Abstract
Rationale
Acute low-dose administration of the NMDA receptor antagonist, ketamine, produces rapid and sustained antidepressant-like effects in humans and rodents. Recently, we found that the long-lasting effect of ketamine on the forced swim test requires ventral hippocampal (vHipp) activity at the time of drug administration. The medial prefrontal cortex (mPFC), a target of the vHipp dysregulated in depression, is important for cognitive flexibility and response strategy selection. Deficits in cognitive flexibility, the ability to modify thoughts and behaviors in response to changes in the environment, are associated with depression. We have shown that chronic stress impairs cognitive flexibility on the attentional set-shifting test (AST), and induces a shift from active to passive response strategies on the shock-probe defensive burying test (SPDB).
Objective
In this study, we tested the effects of ketamine on chronic stress-induced changes in cognitive flexibility and coping behavior on the AST and SPDB, respectively. Subsequently, we investigated vHipp-mPFC plasticity as a potential mechanism of ketamine’s therapeutic action.
Results
Ketamine reversed deficits in cognitive flexibility and restored active coping behavior in chronically stressed rats. Further, high frequency stimulation in the vHipp replicated ketamine’s antidepressant-like effects on the forced swim test and AST, but not on the SPDB.
Conclusion
These results show that ketamine restores cognitive flexibility and coping response strategy compromised by stress. Activity in the vHipp-mPFC pathway may represent a neural substrate for some of the antidepressant-like behavioral effects of ketamine, including cognitive flexibility, but other circuits may mediate the effects of ketamine on coping response strategy.
Keywords: Cognitive flexibility, chronic stress, ventral hippocampus, medial prefrontal cortex, ketamine
Introduction
Major depression is a common and recurrent neuropsychiatric disorder associated with diminished quality of life, productivity, and longevity (Malhi et al., 2000, Kessler et al., 2003, Kessler et al., 2007). Although it affects millions worldwide, the pathology of depression remains unclear, and current antidepressants have poor therapeutic efficacy. Only 60% of depressed patients show improvement in symptom severity, and more than half of patients that respond to traditional antidepressants exhibit residual symptoms after treatment (Nierenberg and Amsterdam, 1990, Richards, 2011). This outcome is particularly alarming in that partial remission is highly correlated with chronic recurrent depression (Paykel et al., 1995, Nierenberg and Wright, 1999). Thus, understanding the mechanisms that underlie both the etiology of depression and effective therapeutic response may improve treatment efficacy.
Chronic stress is a major risk factor for depression (Kendler et al., 1999, Harris, 2001, Heim and Nemeroff, 2001, Caspi et al., 2003). In patients with depression, impairments in cognitive function are associated with hypoactivity and reduced volume in the medial prefrontal cortex (mPFC) and hippocampus, two regions impacted by stress (Siegle et al., 2007, Fales et al., 2009, Disner et al., 2011, MacQueen and Frodl, 2011). Rodents exposed to chronic stress also exhibit changes in mPFC and hippocampal structure, depression-like behaviors, and deficits in cognitive flexibility (Magarinos and McEwen, 1995, Liston et al., 2006, Bondi et al., 2008, Li et al., 2011, Grimm et al., 2012). Cognitive flexibility, the ability to use feedback from the environment to modify established thoughts and behaviors, depends on the function of the mPFC (Austin et al., 2001, Disner et al., 2011). Deficits in cognitive flexibility are associated with the acquisition of negative (Bondi et al., 2007)emotional biases that may contribute to the onset and maintenance of depression (Disner et al., 2011). We have shown previously that chronic unpredictable stress (CUS) impaired mPFC-mediated cognitive flexibility in rats, measured as a deficit in extradimensional (ED) set-shifting on the attentional set-shifting test (AST) (Bondi et al., 2008, Jett and Morilak, 2012). CUS also altered rats’ selection of response strategy on the shock-probe defensive burying (SPDB) test, inducing a shift from an active coping strategy (burying) to a more passive strategy (immobility) (Bondi et al., 2007). The selection of response strategy, and specifically behavior on the SPDB test, may also be subject to top-down modulation by the mPFC (Shah et al., 2004b, a). Thus, both clinical and preclinical data suggest that the mPFC and hippocampus are vulnerable to chronic stress, and functional changes in these regions may contribute to cognitive and behavioral components of depression.
Imaging and postmortem studies suggest that patients with depression exhibit abnormal glutamate neurotransmission in the mPFC and hippocampus (Hasler et al., 2007, Grimm et al., 2012, de Diego-Adelino et al., 2013). Further, acute low-dose administration of the N-methyl-D-aspartate (NMDA) receptor antagonist, ketamine, induces rapid antidepressant-like effects in treatment-resistant patients and rodent models (Machado-Vieira et al., 2009, Li et al., 2011, Carlson et al., 2013, Nosyreva et al., 2013). It has been proposed that the therapeutic effects of ketamine result in part from enhanced glutamate neurotransmission and activation of the principal neurons in the mPFC and hippocampus (Moghaddam et al., 1997, Li et al., 2010). A single dose of ketamine augments glutamate levels in the mPFC while the drug is onboard, and restores stress-induced deficits in glutamate receptor expression, spine density, and excitatory postsynaptic potentials long after the drug is metabolized (i.e., 1-7 days post-administration) (Moghaddam et al., 1997, Maeng and Zarate, 2007, Maeng et al., 2008, Li et al., 2010). We, and others, have shown that a single injection of ketamine reduces immobility on the forced swim test, a measure of antidepressant-like efficacy, up to 7 days after administration (Li et al., 2010, Lodge et al., 2012, Nosyreva et al., 2013). Our lab found recently that either inactivating the ventral hippocampus (vHipp) with lidocaine, or blocking the brain derived neurotrophic factor receptor, TrkB, in the vHipp during ketamine administration prevented the drug’s long-lasting antidepressant-like effect (Lodge et al., 2012). This suggests that plasticity in a circuit involving the vHipp underlies ketamine’s antidepressant effects. The vHipp is a major glutamatergic afferent to the mPFC (Godsil et al., 2013). Therefore, we hypothesized that ketamine may have long-lasting antidepressant-like effects on cognitive function, and that such effects may result from functional plasticity in a pathway from vHipp to mPFC.
In the present study, we assessed the effects of a single sub-anesthetic administration of ketamine on the CUS-induced deficit in cognitive set-shifting. We then examined the generalizability of ketamine’s potential therapeutic effects to another dimension of depressive-like behavior, coping strategy on the SPDB test. Lastly, we investigated whether inducing plasticity in the vHipp-mPFC pathway recapitulated the behavioral effects of ketamine. Portions of this work have been presented in abstract form (Lodge et al., 2012, Jett et al., 2013).
Materials and Methods
Animals
A total of 186 male Sprague-Dawley rats (Harlan, USA) weighing 220-300g upon arrival, were used for these studies. Rats were initially group-housed (3 rats/cage) in 25 × 45 × 15 cm cages and maintained on a 12:12 hr light/dark cycle (lights on at 07:00), then singly-housed prior to experimental procedures. Experiments were conducted during the light phase. Food and water was given ad libitum except when rats were food restricted for the attention set-shifting test. For the social defeat portion of chronic unpredictable stress, 12 Long-Evans retired male breeders were pair-housed with ovariectomized females (Charles river, USA) in large cages (63 × 63 × 40 cm) in a separate room. All procedures were in accordance with National Institute of Health guidelines and approved by the University of Texas Health Science Center at San Antonio’s Institutional Animal Care and Use Committee.
Chronic unpredictable stress
CUS was conducted as previously described (Bondi et al., 2008), unless rats were chronically implanted with electrodes, in which case swim stress was removed (Jett and Morilak, 2012). A different acute stressor was administered daily, at various times of day, for 2 weeks (see Table 1). Following each session, rats recovered for 1 hr in an isolated room, then were transferred to clean cages and returned to housing. Unstressed controls were handled 1-2 min/day. One week before testing, rats were food restricted to 14 g/day for AST procedures.
Table 1.
CUS, ketamine, HFS, and AST treatment schedule
| Day 1 | Restraint (30 min) |
| Day 2 | Shaking/crowding (1 hr; 6 rats/box; 220 back and forth movements/min) |
| Day 3 | Social defeat (45 min continued protected exposure post-defeat) |
| Day 4 | Warm swim ( 15 min) |
| Day 5 | Wet bedding (24 hrs) |
| Day 6 | Cold swim (10 min) |
| Day7 | Shaking/crowding |
| Day 8 | Footshock (15 min; 1.5mA, 5 sec on/ 5 sec off for 30 sec. every 3 min) |
| Day 9 | Social defeat |
| Day 10 | Warm swim (begin food restriction for AST) |
| Day 11 | Footshock |
| Day 12 | Tall pinch (10 min) |
| Day 13 | Cold swim |
| Day 14 | Footshock |
| Day 15 | AST Habituation |
| Day 16 | AST Training |
| Day 17 | Drug injection or High frequency stimulation (HFS) |
| Day 18 | AST Testing (24 hrs after HFS) |
Attentional set-shifting test (AST)
The ED task of the AST is a behavioral readout of mPFC function. We have consistently shown that chronic unpredictable stress (CUS) induces a deficit on ED set-shifting (Bondi et al., 2008, Bondi et al., 2010, Jett and Morilak, 2012). Thus, we investigated if acute ketamine administration can reverse CUS-induced ED impairments.
Following the last stress treatment, rats completed AST procedures as previously described (Lapiz-Bluhm et al., 2008). The test arena was a white wooden box (75 × 44 × 30 cm). The proximal third was a start gate, and a Plexiglas wall divided the distal third. A terracotta pot (diameter 7 cm, depth 6 cm) was placed on each side of the Plexiglas divider. The pots were defined by cues along two stimulus dimensions: the digging medium filling the pot, and an odor applied to the rim of the pot. The reward, a ¼ piece of Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN, USA) was buried in the digging medium of the “positive” pot. Prior to the beginning of each task, Cheerio powder was sprinkled on the medium of the pots to prevent location of the reward by smell. Trials were initiated by lifting the start gate. Rats had 10 min to make a choice. If they chose correctly, they were allowed to retrieve and eat the reward before being returned to the start gate. The test was conducted over 4 days:
Day 15: Habituation - Rats were taught to dig for reward in unscented pots filled with sawdust.
Day 16: Training - Rats learned to make simple discriminations in the arena. The reward was first paired with an odor (i.e., lemon vs. rosewood), then with a medium (i.e., felt vs. paper).
Day 17: Drug treatment - To prevent ketamine from interfering with learning that occurred during training, vehicle (saline 1 mL/kg, i.p.) or ketamine (10 mg/kg) injections were given the day after training, 24 hr before testing. Investigators were blind to drug treatment.
Day 18: Testing - Testing commenced 24 hr after drug administration. The first task was a simple discrimination (SD), with only one stimulus dimension (odor or medium) present (see Table 2). Half of the rats in each treatment started with odor as the salient dimension, while the other half started with medium. The second task was a compound discrimination (CD), in which the same discrimination was required and the second irrelevant dimension was introduced as a distractor. The third task was a reversal (R1). For this task the same media/odors were present, but the previously negative stimulus became positive, and vice versa. For the intradimensional shift (ID) all new media and odors were introduced, and the same stimulus dimension remained relevant. The fifth task was a second reversal (R2). Similar to R1, the positive and negative cues were reversed. In the sixth task, ED, the previously irrelevant dimension (e.g., medium) became relevant, and the previously salient dimension (e.g., odor) became irrelevant. The dependent measure was the number of trials needed to meet criterion of 6 consecutive correct trials on each task.
Table 2.
Representative example of stimulus pairings on the AST1
| DISCRIMINATION STAGE | DIMENSIONS | EXAMPLE COMBINATIONS | ||
|---|---|---|---|---|
| Relevant | Irrelevant | (+) | (−) | |
| Simple (SD) | Odor | Clove | Nutmeg | |
| Compound (CD) | Odor | Medium |
Clove /Raffia Clove/Yarn |
Nutmeg/Yarn Nutmeg/Raffia |
| Reversal 1 (Rl) | Odor | Medium |
Nutmeg/Raffia Nutmeg/Yarn |
Clove/Yarn Clove/Raffia |
| Intradimensional Shift (ID) | Odor | Medium |
Rosemary/Wood balls Rosemary/Plastic beads |
Cinnamon/Plastic beads Cinnamon/Wood balls |
| Reversal 2 (Rev 2) | Odor | Medium |
Cinnamon/Plastic beads Cinnamon/Wood balls |
Rosemary/Wood balls Rosemary/Plastic beads |
| Extradimensional Set-Shift (ED) | Medium | Odor |
Velvet/Citronella Velvet/Thyme |
Crepe/Thyme Crepe/Citronella |
Half the rats began with odor as the initial discriminating factor and shifted to medium, while the other half started with medium and shifted to odor. In this example, odor was the initial discriminating factor. For each task, the positive stimulus is in bold. Once a rat met the criterion of six consecutive correct trials on a task, they proceeded to the next stage of the test.
Shock probe defensive burying test (SPDB)
Two days after the last stress session (Day 16), rats were administered saline (1 ml/kg, i.p.) or ketamine (10 mg/kg). Rats were then tested on SPDB 24 hours after drug administration. SPDB procedures were as previously described. Rats were placed in a cage containing 5 cm of clean bedding, with a shock probe protruding 6 cm into one end of the cage. The probe delivered a shock (2 mA) when touched by the rat, after which the current was shut off. Behavior was recorded with a camera mounted above the cage. The amounts of time spent immobile and actively burying the probe was measured. Immobility was defined as a lack of movement other than that required for breathing (slight scanning movements of the head were permitted). Burying was defined as digging, plowing, pushing, or flicking the bedding toward the probe. The dependent measures were the amount of time the rat exhibited each behavior in the 15 min period following contact with the probe. Investigators were blind to treatment conditions when scoring videos offline.
Forced Swim Test
For this experiment, only unstressed rats were used. Rats were allowed 1 hr to acclimate to the testing room, and then placed into a Plexiglas cylinder (21 × 46 cm) filled with water (25 °C). Behavior was recorded for 10 min by a video camera mounted above the tank, and scored offline. The occurrence of immobility (i.e., floating in the tank) was scored for each 5 sec bin during the first 5 min of the test. The dependent measure was the number of 5 sec bins in which immobility was exhibited. Investigators were blind to drug treatment conditions when scoring videos offline.
Electrophysiology
Rats were exposed to two weeks of CUS or non-stressed control procedures. On Day 17, rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic frame. A bipolar, stainless steel, stimulating electrode was lowered into the right vHipp (from bregma: DV: 7.5, AP: - 5.3, ML +5.0 mm), and a glass recording electrode lowered into the right mPFC (DV: −4.5, AP: 3.0, ML: +0.6 mm) (Paxinos and Watson, 1998). Following a 30 min equilibration period, local field potentials were filtered (low cutoff = 0.3Hz: high cutoff 100Hz) and digitized (power lab: ADInstruments). A current-response curve was established (100-800 μA in 100 μA steps, 260μsec), with 30 pulses delivered at a rate of 0.1 Hz. Baseline responses were collected for 30 mins (260μs, 0.1Hz), at a current that induced ~50% maximal response from the current-response curve. High frequency stimulation (3 trains of 100 monophasic pulses, 1mA, 260μsec, at 50 Hz, inter-train interval 20 sec) was then applied in the vHipp to induce long-term potentiation. After high frequency stimulation, parameters were returned to baseline conditions and recording continued for 1 hr.
High frequency stimulation and behavior
Recently, we found that vHipp activity at the time of ketamine administration was required for the drug’s long-lasting antidepressant-like effect (Lodge et al., 2012). The vHipp is a major glutamatergic afferent to the mPFC (Godsil et al., 2013). Thus, we applied HFS to the vHipp of conscious non-stressed rats to see if inducing long-term potentiation (LTP) in the vHipp-mPFC pathway recapitulates the antidepressant-like effects of ketamine on the forced swim test, AST, and SPDB test. For these experiments, rats were pretreated with atropine (0.1 mg/kg i.p.), then anesthetized with sodium pentobarbital (60 mg/kg i.p.) and placed in a stereotaxic frame. Bipolar, stainless steel, twisted pair stimulating electrodes (MS303/1-B, Plastics One) were implanted bilaterally in the vHipp (from bregma: DV: 7.5mm, AP: −5.3, ML ±5.2 mm (Paxinos and Watson, 1998). The electrode pedestal was anchored to the skull with jeweler screws and dental cement. Rats then recovered for one week post-surgery. For the forced swim test, rats were treated with vehicle (1 ml/kg, i.p.) ketamine (10 mg/kg), or high frequency stimulation bilaterally in the vHipp (10 trains of 50 monophasic pulses, 700μA, 200μsec, at 250 Hz, inter-train interval 10 sec) then tested on forced swim 7 days later. For AST, rats underwent two-weeks of CUS following recovery from surgery. They started food restriction on Day 10 of CUS or non-stressed handling procedures (see Table 1). After the completion of stress procedures, rats were habituated and trained for AST on Day 15 and 16, respectfully (see Table 2). On Day 17, a time course comparable to ketamine treatment, rats were randomly assigned to two treatment conditions, CUS-sham or CUS-high frequency stimulation in the vHipp. For the sham condition, rats were connected to the stimulation cables, but no current applied. Rats were then returned to housing and completed AST testing on Day 18, twenty-four hours after sham or high frequency stimulation treatment. For the SPDB test, rats underwent two-weeks of non-stressed control or CUS procedures following one week of recovery post surgery. On Day 16, two days after the last stress session, non-stressed controls and CUS rats were randomly assigned to sham or high frequency stimulation treatments. Similar to AST, sham controls were connected to stimulating cables, but no stimulation was applied. Rats were then returned to housing and tested on SPDB 24 hr post treatment (Day 17). For all behavioral paradigms, investigators were blind to stimulation treatment conditions.
Statistics and histology
Where applicable, electrode placements were confirmed histologically following experimental procedures. Rats with stimulating or recording electrodes outside of the vHipp and mPFC were removed from analysis. Furthermore, rats that failed to complete the AST, either by not digging for six consecutive trials, or failing to reach criterion within 50 trials on any task, were excluded from analysis. Our a priori hypothesis for the AST experiments was that ketamine treatment and high frequency stimulation in the vHipp would reverse CUS-induced deficits on the ED task. Thus, in Section 2.2 we analyzed the number of trials to criterion on the ED task with a two-way ANOVA (Stress × Drug), while a two-tailed t-test was used to measure effects of high frequency stimulation on the ED task in Section 2.6. Subsequently, performance on the tasks preceding ED (i.e., SD-R2) was assessed in both experiments using an omnibus ANOVA, with repeated measures for Task. For the experiments with shock probe defensive burying (i.e., Sections 2.3 and 2.7), a two-way ANOVA was used to assess immobility. Because the bury time data did not meet criteria for parametric analyses, data were first converted to ranks and then analyzed by two-way ANOVA (Conover and Iman, 1981). A one-way ANOVA was used to analyze immobility on the forced swim test. Lastly, a two-way ANOVA, with repeated measures for Time, was used to analyze LTP following high frequency stimulation in Section 2.5. Where significant main effects or interactions were indicated (p < 0.05), post hoc comparisons were made with the Newman Keuls test.
Results
Ketamine and AST
Figure 1(a) shows the effects of CUS and ketamine treatment on the ED set-shifting task. Two-way ANOVA indicated a main effect of Drug (F1,41 = 4.438, p < 0.042), and a Stress × Drug interaction (F1,41 = 5.334, p < 0.027; n = 9-13/group). As we have shown previously, CUS significantly increased TTC on the ED task compared to non-stressed vehicle controls (p < 0.025). Acute ketamine treatment 24 hr prior to testing reversed the detrimental effects of CUS (p < 0.02), restoring the performance of CUS-rats. Ketamine had no effect on ED performance in unstressed controls (p = 0.8).
Figure 1.
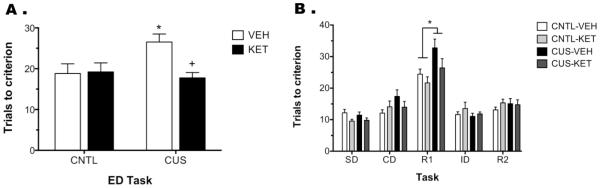
Acute ketamine administration after CUS restores mPFC function on the ED task. A) CUS rats treated with vehicle required significantly more trials to reach criterion on the ED task than non-stressed controls (CNTL, *p < 0.025). Ketamine treatment, 24 hr prior to testing, reversed this CUS-induced deficit (+p < 0.02), making CUS-KET rats comparable to controls. B) Analysis of the tasks preceding the ED set-shift task revealed a significant Stress × Task interaction, in which CUS rats, regardless of drug treatment, had higher TTC than controls on the first reversal (R1, *p < 0.005). Although ketamine appeared to reverse CUS-induced deficits on R1, this difference was not significance. All data are presented as mean ± SEM, n = 9-13/group.
Analysis of the tasks preceding ED revealed, as expected, a main effect of Task (F4,164 = 72.232, p < 0.001), but no effect of Stress (F1,41 = 2.769, p > 0.1) or Drug (F1,41 = 0.999, p = 0.3; Figure 1b). There was a Stress × Task interaction (F4,164 = 4.332, p < 0.003), in which CUS, independent of drug treatment, significantly increased trials to criterion on the first reversal (R1) task compared to non-stressed controls (p < 0.01). Further, although ketamine treatment appeared to restore performance on R1, there was no Stress × Drug × Task interaction (F4,164 = 0.689, p = 0.6). Twelve rats did not complete AST and were excluded from analysis: 4 failed during habituation (3 control, 1 CUS), 6 during training (3 controls, 3 CUS), and 2 failed to complete testing (1 control-vehicle, 1 CUS-ketamine).
Ketamine and the SPDB test
Figure 2 shows the effects of CUS and ketamine treatment on the SPDB test. For immobility, two- way ANOVA indicated a significant main effect of Stress (F1,31 = 34.05, p < 0.001), Drug (F1,31= 8.40, p < 0.007), and a Stress × Drug interaction (F1,31= 11.21, p < 0.003; n = 7-8/group). CUS significantly increased immobility compared to controls (p < 0.001), and ketamine reversed this effect (p < 0.001, Figure 2a). For burying time, there was no main effect Stress (F1,31 = 1.104, p = 0.3) or Drug (F1,31 = 0.047, p > 0.7), but there was a Stress × Drug interaction (F1,31= 6.99, p < 0.02). Post hoc analyses indicated a trend for CUS to reduce burying time compared to unstressed vehicle-controls (p = 0.06; Figure 2b). Ketamine treatment restored burying behavior in CUS rats, which was not different than that observed in controls.
Figure 2.
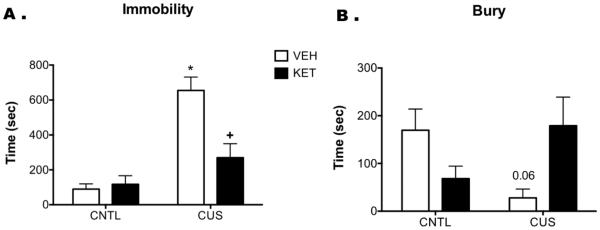
Acute ketamine administration restores active coping strategies in CUS rats on the SPDB test. CUS significantly increased time spent immobile compared to controls (CNTL, *p < 0.001), indicating a shift to a passive coping response to the shock probe. Ketamine, administered 24 hr prior to testing, reversed this CUS-induced increase in passive coping (+p < 0.001). There was also a trend for CUS to reduce bury time compared to VEH-CNTL (p = 0.06), which ketamine also reversed, again indicating a restoration of active coping. All data are presented as mean ± SEM, n = 7-8/group.
High frequency stimulation and the forced swim test
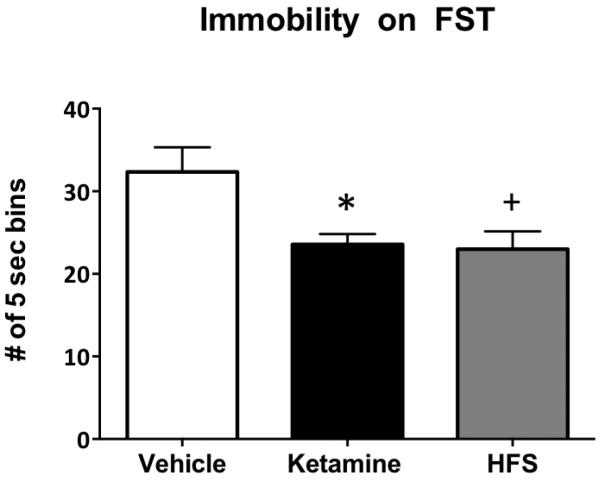
The purpose of this experiment was to test if high frequency stimulation in the vHipp alone could replicate the long-lasting behavioral effects of ketamine on the forced swim test. Figure 3 shows the behavioral effects of high frequency stimulation and ketamine in non-stressed rats on the forced swim (F2,17 = 7.013, p < 0.007, n = 6-7/group). As we have previously shown, ketamine administered 1 week prior to testing significantly reduced immobility (compared to vehicle, p < 0.009). High frequency stimulation of the vHipp alone produced a similar reduction in immobility one week following stimulation (p < 0.006). Histological analysis confirmed that all stimulating electrodes were located in the vHipp.
Figure 3.
High frequency stimulation (HFS) in the vHipp of unstressed rats recapitulates the antidepressant-like effects of ketamine on the forced swim test when assessed one week post treatment. As we have shown previously, ketamine treatment significantly attenuated immobility on the forced swim test compared to saline controls (*p < 0.009). Moreover, HFS in the vHipp induced a similar reduction in immobility on the forced swim test compared to vehicle controls (+p < 0.006). Thus, HFS had an antidepressant-like effect on the forced swim test that was similar to that of ketamine. All data are presented as mean ± SEM, n = 6-7/group.
LTP following CUS
The present study investigated the effects of CUS on the ability to induce LTP following high frequency stimulation in the vHipp. As shown in Figure 4a, two-way ANOVA indicated a main effect of Time (F17,187 = 13.19, p<0.001), but no effect of Stress (F1,11 = 2.46, p = 0.145) nor an interaction (F17,187 = 1.56, p = 0.08). These data suggest that CUS does not compromise the ability to induce LTP in the vHipp-mPFC pathway, allowing us to test the effects of HFS on cognitive set-shifting and behavior on the SPDB test after CUS in subsequent experiments. Figures 4b-c show stimulating electrode placement in the vHipp and recording electrode placement in the mPFC. Any rat with electrodes clearly located outside of the shaded regions were excluded from analysis. Three animals were excluded due to electrode misplacement in the vHipp. Electrode placement in 4 animals could not be determined due to tissue damage during removal or processing. Data from these animals were not excluded.
Figure 4.
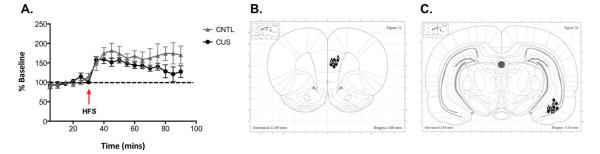
A) CUS exposure does not compromise the induction of long term-potentiation (LTP) in the vHipp-mPFC pathway by high frequency stimulation (HSF) in the vHipp. There was a main effect of Time (p<0.001), in which the responses following HFS were significantly higher than baseline responses, indicating an induction of LTP in the vHipp-mPFC pathway. All data are presented as mean ± SEM, n = 5-8/group. B) Schematic diagrams showing placement of recording electrodes in the mPFC, and C) stimulating electrodes in the vHipp. Any rats with placement outside of these regions were excluded from analysis.
High frequency stimulation and AST
Similar to our findings on the forced swim test, HFS in the vHipp recapitulated ketamine’s therapeutic effects on the ED task, a form of cognitive flexibility specific to mPFC function, after CUS. Figure 5 shows that CUS rats treated with high frequency stimulation, 24-hrs prior to testing, had a significant reduction in trials to criterion on the ED task compared to their CUS sham treated counterparts (p < 0.02). Additionally, analysis of the tasks preceding ED (i.e., SD-R2), showed a main effect of Task (F4,56 = 5.415, p < 0.001), but no effect of Treatment (F1,14 = 0.829, p = 0.378) or a Task × Treatment interaction (F4,56 = 1.538, p = 0.203, n = 8/group). No rats were removed from this analysis due to electrode misplacement or a failure to perform on the AST.
Figure 5.
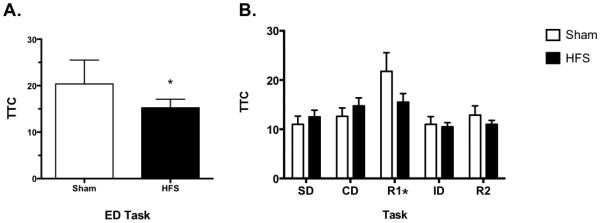
HFS in the vHipp replicated ketamine’s therapeutic effects on the ED task of the AST. A) CUS rats treated with HSF, 24-hrs prior to testing, had a significant reduction in trials to criterion on ED compared to shams (*p < 0.02). B) For the tasks proceeding ED (i.e., SD-R2), there was only a main effect of Task on R1 (*p < 0.001). All data are presented as mean ± SEM, n = 8/group.
High frequency stimulation and the SPDB test
There was a main effect of Stress on immobility in the SPDB (F1,24 = 30.05, p < 0.001). However, there was no effect of Treatment (F1,24 = 0.35, p = 0.55) nor a Stress × Treatment interaction (F1,24 = 0.035, p = 0.85, n = 7/group; Figure 6). Two-way ANOVA for ranks on burying behavior revealed no effects of Stress (F1,24 = 0.002, p = 0.96) or Treatment (F1,24 = 0.684, p = 0.41), and no Stress X Treatment interaction (F1,24 = 1.9, p = 0.18). No rats were removed from this analysis due to misplacement of stimulating electrodes.
Figure 6.
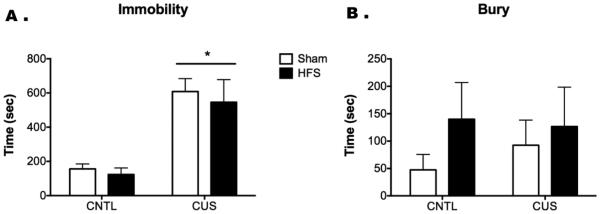
HFS in the vHipp does not recapitulate the antidepressant-like effects of ketamine on the SPDB test in CUS rats. As in Fig. 2, CUS significantly increased immobility in response to the shock probe (*p < 0.001). However, in contrast to the effect of ketamine in reversing this effect (Fig. 2), the passive coping response induced by CUS was not reversed by HFS in the vHipp 24 hr prior to testing. In this experiment, there was no significant effect of either stress or HFS on bury time. All data are presented as mean ± SEM, n = 7-8/group.
Discussion
In the present study we found that a single sub-anesthetic dose of ketamine, administered 24 hours prior to testing, reversed CUS-induced cognitive deficits on the ED set-shifting task. Additionally, ketamine reduced immobility and increased burying behavior in chronically stressed rats on the SPDB test, suggesting a restoration of active coping strategy. We recently found that inactivating the vHipp during ketamine administration prevented the drug’s long-lasting antidepressant-like effects on the forced swim test (Lodge et al., 2012). Thus, in our current studies, we used transient, high-frequency stimulation to investigate if enhanced plasticity in the vHipp alone could recapitulate the long-lasting antidepressant-like effects of ketamine. To test this, we first administered ketamine (i.p.) or high frequency stimulation in the vHipp of non-stressed animals, then tested them on the forced swim test 7 days later. Similar to ketamine, we found that high frequency stimulation in the vHipp significantly reduced immobility on the forced swim test. The vHipp is a primary glutamatergic afferent to the mPFC, therefore our subsequent study verified that high frequency stimulation in the vHipp induced LTP in the mPFC. Furthermore, we determined that CUS exposure does not compromise the ability of HFS to induce LTP in the vHipp-mPFC pathway. We then assessed the effects of high frequency stimulation in the vHipp on a task specific to mPFC function (i.e., ED set-shifting). Results indicated that high frequency stimulation in the vHipp 24 hrs prior to testing significantly attenuated the number of trials to meet criterion in CUS treated rats on the ED task. In contrast, high frequency stimulation after CUS exposure did not restore active coping behavior on the SPDB test. Taken together, these findings indicate that enhanced plasticity in the vHipp-mPFC pathway recapitulates some but not all of ketamine’s antidepressant-like effects on specific behavioral dimensions associated with depression (e.g., cognitive impairment).
Acute ketamine (e.g., 10 or 20 mg/kg) has been shown to disrupt ED set-shifting in rodents when administered 1-4 hours, but not 24 hours, prior to testing (Nikiforuk et al., 2010, Kos et al., 2011, Gastambide et al., 2013). Similarly, we found that ketamine administered 24 hours prior to AST had no effect on ED performance of non-stressed control rats. Previous studies also indicate that repeated ketamine administration during chronic stress exposure can prevent stress-induced cognitive deficits on the AST (Nikiforuk and Popik, 2014). In contrast, the present study demonstrated that a single acute administration of ketamine is sufficient to restore ED set-shifting in rats compromised by two weeks of CUS (Figure 1). Ketamine is rapidly metabolized (total metabolism in 3-4 hours in rats) (White et al., 1975, Gastambide et al., 2013). Thus, the detrimental effects of ketamine on cognitive function may represent a direct effect of the drug while it is present, whereas the long-lasting beneficial effects represent changes that are initiated by the drug and persist beyond its elimination. Preclinical evidence suggests that ketamine enhances glutamate neurotransmission in cortical and limbic regions by blocking NMDA receptors on fast-spiking interneurons, thereby disinhibiting glutamatergic signaling and increasing AMPA receptor activation (Moghaddam et al., 1997, Li et al., 2010, Nosyreva et al., 2013). In healthy humans and rodent models, this ketamine-induced hyper-glutamatergic state has been associated with psychotic-like behaviors and cognitive impairments characteristic of schizophrenia (Krystal et al., 1994, Moghaddam and Javitt, 2012, Meltzer et al., 2013, Schobel et al., 2013). Furthermore, ketamine exacerbates psychotic symptoms and cognitive deficits in schizophrenic patients (Malhotra et al., 1997). Interestingly, the same mechanisms associated with ketamine’s psychotomimetic effects also appear essential to its antidepressant effects long after the drug has been metabolized. Preclinical studies indicate that enhanced non-NMDA receptor-mediated glutamate signaling during ketamine administration elevates synaptic AMPA receptor expression, spine density, and markers of synaptic plasticity for days following acute drug administration (Moghaddam et al., 1997, Li et al., 2010, Nosyreva et al., 2013). Depressed patients exhibit hypoactivity and reduced glutamate levels in the prefrontal cortex (Hasler et al., 2007, Siegle et al., 2007, Fales et al., 2009). Additionally, expression of glutamate receptors and markers of synaptic plasticity are decreased in the prefrontal cortex of both depressed patients and chronically stressed rats (Feyissa et al., 2009, Lee and Goto, 2011, Li et al., 2011). Thus, ketamine’s long-lasting therapeutic effects in CUS-treated rats and in depressed patients may result from a restoration of glutamate transmission and plasticity in the mPFC to a level that is optimal for cognitive function (Li et al., 2011, Cornwell et al., 2012). Conversely, ketamine may induce cognitive impairments while the drug is on board by over stimulating glutamate signaling in this region (Moghaddam and Javitt, 2012, Schobel et al., 2013).
Coping behavior on the SPDB test may also be subject to modulation by the mPFC. Lesioning or pharmacologically reducing activity in the mPFC of rodents decreased burying behavior on the SPDB test (Shah et al., 2004a, b, Siegle et al., 2007). Our lab and others have shown that active coping, i.e., burying the probe following shock, reduced hypothalamic-pituitary adrenal axis activation (Bondi et al., 2007). By contrast, the passive immobility response was associated with elevated stress hormone levels, and thus represents a less adaptive coping response (Bondi et al., 2007). Clinical evidence has also shown in humans that active coping, as opposed to avoidant coping, is associated with attenuated HPA activity, greater ability to overcome challenge, and improved long-term mental and physical health outcomes (LeDoux and Gorman, 2001, Charney, 2004, Olff et al., 2005). In the present study, ketamine decreased immobility and increased burying behavior after CUS, suggesting that ketamine restores coping strategies that have been compromised by chronic stress (Figure 2). Given the evidence discussed above, a ketamine-induced restoration of active coping after CUS may also result from enhanced activity in the mPFC. It is possible that ketamine may have had a mild anxiolytic effect in control animals, as burying was reduced, albeit non-significantly, with no change in immobility. However, further investigation using validated measures of anxiety would be required to address this definitively.
Rodent models suggest that ketamine’s antidepressant-like effects are associated with an increase in spine densities and excitatory postsynaptic currents in the mPFC (Li et al., 2010, Li et al., 2011, Nosyreva et al., 2013). However, we recently found that inactivating the vHipp with lidocaine during ketamine administration prevented the drug’s long-lasting antidepressant-like effects on the forced swim test (Lodge et al., 2012). Our lab and others have obtained similar results by targeting mechanisms that modulate brain derived neurotrophic factor signaling in the vHipp while ketamine was onboard (Autry et al., 2011, Lodge et al., 2012, Nosyreva et al., 2013). Furthermore, our present study found that high frequency stimulation in the vHipp of non-stressed rats was sufficient to recapitulate ketamine’s antidepressant-like effects on the forced swim test (Figure 3). Enhanced plasticity in the hippocampal-prefrontal cortical circuitry is commonly associated with antidepressant efficacy (Ohashi et al., 2002, Li et al., 2011, Cornwell et al., 2012, Carlson et al., 2013, Nosyreva et al., 2013). Additionally, imaging studies in humans and rodents suggest that ketamine enhances connectivity in hippocampal and cortical regions (Cornwell et al., 2012, Carlson et al., 2013, Gass et al., 2014). Given that ketamine restored mPFC-mediated cognitive function on the AST in CUS-treated rats (Figure 1a), enhanced plasticity in the vHipp-mPFC pathway could be a mechanism modulating ketamine’s therapeutic effects.
To assess the effects of CUS on plasticity in the vHipp-mPFC pathway, we measured mPFC response to high frequency stimulation in the vHipp, at a time point that was comparable to our behavioral studies (Day 17). Our findings verified that CUS does not compromise the ability to induce LTP in the vHipp-mPFC pathway (Figure 4). It appears that the response may have declined more rapidly in CUS compared to control animals, perhaps indicating a potential change in mechanisms underlying late vs. early LTP, although this was not a significant difference, and further investigation is required to address this possibility. To then determine if enhanced plasticity in the vHipp-mPFC pathway facilitates behavior specific to mPFC function, we applied high frequency stimulation to the vHipp after CUS and tested rats on the AST 24 hours later. Similar to the effects of ketamine, high frequency stimulation in the vHipp significantly decreased trials to criterion on the ED set-shifting task in CUS treated rats, indicating enhanced cognitive flexibility associated with mPFC function (Figure 5). To determine if the therapeutic response to high frequency stimulation in the vHipp could be generalized across behavioral dimensions, we then tested its effects on the SPDB test. In contrast to the forced swim test and AST, high frequency stimulation in the vHipp did not affect immobility or burying behavior on the SPDB test after CUS (Figure 6). The inability of high frequency stimulation to recapitulate ketamine’s’ therapeutic effects on the SPDB test suggests that enhanced plasticity in the vHipp-mPFC pathway is not the only mechanism underlying ketamine’s therapeutic effects, and that vHipp-related circuits may not directly impact coping strategy on the SPDB test.
In summary, our findings indicate that the antidepressant-like effects of ketamine are evident across several higher-order behavioral dimensions associated with mPFC function, such as cognitive flexibility and active coping responses. Further, high frequency stimulation in the vHipp alone was sufficient to recapitulate the effects of ketamine on the forced swim test and ED task of the AST. However, it is clear that plasticity in the vHipp-mPFC pathway is not the sole mechanism underlying the behavioral response to ketamine, as high frequency stimulation in the vHipp did not recapitulate the drug’s effect on the SPDB test. Further, additional studies are needed to delineate the circuitry associated with ketamine’s antidepressant-like effects in other behavioral dimensions, such as anhedonia. Elucidating ketamine’s therapeutic effects at the circuit level will enhance our understanding of the pathology underlying depression, as well as potentially provide novel strategies for enhancing antidepressant efficacy.
Acknowledgments
We would like to acknowledge and thank Lauren Evans for her superb technical assistance.
Funding
Funding for this work was provided by research grants MH090067 (DJL), MH053851 and MH072672 (DAM) from the National Institutes of Health, and by a University of Texas Health Science Center President’s Council Faculty Scholar Award (DAM).
Footnotes
Conflict of interest
There are no conflicts of interest to report
References
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Barrera G, Lapiz MD, Bedard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:482–495. doi: 10.1016/j.pnpbp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:913–923. doi: 10.1016/j.pnpbp.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, Herscovitch P, Manji HK, Zarate CA, Jr., Drevets WC. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician. 1981;35:124–129. [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA., Jr. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Diego-Adelino J, Portella MJ, Gomez-Anson B, Lopez-Moruelo O, Serra-Blasco M, Vives Y, Puigdemont D, Perez-Egea R, Alvarez E, Perez V. Hippocampal abnormalities of glutamate/glutamine, N-acetylaspartate and choline in patients with depression are related to past illness burden. J Psychiatry Neurosci. 2013;38:107–116. doi: 10.1503/jpn.110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass N, Schwarz AJ, Sartorius A, Schenker E, Risterucci C, Spedding M, Zheng L, Meyer-Lindenberg A, Weber-Fahr W. Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology. 2014;39:895–906. doi: 10.1038/npp.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Mitchell SN, Robbins TW, Tricklebank MD, Gilmour G. Temporally distinct cognitive effects following acute administration of ketamine and phencyclidine in the rat. Eur Neuropsychopharmacol. 2013;23:1414–1422. doi: 10.1016/j.euroneuro.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol. 2013;23:1165–1181. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Grimm S, Luborzewski A, Schubert F, Merkl A, Kronenberg G, Colla M, Heuser I, Bajbouj M. Region-specific glutamate changes in patients with unipolar depression. J Psychiatr Res. 2012;46:1059–1065. doi: 10.1016/j.jpsychires.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Harris T. Recent developments in understanding the psychosocial aspects of depression. Br Med Bull. 2001;57:17–32. doi: 10.1093/bmb/57.1.17. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Jett JD, Evans L, Lodge DJ, Morilak DA. Effects of ketamine administration on chronic stress-induced cognitive deficits in rats. Soc Neurosci Abstr Program number 730.7. 2013 [Google Scholar]
- Jett JD, Morilak DA. Too much of a good thing: Blocking noradrenergic facilitation in medial prefrontal cortex prevents the detrimental effects of chronic stress on cognition. Neuropsychopharmacology. 2013;38:585–595. doi: 10.1038/npp.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol. 2007;3:137–158. doi: 10.1146/annurev.clinpsy.3.022806.091444. [DOI] [PubMed] [Google Scholar]
- Kos T, Nikiforuk A, Rafa D, Popik P. The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacology (Berl) 2011;214:911–921. doi: 10.1007/s00213-010-2102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry. 2001;158:1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- Lee YA, Goto Y. Chronic stress modulation of prefrontal cortical NMDA receptor expression disrupts limbic structure--prefrontal cortex interaction. Eur J Neurosci. 2011;34:426–436. doi: 10.1111/j.1460-9568.2011.07750.x. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Carreno F, Shah A, Jett JD, Delgado PL, Morilak DA, Frazer A. Neuronal systems underlying the antidepressant response of ketamine. Neuropsychopharmacology. 2012;38 Online:S104. [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA., Jr. The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr., Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Moore J, McGuffin P. The genetics of major depressive disorder. Curr Psychiatry Rep. 2000;2:165–169. doi: 10.1007/s11920-000-0062-y. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M. Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2013;16:2181–2194. doi: 10.1017/S1461145713000928. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, Amsterdam JD. Treatment-resistant depression: definition and treatment approaches. J Clin Psychiatry. 1990;51(Suppl):39–47. discussion 48-50. [PubMed] [Google Scholar]
- Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(Suppl 22):7–11. [PubMed] [Google Scholar]
- Nikiforuk A, Golembiowska K, Popik P. Mazindol attenuates ketamine-induced cognitive deficit in the attentional set shifting task in rats. Eur Neuropsychopharmacol. 2010;20:37–48. doi: 10.1016/j.euroneuro.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P. Ketamine prevents stress-induced cognitive inflexibility in rats. Psychoneuroendocrinology. 2014;40:119–122. doi: 10.1016/j.psyneuen.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Otani H, Mori K, Togashi H, Ueno K, Kaku A, Yoshioka M. Changes in synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway induced by repeated treatments with fluvoxamine. Brain Res. 2002;949:131–138. doi: 10.1016/s0006-8993(02)02973-6. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BP. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neurosci Biobehav Rev. 2005;29:457–467. doi: 10.1016/j.neubiorev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Academic Press; San Diego: 1998. [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev. 2011;31:1117–1125. doi: 10.1016/j.cpr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated w and attenuates shock-probe burying in rats. Brain Res. 2004a;1028:112–115. doi: 10.1016/j.brainres.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Selective antagonism of medial prefrontal cortex D4 receptors decreases fear-related behaviour in rats. Eur J Neurosci. 2004b;19:3393–3397. doi: 10.1111/j.0953-816X.2004.03447.x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- White PF, Johnston RR, Pudwill CR. Interaction of ketamine and halothane in rats. Anesthesiology. 1975;42:179–186. doi: 10.1097/00000542-197502000-00011. [DOI] [PubMed] [Google Scholar]