Abstract
Background
The development of ethanol dependence is associated with alterations in hypothalamic-pituitary-adrenal (HPA) axis and activation of type II glucocorticoid receptors (GR). These effects may contribute to withdrawal-associated anxiety, craving and relapse to drinking. The present studies examined acute and oral administration of the novel, selective and competitive GR antagonist ORG 34517 on the severity of ethanol withdrawal.
Methods
Adult, male Sprague-Dawley rats were administered ethanol (4g/kg/i.g.) twice daily for 5 days followed by 2 days of withdrawal for 1, 2 or 3 consecutive cycles. Blood ethanol levels (BELs) were determined at 0930 on Day 4 of each week, while blood corticosterone levels (BCLs) were obtained at 1100 hrs on the first day of each ethanol withdrawal. During early withdrawal, subjects received oral administration of ORG 345617 (60 mg/kg/i.g.) or a placebo and withdrawal was monitored.
Results
Peak BELs of 225.52 mg/dl were observed during the third week. Withdrawal from three cycles of the regimen produced marked behavioral abnormalities (e.g. aggression, rigidity, and hypoactivity) and significant increases in BCLs of ethanol-dependent subjects. Acute, oral administration of ORG 34517 during early withdrawal significantly reduced both the severity of ethanol withdrawal, as reflected in reduced rigidity, aggression, and hypoactivity, and elevations in BCL without producing any sedative-like effects.
Conclusions
The present findings demonstrate that repeated ethanol exposure and withdrawal is associated with significant behavioral abnormalities and dysregulation of HPA axis activation. Further these data suggest that selective GR antagonists should be further considered as putative pharmacotherapies for treatment of ethanol dependence.
Keywords: ethanol, dependence, withdrawal, glucocorticoid, hypothalamic-pituitary-adrenal-axis
1. INTRODUCTION
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is associated with the development of alcohol dependence and relapse to drinking in abstinent individuals with an alcohol use disorder (Adinoff et al., 2005; Junghanns et al.). In humans, increases in cortisol are detected during periods of ethanol intoxication and during periods of detoxification in alcohol-dependent individuals (Adinoff et al., 1991, 2003). In contrast, decreases in salivary cortisol have been observed in alcohol-dependent individuals following four weeks of protracted abstinence (Adinoff et al., 2005) as well as following stress and alcohol cue exposure (Sinha et al., 2009). In rodents, increases in the release of corticosterone (CORT; Ellis, 1966) have been observed after acute ethanol administration, as well as during periods of withdrawal in ethanol-dependent animals (Cippitelli et al 2012; Rasmussen et al., 2000; Sharrett-Field et al., 2013).
Prior studies employing a 4-day binge-like model of ethanol dependence (9–15 grams/kg/day) demonstrated that behavioral and physiological manifestations of withdrawal, including increases in plasma CORT levels, in ethanol-dependent rats were attenuated by prior administration of the non-selective glucocorticoid receptor (GR) antagonist mifepristone (Sharrett-Field et al., 2013). In vitro, mifepristone application reduced withdrawal-associated and CORT-potentiated hippocampal neurotoxicity (Mulholland et al., 2005). Other studies demonstrated that mifepristone is effective in reducing cognitive deficits in ethanol-dependent mice (Jacquot et al., 2008), and decreasing voluntary intake of ethanol in other rodent models (Koenig and Olive, 2004; Vendruscolo et al., 2012). These studies support the possibility that GRs plays an important role in the development of ethanol dependence. However, studies suggest mifepristone modulates progesterone receptors, as well (Gagne et al., 1985). Thus, the selectivity of these effects is not certain. In addition, prior work has shown that mifepristone acts as a partial agonist at GRs under some circumstances, at least in in mouse pituitary AtT20 cells (Peeters et al., 2008) and osteosarcoma cells (Fryer et al., 2000). A structurally similar and highly novel, selective GR antagonist ORG 34517, a 11,21-Bisphenyl-19-norpregnane steroid, has been synthesized and demonstrated to be highly selective for the GR (Peeters et al., 2008, 2004). Importantly, ORG 34517 has negligible affinity for human progesterone receptors (PRs) and possess a nearly 500-fold greater affinity for human GRs than human PRs (Gebhard et al., 1997). In contrast, mifepristone was shown to have only a 5.4-fold greater affinity for GRs than progesterone receptors (Gebhard et al., 1997). The present studies examined the effects of acute, oral treatment with ORG 34517 on the severity of alcohol withdrawal behavioral abnormalities and plasma corticosterone elevations following chronic, intermittent alcohol intoxication in adult male rodents.
2. MATERIALS AND METHODS
2.1. Animals
Twenty-five adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing between 300 and 325 grams upon arrival were used as subjects. Animals were allowed to acclimate to the colony for 1 week prior to handling and then handled once daily for 3 consecutive days prior to any experimentation. Animals were allowed ad libitum access to standard rat chow and water throughout the entire duration of the experiments. Care of all animals was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), as well as the University of Kentucky’s Institutional Animal Care and Use Committee.
2.2. Administration of Ethanol and Control Diets
Ethanol and control diets were composed of 30% v/v Vanilla Ensure® (Abbott Nutrition, Columbus, Ohio) and an equal quantity of double-distilled water. The ethanol-containing diet was composed of 40% v/v 200 proof ethanol (Sigma-Aldrich, San Diego, CA). Control diets were isocalorically matched to ethanol-containing diets via substitution of maltose (Sigma-Aldrich) dissolved in double-distilled water yielding equivalent caloric intake. In order to determine how many withdrawals were required to produce reliable behavioral manifestations of withdrawal, animals received ethanol (4 g/kg/i.g.) or an isocaloric-containing control diet twice daily at 0800 and 1600 for 5 consecutive days followed by a 2-day period of withdrawal. This regimen was repeated either one, two, or three times (i.e., 1, 2 CIE, or 3 CIE). This study aimed to achieve peak blood ethanol levels (BELs) of approximately 200 mg/dl to reflect patterns of binge-alcohol use in humans (Eckardt et al., 1998; Jones and Sternebring, 1992). Further, the relatively moderate ethanol dose employed (i.e., 4g/kg twice daily) allowed for each animal to receive every ethanol dose in the current study; thereby reducing mortality rates known to be inherent to binge-like ethanol administration. Animal weights were recorded prior to 0800 dosing daily.
2.3. ORG 34517 Administration
To assess the effects of acute, oral administration of ORG 34517 on behavioral effects of withdrawal and HPA axis activation ORG 34517 (PopTest, LLC, Cliffside Park, NJ) (60 mg/kg) or placebo ([PL] 60 mg/kg) were administered to animals (N = 5–6/group) via oral gavage at 0800 on the first withdrawal day (day 6 of week 1, 2, and 3) for 3 consecutive cycles of CIE. ORG 34517 (60 mg/kg) and PL (60 mg/kg) were suspended in the control diet (described in detail above) sodium starch glycolate (9.6 mg), microcrystalline cellulose (48 mg), polyvinylpyrolidone (7.2 mg), lactose (24 mg), magnesium stearate (1.2 mg), sodium lauryl sulfate (.24 mg) mixed thoroughly for 30 minutes, and stored at 4°C until used. Drugs were mixed thoroughly again 10 to 15 minutes prior to oral gavage administration. ORG 34517 has a cmax of 663.27 ng/mL; a tmax of 0.17 h and t1/2 of 4.27 h in male Charles River mice (Personal Communication; PopTest, LLC). In the present report, acute doses of ORG 34517 and PL (i.e., 60 mg/kg) were selected based on prior reports suggesting chronic ORG 34517 (20 mg/kg/day) administration for up to five weeks did not produce changes in HPA activity in the rodent (Bachmann et al., 2003).
2.4. Behavioral Effects of Withdrawal
Behavioral effects of withdrawal were assessed 16 hours after the last ethanol administration (i.e., 0900 hour) on the first withdrawal day (day 6 of week 1, 2, and 3) using a modified (from Majchrowicz, 1975) behavioral scale that has been reported previously (Self et al., 2009; Sharrett-Field et al., 2013) for either 1, 2, or 3 consecutive withdrawals from CIE. Animals were individually placed in a square Plexiglas chamber for 2 minutes and evaluated for behaviors typically observed in the rodent during withdrawal by two experimenters blinded to experimental conditions. The assessment was limited to two minutes based on prior studies which demonstrated that duration is sufficient to distinguish between dependent and non-dependent animals. Further, use of a brief observation period allows for uniformity in the post-dosing interval between all animals. All of these behaviors were included in the analysis if both experimenters observed it. If a given behavior was identified by only one blinded experimenter, it was not considered to be present. These behaviors include rigidity, tremor, stereotypy, retropulsion, dystonic gait, hypoactivity, aggression, splayed paws, vocalization, and seizure. These measures were scored using all or none criteria, such that the behavior was either present or absent during the observation period.
2.5. Analyses of BELs and Blood Corticosterone Concentrations (BCLs)
In order to assess BELs and BCLs, approximately 200 µL of tail blood was collected in two Fisherbrand heparinized micro-hematocrit capillary tubes (Fisher Scientific, Waltham, MA) on day 4 of week 1, 2, and 3 at 0930 hours (90 minutes after ethanol administration). Samples were immediately placed on ice and then centrifuged for 5 minutes at 21, 8900g using an Analox benchtop centrifuge (Analox Instruments, London, England). Blood plasma was collected and stored at −80° until further analyses of BELs was conducted using an Analox AM1 apparatus. This device indirectly measures BELs, as oxygen consumption is directly proportional to ethanol concentrations in each blood plasma sample.
Blood CORT levels (BCLs) were assessed on the first withdrawal day (day 6 of week 1, 2, and 3) at 1100 hours (i.e., ~18 hours following the last ethanol administration) using a competitive enzyme immunosorbant assay for corticosterone (IDS Limited, Fountain Hills, AZ). All samples were run in triplicate and first diluted using a 1:20 ratio. Next, diluted samples, calibrators, and controls (100 µL) and 100 µL of enzyme (i.e., CORT labeled with horseradish perioxidase) were pipetted into a 96-well antibody-coated plate (i.e., polyclonal rabbit anti-CORT) and incubated for 24 hours at 4°C. Plates were then rinsed three times with a wash solution with 200 µL of substrate composed of tetramethylbenzidine and sodium peroxide added for 30 minutes. After this incubation period, a stop solution composed of 0.5 M hydrocloric acid was added to plates. Plates were read using a Beckman Coulter DTX 880 Multimodal Detector (Lagerhausstrasse, Austria) using Beckman Coulter Multimode Detection Software (v.20.0.12). Absorbance was detected at 450 nm for samples, controls, and calibrators. Mean absorbance for each value was determined and then averaged so as to yield one measurement for each sample. Percent binding was then calculated via formulae (B/B%=[(mean absorbance)/(mean absorbance for “0” calibrator)]× 100). Mean CORT concentrations were then determined from each sample (ng/ml) based on the generated calibration curve.
2.6. Statistical Analyses
Body weight, BCL, and withdrawal behavioral data were analyzed by a three-factor repeated-measure ANOVA with week (i.e., week 1, 2, and 3) as the within-subjects factor and diet (i.e., control and ethanol) and drug (i.e., PL and ORG 34516) as the between-subject factors. BEL data were analyzed by a two-factor repeated-measure ANOVA with week (i.e., week 1, 2, and 3) as the within-subjects factor and drug (i.e., PL and ORG 34517) as the between-subject factors. Pairwise comparisons of means were conducted using Bonferroni. For graphical representation and interpretation, data are presented as group mean +/− the standard error of the mean (SEM).
3. RESULTS
3.1. Effects of Binge-like Ethanol Administration on Body Weights
Body weights were determined daily at 0800 hours. ANOVA revealed a significant week-by-diet interaction (F[2,40]= 4.38, p<0.05) and a significant week-by-diet interaction (F[2,40]= 17.90, p<0.001). Drug administration (PL or ORG 34517) did not produce any marked changes in body weight at any point during the regimen Figure 1 shows that binge-like ethanol administration (8g/kg/day) produced significant decreases in body weight by an average of 23, 36, and 37 grams at week 1, 2, and 3 time points, respectively, as compared to animals administered the control diet. Further, body weights for all animals were significantly higher during week 3 than during week 1.
Figure 1.
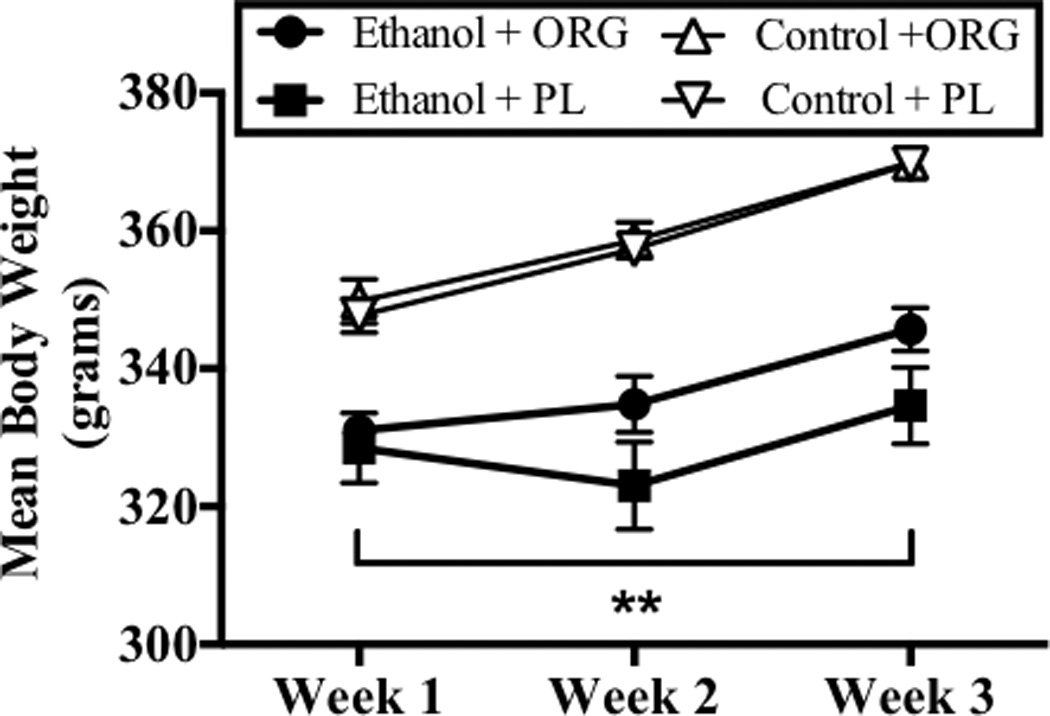
Body weight data were obtained daily on week 1, 2, and 3. Data points show mean weekly body weights for subjects exposed to ethanol and ORG 34517 (60 mg/kg; n=6; empty circle), ethanol and PL (60 mg/kg; n=5; filled circle), control and ORG 34517 (60 mg/kg; n=6; empty triangle), and control and PL (60 mg/kg; n= 5; filled triangle). Two asterisks indicate a significant interaction of diet and week.
3.2. Effects of Binge-like Ethanol Administration on BELs and BCLs
Analysis of variance revealed a significant main effect of week (F[2,14]= 11.58, p<0.001) on BELs. Figure 2 shows increases in BELs were observed on week 3, as compared to week 1 and week 2 (p<0.05; Bonferroni adjustment for multiple comparisons). BELs of 140 mg/dl to 228 mg/dl were achieved in ethanol dependent rats and these were not altered by ORG 34517 administration. Figure 2 also shows that drug (i.e., PL and ORG 34517) did not significantly alter BELs at any time point during the regimen. Plasma samples were also obtained at 1100 hours on day 6 of week 1, 2, and 3 in order to assess BCLs during withdrawal. Analysis of variance revealed significant week-by-diet-by-drug interaction (F[2,36]= 5.32, p<0.05) on BCLs. Figure 3 shows that binge-like ethanol administration increased BCLs during withdrawal on week 3, an effect that was blocked by acute oral administration of ORG (60 mg/kg). Blood corticosterone levels reached 32 ng/ml in ethanol-dependent rats administered the PL treatment, nearly 10-fold higher than those observed in all other animals, during the third withdrawal period.
Figure 2.
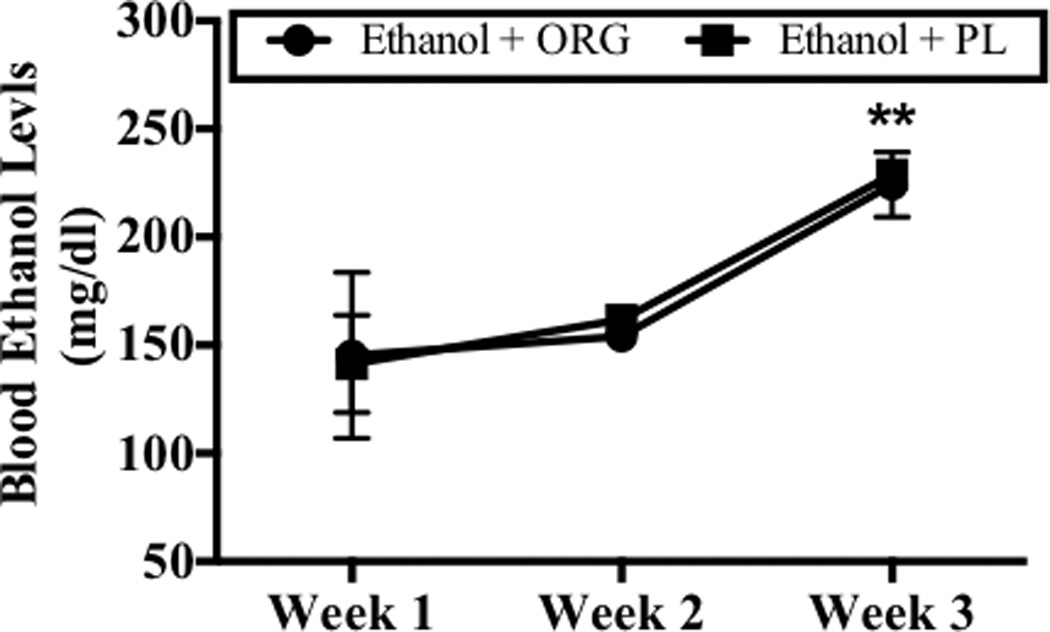
Peak BELs were assessed 90 minutes post-ethanol administration on day 4 of week 1, 2, and 3. Data points show mean score BELs for subjects exposed to ethanol and ORG 34517 (60 mg/kg; n=6; empty circle) or ethanol and PL (60 mg/kg; n=3; filled circle). Two asterisks indicate that there is a significant main effect of week.
Figure 3.
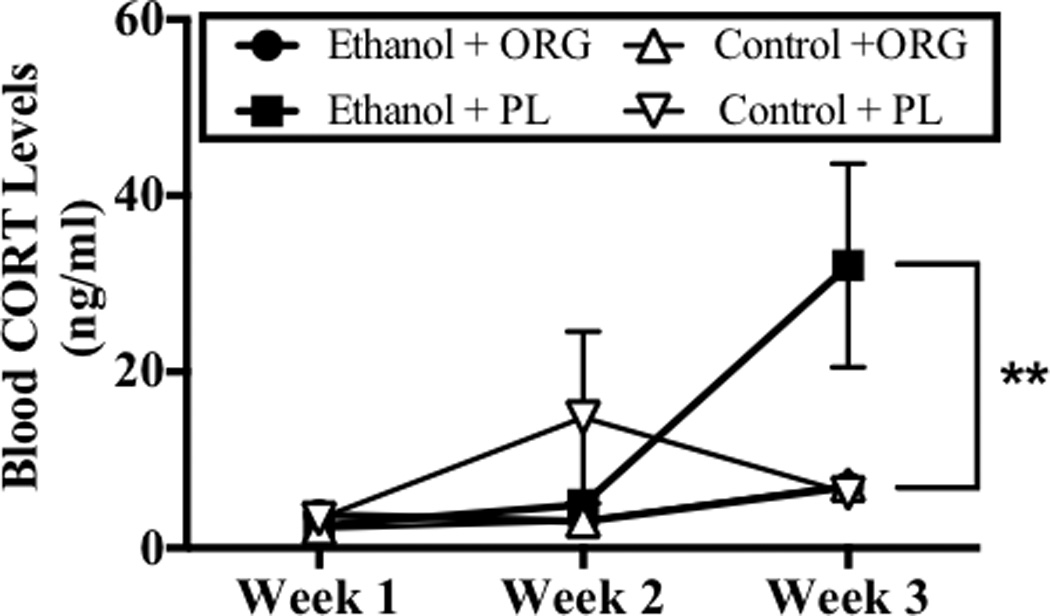
Peak blood CORT levels (BCLs) were assessed during withdrawal on day 6 of week 1, 2, and 3. Data points show mean score BCLs for subjects exposed to ethanol and ORG 34517 (60 mg/kg; n=6; empty circle), ethanol and PL (60 mg/kg; n=5; filled circle), ethanol and ORG 34517 (60 mg/kg; n=6; empty circle), ethanol and PL (60 mg/kg; n=5; filled circle). Two asterisks indicate a significant week-by-diet-by-drug interaction.
3.3. Effects of Binge-like Ethanol Administration and ORG 34517 on Withdrawal Behavior
Analysis of variance revealed a significant week-by-diet-by-drug interaction (F[2,38]= 7.63, p<0.05). Post hoc analyses revealed that withdrawal from binge-like ethanol administration was associated with the presence of significant behavioral abnormalities, as compared to non-dependent subjects, during the second and third withdrawal periods (p<0.001; Bonferroni adjustment for multiple comparisons). Animals administered ethanol had a mean of approximately 2.5 and 5 withdrawal symptoms per animal during the second and third withdrawal, respectively (Figure 4). Figure 4 also shows that ORG 34517 administration (60 mg/kg) significantly decreased the severity of withdrawal during the third withdrawal period, but did not alter the behavior of non-dependent animals. At this time, ORG 34517-treated ethanol-dependent animals presented with a mean of approximately 2 withdrawal symptoms. No effect of ORG 34517 was observed during the second withdrawal period.
Figure 4.
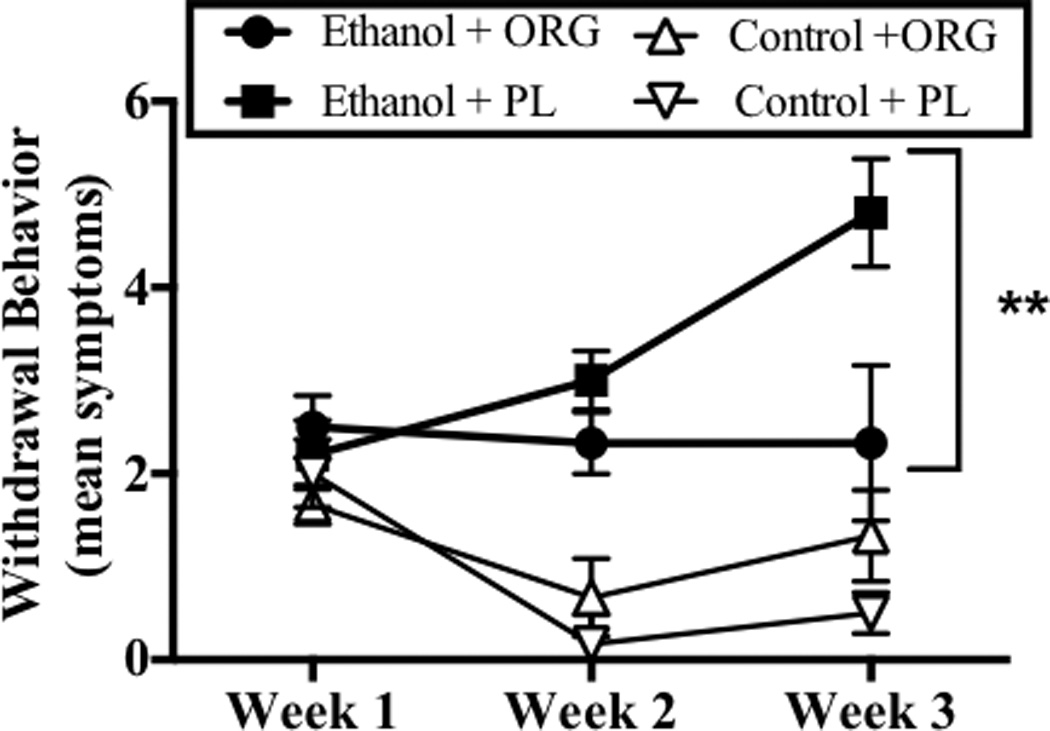
Withdrawal behavior was assessed during withdrawal on day 6 of week 1, 2, and 3. Data points show mean withdrawal behavior score for subjects exposed to ethanol and ORG 34517 (60 mg/kg; n=6; empty circle), ethanol and PL (60 mg/kg; n=5; filled circle), ethanol and ORG 34517 (60 mg/kg; n=6; empty circle), ethanol and PL (60 mg/kg; n=5; filled circle). Two asterisks indicate a significant week-by-diet-by-drug interaction.
The most common withdrawal symptoms were rigidity, aggression (e.g., clawing, escape behavior, bite attempts), abnormal motor activity including hypoactivity, and tremor. In analyzing the pattern of behavioral changes associated with ORG 34517 administration, it is evident that the primary effect of ORG 34517 was in reducing the frequency of aggressive behaviors (which were reduced by 80%) and hypoactivity (which was reduced by 50%), though the frequency of tremor and splayed paws was also moderately reduced (Figure 5).
Figure 5.

Frequency of specific ethanol withdrawal behaviors in ethanol-dependent animals that received oral administration of either a placebo (PL) or ORG 34517 (ORG) during ethanol withdrawal.
4. DISCUSSION
Findings of both pre-clinical and human subjects work have demonstrated that the development of ethanol dependence is associated with HPA axis dysregulation. Ellis (1966) first reported that acute ethanol exposure increases CORT secretion in rodents. The work of Rivier and colleagues (e.g., Lee et al., 2004, among others) has eloquently delineated the diverse myriad effects of ethanol on HPA access function. Further, the work of Heilig and Koob (e.g., Heilig and Koob, 2007), among others (Lowery et al., 2010) has characterized the role that corticotropin release factor systems have in the development of dependence and voluntary ethanol intake. Little et al. (2008) reported that corticosterone levels were markedly elevated in several brain regions of mice and rats during prolonged withdrawal from chronic ethanol intake. Rasmussen et al (2000) reported that free CORT was elevated in plasma during the early phases of withdrawal from continuous exposure to modest ethanol doses. Cippitelli et al (2014) demonstrate elevations in CORT plasma levels of over 100 ng/ml (i.e., 288 nM) in ethanol-dependent rats achieving BELs near 350 mg/dl. Sharrett-Field et al., (2013) demonstrated that CORT levels were elevated during withdrawal from a 4-day binge-ethanol regimen. In humans, increases in the stress hormone cortisol were detected during periods of ethanol intoxication and during periods of detoxification in alcohol-dependent individuals (Adinoff et al., 2003, 1991). However, others have reported an insensitivity of the HPA access following prolonged ethanol exposure in rodents (Richardson et al., 2008) and humans (Adinoff et al., 2005), including after stress and alcohol cue exposure (Sinha et al., 2009).
The role that these alterations in CORT and CRF levels, for example, have in the development of dependence is not well understood, however. Mulholland et al. (2005) did report that exposure to stress-relevant concentrations of CORT promoted intracellular calcium accumulation and pyramidal cell neurotoxicity in hippocampal explants and Little et al. (2008) reported that a single administration of mifepristone during ethanol withdrawal reduced subsequent memory deficits observed in dependent mice. Thus, one consequence of this disruption of HPA axis function may be cognitive disturbances. However, the application of mifepristone as a GR antagonist is limited due to partial agonist-like activity at the GR (Gruol and Altschmied, 1993; Havel et al., 1996) and known effects at progesterone receptors (Gagne et al., 1985).
In the present studies, we examined the effects of acute and oral administration of the novel, competitive and selective GR antagonist ORG 34517 on ethanol withdrawal severity and HPA axis activation. Oral administration of GR antagonist ORG 34517 acutely attenuates the behavioral effects of withdrawal by more than four-fold. More specifically, oral ORG 34517 administration completely prevented prototypical mild to moderate withdrawal symptoms, while more severe symptoms (e.g., spasticity) were partially reduced in ethanol-dependent rats. Worthwhile to note, is that ORG 34517 essentially restored decreases in activity typically associated with withdrawal. Further, administration of ORG 34517 did not alter blood ethanol levels. It should be noted that BELs for animals in all groups were elevated during week 3 of the regimen, as compared to weeks 1 and 2. While it is not clear what underlies this increase in mean BELs, it may be related to hepatic damage or alterations in water intake associated with prolonged intoxication. Regardless, the effects of ORG 34517 are not associated with pharmacokinetic considerations or depressant effects of the compound. It is of interest to note the pattern of behaviors that were altered by ORG 34517 during ethanol withdrawal. Aggression is a common behavioral correlate of ethanol withdrawal in rodents (e.g., Hwa et al., 2015) and the present findings are consistent with this given that aggression was the most prominent withdrawal symptom observed, though other common motor abnormalities were observed. Baars et al. (2013) have reported that aggression is highly correlated with mood disorders and risk of relapse in a sample of male alcohol-dependent patients. The present findings also demonstrated that ORG 34517 attenuated increases in CORT plasma levels during the third withdrawal period from the chronic, intermittent intoxication regimen. Though manifestations of withdrawal were clearly observed during the second withdrawal period, no concomitant increase in serum CORT levels was observed, likely to do the lesser severity of second withdrawal. More specifically, BCLs were increased in ethanol-dependent rats by more than ten-fold during the third withdrawal period. These levels were reduced more than four-fold (i.e., from 32 ng/ml [~92 nM] to 7 ng/ml [~20 nM]) in rats administered ORG 34517. Though it might be presumed that GR antagonism would prevent GR-mediated negative feedback to adrenal CORT release, resulting in elevated CORT levels, prior work with rodents demonstrated impairment of this feedback in highly anxious mice, a finding which may be relevant to the current observation that ORG 34517 reduced serum CORT levels during ethanol withdrawal. Jakovcevski et al. (2011) reported that acute administration of the less selective GR antagonist mifepristone reduced stressor-induced HPA axis activation and anxiety-like behavior in mice with high trait anxiety, but not in mice with low trait anxiety. The present findings, and those of Jakovcevski et al. (2011) may well demonstrate dysregulation of HPA feedback moderating CORT release, particularly in brain regions known to produce regulatory feedback to the paraventricular nucleus of the hypothalamus, such as the nucleus of the solitary tract (Ghosal et al. 2014). Thus, the present findings may suggest that acute antagonism of GRs can moderate the severity of affective disruption and activation of the stress axis during ethanol withdrawal.
It is possible that the efficacy of ORG 34517 in sparing withdrawal symptoms in ethanol-dependent rats in the present studies reflects GR-dependent modulation of N-methyl-D-aspartate (NMDA) receptors (reviewed in Prendergast and Mulholland, 2012). For example, Mulholland and colleagues (2005) demonstrate that CORT potentiates NMDA-induced hippocampal damage in a GR-dependent manner and that CORT potentiation of ethanol withdrawal in vitro is NMDA receptor-dependent. Other studies have shown that CORT application potentiates NMDA receptor activity of dopaminergic neurons in the ventral tegmental area (Cho and Little, 1999). Collectively, these findings suggest that ethanol dependence is accompanied by activation of the HPA axis during withdrawal following binge-like ethanol administration and that ethanol withdrawal symptoms were blunted via acute and oral administration of the novel, selective and competitive GR antagonist ORG 34517.
Highlights.
We model kindling-like effects of repeated ethanol intoxication and withdrawal.
We examine behavioral indices of withdrawal and hypothalamic-adrenal-axis activity.
Selective antagonism of glucocorticoid receptors attenuates withdrawal severity.
Acute antagonism of these receptors reduces hypothalamic-adrenal-axis activation.
Acknowledgements
We thank PopTest, LLC for the gift of ORG 34517.
Role of funding source:T32 DA035200, AA013388
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Conflict of Interest Statements:
Anna Reynolds: 'Conflicts of interest: none'.
Meredith Saunders: 'Conflicts of interest: none'.
Honoree Brewton: 'Conflicts of interest: none'.
Sydney Winchester: 'Conflicts of interest: none'.
Ibrahim Egulmati: 'Conflicts of interest: none'.
Mark Prendergast: 'Conflicts of interest: none'.
Contributors:
Anna Reynolds: I directed design, implementation, and conduct of this study and approve the final article.
Meredith Saunders: I helped with all data collection and approve the final article.
Honoree Brewton: I helped with all data collection and approve the final article.
Sydney Winchester: I helped with all data collection and approve the final article.
Ibrahim Egulmati: I helped with all data collection and approve the final article.
Mark Prendergast: As Director of this laboratory, I worked closely with Ms. Reynolds in the design and interpretation of all studies and with writing the manuscript. I approve the final article.
REFERENCES
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol. Clin. Exp. Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GHA, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am. J. Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol. Clin. Exp. Res. 2003;27:1420–1427. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Baars MY, Müller MJ, Gallhofer B, Netter P. Relapse (number of detoxifications) in abstinent male alcohol-dependent patients as related to personality traits and types of tolerance to frustration. Neuropsychobiology. 2013;67:241–248. doi: 10.1159/000350483. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Linthorst ACE, Holsboer F, Reul JMHM. Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology. 2003;28:1056–1067. doi: 10.1038/sj.npp.1300158. [DOI] [PubMed] [Google Scholar]
- Cho K, Little H. Effects of corticosterone on excitatory amino acid responses in dopamine-sensitive neurons in the ventral tegmental area. Neuroscience. 1999;88:837–845. doi: 10.1016/s0306-4522(98)00264-4. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hamelink C, Brunnquell M, Thorsell A, Heilig M, Eskay RL. Binge-like ethanol consumption increases corticosterone levels and neurodegneration whereas occupancy of type II glucocorticoid receptors with mifepristone is neuroprotective. Addict. Biol. 2014;19:27–36. doi: 10.1111/j.1369-1600.2012.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol. Clin. Exp. Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. J. Pharmacol. Exp. Ther. 1966;153:121–127. [PubMed] [Google Scholar]
- Fryer CJ, Kinyamu HK, Rogatsky I, Garabedian MJ, Archer TK. Selective activation of the glucocorticoid receptor by steroid antagonists in human breast cancer and osteosarcoma cells. J. Biol. Chem. 2000;275:17771–17777. doi: 10.1074/jbc.M908729199. [DOI] [PubMed] [Google Scholar]
- Gagne D, Pons M, Philibert D. RU 38486: a potent antiglucocorticoid in vitro and in vivo. J. Steroid Biochem. 1985;23:247–251. doi: 10.1016/0022-4731(85)90401-7. [DOI] [PubMed] [Google Scholar]
- Gebhard R, van der Voort H, Schuts W, Schoonen 11,21-Bisphenyl-19-norpregnane derivatives are selective antiglucocorticoids. Bioorganic Med. Chem. Lett. 1994;7:2229–2234. [Google Scholar]
- Ghosal S, Bundzikova-Osacka J, Dolgas CM, Myers B, Herman JP. Glucocorticoid receptors in the nucleus of the solitary tract (NTS) decrease endocrine and behavioral stress responses. Psychoneuroendocrinology. 2014;45:142–153. doi: 10.1016/j.psyneuen.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DJ, Altschmied J. Synergistic induction of apoptosis with glucocorticoids and 3’,5'-cyclic adenosine monophosphate reveals agonist activity by RU 486. Mol. Endocrinol. 1993;7:104–113. doi: 10.1210/mend.7.1.8383286. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Busch BL, Curry DL, Johnson PR, Dallman MF, Stern JS. Predominately glucocorticoid agonist actions of RU-486 in young specific-pathogen-free Zucker rats. Am. J. Physiol. 1996;271:R710–R717. doi: 10.1152/ajpregu.1996.271.3.R710. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Nathanson AJ, Shimamoto A, Tayeh JK, Wilens AR, Holly EN, Newman EL, DeBold JF, Miczek KA. Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology (Berl.) 2015 doi: 10.1007/s00213-015-3925-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ. Effects of the glucocorticoid antagonist, mifepristone, on the consequences of withdrawal from long term alcohol consumption. Alcohol. Clin. Exp. Res. 2008;32:2107–2116. doi: 10.1111/j.1530-0277.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- Jakovcevski M, Schachner M, Morellini F. Susceptibility to the long-term anxiogenic effects of an acute stressor is mediated by the activation of the glucocorticoid receptors. Neuropharmacology. 2011;61:1297–1305. doi: 10.1016/j.neuropharm.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Jones AW, Sternebring B. Kinetics of ethanol and methanol in alcoholics during detoxification. Alcohol Alcohol. 1992;27:641–647. [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, Blank S, Backhaus J. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Lee S, Selvage D, Hansen K, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145:4470–4479. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Self RL, Harris BR, Little HJ, Littleton JM, Prendergast MA. Corticosterone increases damage and cytosolic calcium accumulation associated with ethanol withdrawal in rat hippocampal slice cultures. Alcohol. Clin. Exp. Res. 2005;29:871–881. doi: 10.1097/01.alc.0000163509.27577.da. [DOI] [PubMed] [Google Scholar]
- Peeters BWMM, Ruigt GSF, Craighead M, Kitchener P. Differential effects of the new glucocorticoid receptor antagonist ORG 34517 and RU486 (mifepristone) on glucocorticoid receptor nuclear translocation in the AtT20 cell line. Ann. N. Y. Acad. Sci. 2008;1148:536–541. doi: 10.1196/annals.1410.072. [DOI] [PubMed] [Google Scholar]
- Peeters BWMM, Tonnaer JADM, Groen MB, Broekkamp CLE, van der Voort HAA, Schoonen WGFJ, Smets RJM, Vanderheyden PML, Gebhard R, Ruigt GSF. Glucocorticoid receptor antagonists: new tools to investigate disorders characterized by cortisol hypersecretion. Stress. 2004;7:233–241. doi: 10.1080/10253890400019672. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Mulholland PJ. Glucocorticoid and polyamine interactions in the plasticity of glutamatergic synapses that contribute to ethanol-associated dependence and neuronal injury. Addict. Biol. 2012;17:209–223. doi: 10.1111/j.1369-1600.2011.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol. Clin. Exp. Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur. J. Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self RL, Smith KJ, Butler TR, Pauly JR, Prendergast MA. Intra-cornu ammonis 1 administration of the human immunodeficiency virus-1 protein trans-activator of transcription exacerbates the ethanol withdrawal syndrome in rodents and activates N-methyl-D-aspartate glutamate receptors to produce persisting spatial. Neuroscience. 2009;163:868–876. doi: 10.1016/j.neuroscience.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Berry JN, Reynolds AR, Prendergast MA. Mifepristone pretreatment reduces ethanol withdrawal severity in vivo. Alcohol. Clin. Exp. Res. 2013;37:1417–1423. doi: 10.1111/acer.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


