Sphere, N-myc
INTRODUCTION
Neuroblastoma is the most common extracranial solid tumor in infants and children, arising from neural crest precursors of the sympathetic nervous systems.1 Clinical heterogeneity is a unique feature of neuroblastoma with some tumors spontaneously regressing, while others are refractory to even the most aggressive therapies. Risk-group categorization for neuroblastoma was developed to predict disease response to therapy and to treat patients based on tumor biology. The prognosis for children with high-risk disease remains dismal with 50% overall survival.1 Key features of high-risk disease include age at diagnosis >18 months, tumor stage, and MYCN amplification.1 MYCN is amplified in approximately 30% of all high-risk neuroblastomas. Increased expression of N-myc drives neuroblastoma proliferation, thus making it an attractive target for therapeutic intervention. However, transcription factor of oncoproteins, such as N-myc, have historically been resistant to conventional drug development strategies due to a lack of high affinity binding sites for small molecules.2 Recent drug discovery strategies have attempted to subvert these limitations by targeting epigenetic regulators of oncoprotein transcription factors.2
The bromodomain and extraterminal (BET) proteins are a family of epigenetic modifiers that have recently been shown to regulate MYC expression.3 BETs feature bromodomains that selectively recognize histone acetylated lysine residues and serve as co-activators that regulate gene expression.4 Small molecule BET inhibitors have been identified and shown to block MYC expression in a variety of cancers, including multiple myeloma, acute myeloid leukemia, and lymphoma.3, 5, 6 JQ1, a novel thieno-triazolo-1,4-diazepine, is a potent BET inhibitor whose efficacy in neuroblastoma was recently investigated by Puissant et al.7 They described that JQ1 inhibits N-myc expression, arrests cell proliferation, and induces apoptosis in vitro in neuroblastomas and suppressed tumorigenesis in vivo.7
Although the use of pro-differentiating agent (i.e., retinoic acid) has become a part of standard therapy for neuroblastoma, the molecular mechanisms have not been fully explored. N-myc is a key driver of neuroblastoma proliferation and blocks neuronal differentiation.8 As such, we hypothesized that inhibition of N-myc with the small molecule BET inhibitor JQ1 would block neuroblastoma progression and induce differentiation. Establishing neuroblastoma cells as neurospheres in serum-free media has been touted as an in vitro selection process that alters the cancer differentiation profile and increases the expression of stem cell markers. Furthermore, rearing neuroblastomas as neurospheres has been shown to enrich for tumor-initiating capacity of in vitro culture methods.9
Here, we have validated the anti-tumor effects of JQ1 by demonstrating that JQ1 inhibits N-myc expression and blocks neuroblastoma growth as both monolayers and neurospheres and our in vivo model of primary tumor formation. Moreover, we have demonstrated that JQ1 enhances the expression of key differentiation markers, suggesting that JQ1 may act, in part, by promoting neuroblastoma differentiation. These findings provide further preclinical evidence of the efficacy of JQ1 in neuroblastoma and insight into the mechanism by which JQ1 inhibits neuroblastoma growth.
MATERIALS AND METHODS
Materials
Antibodies against N-myc, CD133, Sox2 were purchased from Cell Signaling (Beverly, MA). Antibodies against ZNF423, Nestin, and vimentin were purchased from Abcam (Cambridge, MA). Antibody against NF-M and NF-L was from Novus (Littleton, CO). Nanog and secondary antibodies against mouse and rabbit IgG were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against β-actin and CD57 were from Sigma-Aldrich (St. Louis, MO). JQ1 was synthesized by Dr. Jun Qi and kindly provided by Dr. James Bradner (Dana-Farber Cancer Institute, Boston, MA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc. (Santa Clara, CA). Agarose (SeaPlaque®) was from Lonza Inc. (Allendale, NJ).
Cell culture and tumor sphere formation
Human neuroblastoma cell lines, BE(2)-C, IMR-32, BE(2)-M17, SK-N-SH, and SK-N-DZ were purchased from ATCC (Manassas, VA). JF is a primary neuroblastoma cell line established from resected tumor that was a gift from Dr. Jason M. Shohet (Baylor College of Medicine, Houston, TX). Monolayer cells were maintained in RPMI 1640 media with L-glutamine (Cellgro Mediatech, Inc. Herndon, VA) supplemented with 10% FBS. Cells were maintained at 37 °C in a humidified atmosphere of 95% air and 5% CO2. For tumor sphere assays, 1×103 cells were plated on ultra-low attachment culture dishes and grown in neurobasal media supplemented with B27 serum substitute, 20 ng/mL epidermal growth factor and 20 ng/mL basic fibroblast growth factor (Invitrogen, Carlsbad, CA). Tumor spheres were imaged using a bright-field microscope and then stained with 0.05% crystal violet for quantification. Limiting dilution analysis was completed to quantify tumor-forming capacity with and without JQ1 (1 μM). Serial dilutions were performed to attain estimated cell-plating doses of 500, 250, 125, 62, 31, 16, 8 and 4 cells/well (6 wells/group) in 100 μl of sphere-promoting media on an ultra-low attachment 96-well plate (Corning, Manassas, VA). After 4 days incubation, each well was scored for presence of sphere formation. The frequency of sphere forming cells in each group was estimated from a plot of the estimated cells/well and the fraction of negative wells (those without sphere formation) on a log scale. This analysis was performed online using the Walter and Eliza Hall Institute, Bioinformatics Division (accessed at http://bioinf.wehi.edu.au/software/elda/).10
Cell viability and anchorage-independent growth
To assess for cellular proliferation, a modified MTT assay was used. Cells were seeded onto 96-well plates (3 × 103 cells/well) in RPMI 1640 culture media containing 10% FBS and grown for up to 4 days. Cell viability was assessed using CCK-8 kit daily. The values, corresponding to the number of viable cells, were read at OD450 with the FlexStation 3 Microplate Reader (Molecular Devices, Sunnyvale, CA). For anchorage-independent growth assay, cells were trypsinized and resuspended in RPMI 1640 media containing 0.4% agarose and 7.5% FBS. Transfected BE(2)-C cells were overlaid onto a bottom layer of solidified 0.8% agarose in RPMI 1640 media containing 5% FBS (3 × 103 cells/well) in 12-well plates and incubated for 3 weeks. Colonies were stained with 0.05% crystal violet dissolved in 70% methanol, photographed, and quantified.
Western blotting
Cells were collected using cell lysis buffer with 1 mM PMSF and denatured samples were prepared for immunoblotting. Nonspecific binding sites were blocked with 5% milk in TBS-T (120 mM Tris–HCl, pH 7.4, 150 mM NaCl, and 0.05% Tween 20) for 2 h at room temperature or overnight at 4°C. Target proteins were detected by using rabbit or mouse anti-human antibodies (1:500–2000 dilution) overnight at 4°C. Anti-rabbit or anti-mouse secondary antibodies conjugated with HRP were incubated for 1 h and visualized using an enhanced chemiluminescence system. Equal loading and transfer were confirmed with β-actin.
Immunohistochemistry
Four-micrometer-thick tissue sections were prepared and placed on charged slides. After paraffin removal, the sections were rehydrated and placed in heated citrate buffer for 25 min. Paraffin-embedded sections were stained with monoclonal antibodies for anti-vimentin (1:250 dilutions, Cell Signaling) or anti-NF-L (1:500 dilutions, EMD Millipore Corporation) overnight at 4°C using DAKO EnVision Kit (Dako Corp., Carpinteria, CA). After 4 washes with PBS, the sections were incubated for 30 min with secondary antibody labeled with peroxidase. Lastly, tissue specimens were covered with substrate-chromogen solution (1 ml) added with liquid DAB (20 μl), counterstained with hematoxylin and observed under light microscopy (400X). For negative controls, primary antibody was omitted from the above protocol.
In vivo tumor study
Male athymic nude mice (4–6 weeks old) were maintained as described.11 All studies were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with guidelines issued by the NIH. BE(2)-C cells (1 × 106) in 50 μl of HBSS were subcutaneously injected into the bilateral flanks using a 26-gauge needle as described.12 Ten days after tumor inoculation, mice with established tumors were divided into two groups (n = 5 per group): control (vehicle: 10% DMSO in 5% dextrose) or JQ1 (50 mg/kg/day) group. Vehicle or JQ1 was administered intraperitoneally on a daily basis. Tumor volume was assessed as previously described13 and body weights were recorded weekly. At sacrifice, tumors were excised, weighed, and fixed in formalin for further analyses.
Statistical analysis
Student's paired t test was used for comparisons between the treatment groups in vitro and xenograft experiments were analyzed as described.11, 12 Data represent the means ± SEM. A p value of <0.05 was considered significant.
RESULTS
JQ1 treatment decreased neuroblastoma cell proliferation and inhibited N-myc expression
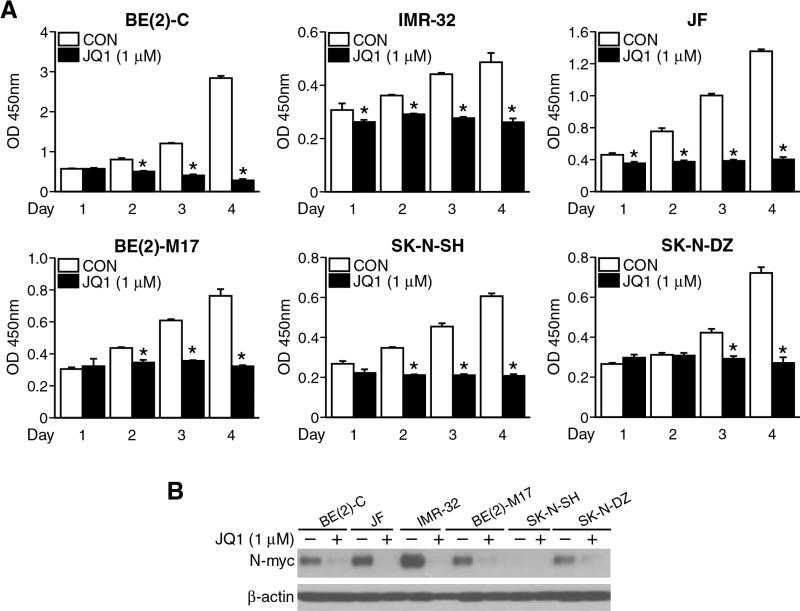
We first characterized the effects of JQ1 on neuroblastoma cell growth and survival in vitro using established cell lines reared as monolayers in serum-enriched media. After plating cells on 96-well plates with serum-supplemented media, attached monolayer cells were treated with JQ1 at 1 μM and harvested over a time course (1-4 days). Significant decreases in cell viability were observed with JQ1 treatment in all neuroblastoma cell lines examined (Fig. 1A). As such, proliferation was halted in both MYCN amplified, BE(2)-C, IMR, JF, BE(2)-M17, SK-N-DZ, as well as MYCN non-amplified, SK-N-SH, neuroblastoma cell lines. JQ1 treatment also decreased N-myc protein levels as measured by Western blotting. Specifically, N-myc protein expression was suppressed with JQ1 treatment in all of our experimental MYCN-amplified cell lines (Fig. 1B). N-myc protein level was undetectable in SK-N-SH, a MYCN non-amplified cell line. These findings validate those previously reported by Puissant et al.7 that JQ1 effectively inhibits neuroblastoma cell growth in vitro and blocks N-myc protein expression.
Figure 1. JQ1 decreased neuroblastoma cell viability.
(A) JQ1 treatment (1 μM) decreased cell viability of all human neuroblastoma cell lines examined, BE(2)-C, IMR-32, JF, BE(2)-M17, SK-N-SH, and SK-N-DZ (mean ± SEM; *=p < 0.05 vs. CON). Cell viability was measured over a four-day period using CCK-8 assay. (B) JQ1 treatment (1 μM) decreased N-myc protein levels in all neuroblastoma cell lines examined except in MYCN-non-amplified SK-N-SH cells. β-actin was probed for protein loading control.
JQ1 induced neuronal differentiation in vitro
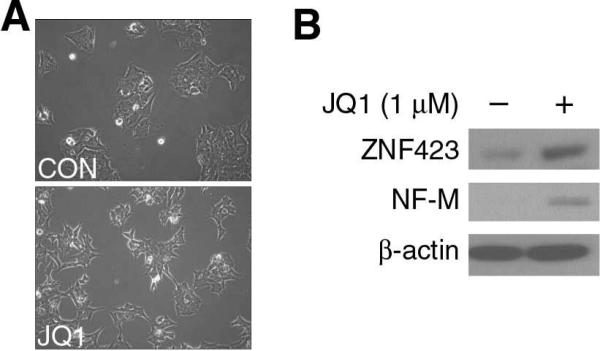
It has been speculated that neuroblastoma derives from neural crest stem cells that fail to undergo differentiation during development in infants and children. Therefore, one clinical treatment strategy is to induce differentiation with agents such as retinoic acid.14 Studies have demonstrated that JQ1 treatment causes cell cycle arrest and apoptosis.7 We examined whether JQ1 induces neuroblastoma cell differentiation and found morphologic changes in MYCN amplified BE(2)-C cell after JQ1 treatment. Specifically, BE(2)-C cells treated with JQ1 adopted a polarized phenotype with longer neurites (Fig. 2A). Furthermore, we investigated the effect of JQ1 on the expression of neural differentiation markers. The protein expression of ZNF423 and NF-M, two known markers of neuroblastoma differentiation, were increased at 72 h after JQ1 treatment (Fig. 2B). Taken together, our results demonstrate that JQ1 induced cell morphology changes consistent with neural differentiation, and this was validated by increased expression of known neural differentiation markers. Our findings demonstrate that JQ1 treatment enhances differentiation of neuroblastoma cells grown under standard in vitro conditions.
Figure 2. JQ1 induced neuroblastoma cell differentiation in BE(2)-C cells.
(A) Human neuroblastoma BE(2)-C cells were plated as monolayer cells in serum-supplemented media and treated with JQ1 (1 μM) for 3 days. JQ1 treatment (bottom panel) induced neuroblastoma cellular differentiation characterized by a polar appearance with longer, outbranching neurites. (B) JQ1 treatment increased the expression of neural differentiation markers, ZNF423 and NF-M, as determined by immunoblotting. β-actin served as our loading control.
JQ1 inhibited neurosphere formation and attenuated stem cell markers
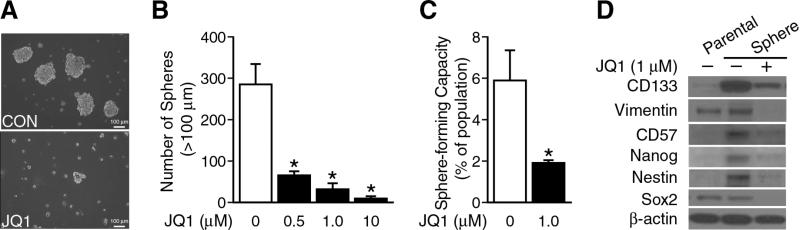
We further characterized the in vitro effects of JQ1 treatment on BE(2)-C cells reared in serum-free media and ultra-low attachment culture dishes that permit formation of neurospheres. Rearing cells as neurospheres has been shown to alter the differentiation profile of neuroblastomas, favoring the expression of stem cell markers and enriching for tumor-initiating cells.9 First, neurospheres were grown in ultra-low attachment plates using serum-free media, and sphere formation was quantified at one week. Our results showed that JQ1 treatment significantly reduced both tumor sphere size (Fig. 3A) and number in a dose-dependent manner (Fig. 3B).
Figure 3. JQ1 inhibited neuroblastoma neurosphere formation.
(A) BE(2)-C cells were cultured in sphere formation media with or without JQ1 (1 μM; bottom panel) for 7 days. Floating spheres were observed using inverted microscopy. (B) JQ1 inhibited neurosphere formation. Spheres with a diameter >100 μm were counted and quantified. No difference in sphere inhibition was observed between three dosages (mean ± SEM; *=p < 0.05 vs. no JQ1). (C) BE(2)-C cells were plated in sphere-promoting media with or without JQ1 (1 μM) and limiting dilution analysis was performed. Data represent frequency of sphere-forming capacity (%) determined by 8-dose dilution and 6 wells/group in control and treatment groups (mean ± SEM; *=p < 0.05 vs. control). (D) Sphere formation selected for subpopulations of BE(2)-C cells demonstrated increased expression of several stem cell markers by Western blotting. The protein levels of stem cell markers decreased with JQ1 (1 μM) treatment in the sphere cells. β-actin served as our protein loading control.
To better quantify the effects of JQ1 on sphere-forming capacity, we utilized the principle of limiting dilution analysis to assess the sphere-forming capacity of BE(2)-C cells reared in the presence and absence of JQ1. Ninety-six well plates were scored for sphere formation after 4 days incubation. Treatment with JQ1 (1 μM) induced a three-fold decrease in sphere forming capacity of BE(2)-C measured after 4 days of incubation (Fig. 3C). Taken together, these findings demonstrate that JQ1 alters the sphere-forming potential of BE(2)-C cells.
Rearing neuroblastomas as neurospheres alters their differentiation profile and enriches for the expression stem cell markers. We next set out to determine whether JQ1 inhibited the expression of these stem cells markers in BE(2)-C spheres, as evidence that JQ1 promotes a more differentiated protein expression profile. First, we compared the protein levels of CD133, vimentin, CD57, Nanog, Nestin, and Sox2, by Western blotting between tumor spheres and parental cells. Expression of the neural stem cell markers CD133, CD57, Nanog, and Nestin were enhanced in our tumor spheres in comparison to parental monolayer cells. However, Sox2 expression levels were not increased in BE(2)-C spheres. JQ1 treatment strikingly decreased protein levels of all tested stem cell markers (CD133, vimentin, CD57, Nanog, Nestin, and Sox2) in tumor spheres (Fig. 3D).15-19 These findings demonstrate that JQ1 blocks stem cell marker expression in BE(2)-C cells grown under sphere-promoting conditions.
JQ1 inhibited anchorage-independent colony growth
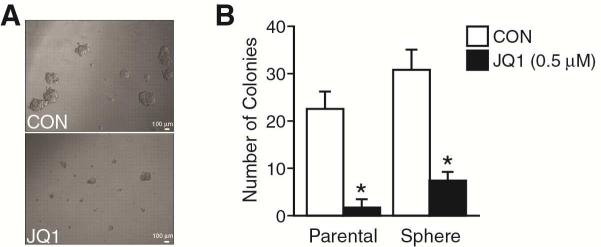
Anchorage-independent growth is one of the hallmarks of cell transformation. In order to evaluate the effect of JQ1 on tumorigenesis, we examined soft agar colony growth as an in vitro surrogate for malignant phenotype. We used both BE(2)-C cells reared as either monolayers or spheres to assess anchorage-independent growth colony forming capacity in semisolid media. The parental cells were trypsinized and sphere cells were dissociated, and single cells were cultured in semisolid agar plate in RPMI media with or without JQ1 addition for 3 weeks. Our results showed that sphere cells formed 40% more colonies than parental cells (Fig. 4), suggesting that rearing cells in sphere-forming conditions selects for a more aggressive phenotype. These findings are consistent with recent reports that rearing neuroblastomas in sphere-forming conditions enhances their tumor-initiating capacity.9 Importantly, JQ1 treatment significantly decreased the cell colony size and number for both spheres and monolayer cells (Fig. 4). Overall, these results provide a functional measure of anchorage-independence, demonstrating that JQ1 alters the aggressiveness of neuroblastomas reared both as serum-free spheres or attached in serum-supplemented conditions.
Figure 4. JQ1 blocked anchorage-independent neuroblastoma colony growth.
(A) BE(2)-C parental monolayer and BE(2)-C sphere cells were cultured in soft agar for 3 weeks in media supplemented with JQ1 (0.5 μM; bottom panel). Representative images of colony formed in soft agar (scale bar = 100 μm). (B) The colony formation was assessed and quantitated (mean ± SEM; *=p < 0.05 vs. CON).
JQ1 inhibited in vivo tumor growth and promoted differentiation
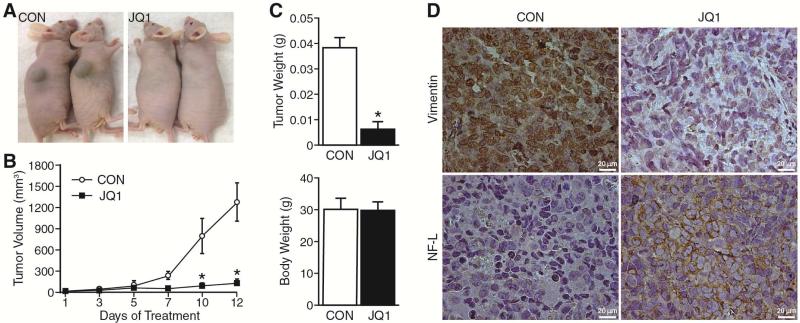
Given our in vitro findings that JQ1 blocks neuroblastoma growth by promoting differentiation, we next examined the effect of JQ1 treatment on neuroblastoma subcutaneous xenografts. After establishing subcutaneous xenografts with BE(2)-C cells, athymic nude mice were randomized to receive either JQ1 (50 mg/kg) or vehicle (control) daily. Tumor volume was measured twice weekly, and the xenografts were observed daily until sacrifice. Smaller tumor volumes were noted after 7 days of treatment with JQ1, and this became statistically significant by treatment day 10 (Fig. 5A, B). At sacrifice, tumor weight in mice receiving JQ1 was significantly less in comparison to vehicle alone (Fig. 5C). Body weight of mice treated with JQ1 was similar to control group indicating that JQ1 treatment caused no gross toxic side effects. Our results are consistent with previous studies and confirm that JQ1 inhibits neuroblastoma growth in an in vivo murine model.
Figure 5. JQ1 inhibited tumor growth in vivo.
(A) Subcutaneous neuroblastoma xenografts were established with BE(2)-C cells injected in the scapular regions of athymic nude mice. A representative photo is shown at 12 days post-treatment demonstrating tumor growth inhibition with JQ1 (right panel). (B) Tumor volume of xenografts of mice treated with daily injections (i.p.) of JQ1 (50 mg/kg/day) or vehicle (CON; 10% DMSO in 5% dextrose). Data are presented as the means ± SEM (n=5/group, *=p < 0.05 vs. CON). (C) Tumor explant and total body weights were measured at sacrifice, 12 days post-treatment with JQ1 (50 mg/kg/day; n=5/group, *=p < 0.05 vs. CON). (D) Representative immunohistochemistry of tumor sections from JQ1 and control group stained with anti-mouse vimentin and NF-L antibodies (brown stain; scale bar = 20 μm).
We next examined the expression of the neural crest stem cell marker, vimentin, and neural differentiation marker, neurofilament-L (NF-L), in tumor sections by immunohistochemistry staining. Our results showed that JQ1 treatment blocked vimentin expression (Fig. 5D; upper panels) while markedly enhancing NF-L staining in tumor sections (Fig. 5D; bottom panels). These results support the findings of our in vitro sphere and monolayer conditions demonstrating enhanced expression of neural differentiation markers with JQ1 treatment.
DISCUSSION
Recent studies have identified that recurrent somatic mutations are rare in high-risk neuroblastoma, indicating that epigenetic modifications may play a role in neuroblastoma tumorigenesis and progression.20 Epigenetic aberrations are an increasingly recognized feature of cancer cells, where chromatin regulatory machinery is exploited to enforce oncogenic gene expression programs.21 Understanding cancer-specific mechanisms of epigenetic regulators in neuroblastoma may lead to the development of novel therapeutic agents to prevent tumor progression and subsequently improve outcomes for patients.
Members of the MYC family of proteins are among the most common amplified or mutated oncoproteins in cancers, including neuroblastoma.22 However, it has been a challenge to develop drugs that inhibit this oncogenic transcription factor.2 BET inhibitors, such as JQ1, are now recognized as one of the first drugs to inhibit this elusive cancer target and have been shown to inhibit tumor progression in multiple different cancer models by modulating MYC expression.3, 6 In neuroblastoma, JQ1 has generated considerable excitement based as its ability to block transcription of MYCN, a key driver of neuroblastoma tumorigenesis whose amplification status is a marker of poor prognosis.
Here we sought to validate the existing literature supporting the preclinical efficacy of JQ1 in neuroblastoma using by in vitro and in vivo models of proliferation, aggressiveness and tumor formation. The purported mechanism of JQ1 is induction of cell cycle arrest and initiation of apoptosis. However, we have demonstrated that JQ1 also alters the protein expression profiles towards a more differentiated state in two in vitro conditions and one in vivo model. Specifically, JQ1 promoted neurofilament and ZNF423 expression in monolayer cells and blocked the expression of several purported stem cell markers that were enriched by rearing cells in serum-free, low-attachment sphere conditions. We have also demonstrated that JQ1 treatment enhanced neurofilament protein expression in our subcutaneous flank tumors with reciprocal downregulation of the mesenchymal marker, vimentin. Taken together, our findings suggest that the anti-tumorigenic effects of JQ1 are, at least in part, by promoting differentiation of neuroblastoma towards a neural fate.
Intriguingly, JQ1 halted proliferation in our non MYCN-amplified SK-N-SH cell line, demonstrating that the anti-tumorigenic effects of BET inhibitors in neuroblastoma are not exclusively attributable to blocking N-myc expression. These findings are consistent with previous trials demonstrated the efficacy of IBET-726 in non MYCN-amplified SK-N-SH and SH-SY5Y cell lines.23 Repression of the anti-apoptotic gene BCL2 was reported to be a non-MYCN target of BET inhibition in these cell lines. Taken together, our findings merit further investigations into identifying alternative aberrant signaling pathways that may be targeted by this family of emerging anticancer therapeutics.
To our knowledge, this is the first evidence of BET inhibitors inducing neuronal differentiation in neuroblastoma, highlighting a novel mechanism by which BET inhibitors may yield therapeutic benefit. Interestingly, ZNF423 expression has previously been identified as a critical mediator of retinoic acid-mediated neuroblastoma growth inhibition and differentiation.24 As such, our findings that JQ1 enhances ZNF423 expression invite further inquiry into the potential synergistic role of JQ1 and retinoic acid in mediating neuroblastoma differentiation. HDAC inhibition was recently demonstrated to enhance neuroblastoma differentiation.25 Our investigation provides further evidence to highlight the therapeutic potential of targeting epigenetic regulators to promote neuroblastoma differentiation. Overall, preclinical trials, including our own, have demonstrated the pleotropic effects of JQ1 on neuroblastoma apoptosis, growth arrest, and differentiation and provide guidance for therapeutic strategies for the use of BET inhibitors in the treatment of high-risk neuroblastoma.
ACKNOWLEDGMENTS
This work was supported by a grant R01 DK61470 from the National Institutes of Health and the Rally Foundation for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 10th Annual Academic Surgical Congress in Las Vegas, NV, February 3-5, 2015
REFERENCES
- 1.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher S, Prochownik EV. Small-molecule inhibitors of the Myc oncoprotein. Biochim Biophys Acta. 2014 Mar; doi: 10.1016/j.bbagrm.2014.03.005. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–17. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282:13141–5. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- 5.Ott CJ, Kopp N, Bird L, et al. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood. 2012;120:2843–52. doi: 10.1182/blood-2012-02-413021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertz JA, Conery AR, Bryant BM, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A. 2011;108:16669–74. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puissant A, Frumm SM, Alexe G, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3:308–23. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westermark UK, Wilhelm M, Frenzel A, et al. The MYCN oncogene and differentiation in neuroblastoma. Semin Cancer Biol. 2011;21:256–66. doi: 10.1016/j.semcancer.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Bate-Eya LT, Ebus ME, Koster J, et al. Newly-derived neuroblastoma cell lines propagated in serum-free media recapitulate the genotype and phenotype of primary neuroblastoma tumours. Eur J Cancer. 2014;50:628–37. doi: 10.1016/j.ejca.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–8. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Kang J, Ishola TA, Baregamian N, et al. Bombesin induces angiogenesis and neuroblastoma growth. Cancer Lett. 2007;253:273–81. doi: 10.1016/j.canlet.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao J, Kang J, Ishola TA, et al. Gastrin-releasing peptide receptor silencing suppresses the tumorigenesis and metastatic potential of neuroblastoma. Proc Natl Acad Sci U S A. 2008;105:12891–6. doi: 10.1073/pnas.0711861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Qiao J, Paul P, et al. FAK is a critical regulator of neuroblastoma liver metastasis. Oncotarget. 2012;3:1576–87. doi: 10.18632/oncotarget.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redfern CP, Lovat PE, Malcolm AJ, et al. Gene expression and neuroblastoma cell differentiation in response to retinoic acid: differential effects of 9-cis and all-trans retinoic acid. Eur J Cancer. 1995;4:486–94. doi: 10.1016/0959-8049(95)00066-r. [DOI] [PubMed] [Google Scholar]
- 15.Takenobu H, Shimozato O, Nakamura T, et al. CD133 suppresses neuroblastoma cell differentiation via signal pathway modification. Oncogene. 2011;30:97–105. doi: 10.1038/onc.2010.383. [DOI] [PubMed] [Google Scholar]
- 16.Schlitter AM, Dorneburg C, Barth TF, et al. CD57(high) neuroblastoma cells have aggressive attributes ex situ and an undifferentiated phenotype in patients. PLoS One. 2012;7:10. doi: 10.1371/journal.pone.0042025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peh GS, Lang RJ, Pera MF, et al. CD133 expression by neural progenitors derived from human embryonic stem cells and its use for their prospective isolation. Stem Cells Dev. 2009;18:269–82. doi: 10.1089/scd.2008.0124. [DOI] [PubMed] [Google Scholar]
- 18.Thomas SK, Messam CA, Spengler BA, et al. Nestin is a potential mediator of malignancy in human neuroblastoma cells. J Biol Chem. 2004;279:27994–9. doi: 10.1074/jbc.M312663200. [DOI] [PubMed] [Google Scholar]
- 19.Ellis P, Fagan BM, Magness ST, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–65. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 20.Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45:279–84. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17:330–9. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]
- 22.Wolfer A, Ramaswamy S. MYC and metastasis. Cancer Res. 2011;71:2034–7. doi: 10.1158/0008-5472.CAN-10-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyce A, Ganji G, Smitheman KN, et al. BET inhibition silences expression of MYCN and BCL2 and induces cytotoxicity in neuroblastoma tumor models. PLoS One. 2013:8. doi: 10.1371/journal.pone.0072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S, Laoukili J, Epping MT, et al. ZNF423 is critically required for retinoic acid-induced differentiation and is a marker of neuroblastoma outcome. Cancer Cell. 2009;15:328–40. doi: 10.1016/j.ccr.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frumm SM, Fan ZP, Ross KN, et al. Selective HDAC1/HDAC2 inhibitors induce neuroblastoma differentiation. Chem Biol. 2013;20:713–25. doi: 10.1016/j.chembiol.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]