Highlights
-
•
Daily sleep was measured 1.5 years and a few months prior to a DTI scan.
-
•
Sleep variability in adolescence was associated with lower FA.
-
•
Sleep duration in adolescence was not associated with FA.
-
•
Sleep variability may have lasting impact on brain development.
Keywords: Sleep, Adolescence, Brain development, DTI
Abstract
Despite the known importance of sleep for brain development, and the sharp increase in poor sleep during adolescence, we know relatively little about how sleep impacts the developing brain. We present the first longitudinal study to examine how sleep during adolescence is associated with white matter integrity. We find that greater variability in sleep duration one year prior to a DTI scan is associated with lower white matter integrity above and beyond the effects of sleep duration, and variability in bedtime, whereas sleep variability a few months prior to the scan is not associated with white matter integrity. Thus, variability in sleep duration during adolescence may have long-term impairments on the developing brain. White matter integrity should be increasing during adolescence, and so sleep variability is directly at odds with normative developmental trends.
Inadequate sleep is endemic in adolescence (Colrain and Baker, 2011). In addition to receiving less than optimal average sleep per night, variability in sleep duration and sleep–wake rhythms peak during adolescence (Dahl and Lewin, 2002, Thorleifsdottir et al., 2002). Children and adolescents often need more sleep than adults, which is attributed to the putative function sleep serves in brain maturation (Iglowstein et al., 2003, Huber and Born, 2014). Indeed, researchers have proposed that the primary purpose of sleep is the promotion of brain development (Roffwarg et al., 1996, Jan et al., 2010, Dahl and Lewin, 2002). Unfortunately, adolescents may not receive the quality environmental context needed for restorative sleep and optimal neuronal development (Dahl and Lewin, 2002).
Experimental research utilizing animal models and human adults has shown that impaired sleep, including sleep deprivation and variability in sleep–wake cycles, alters synaptic plasticity resulting in transcriptional alterations in protein synthesis, reduced cerebral metabolism, gray matter loss in cortical and subcortical structures, as well as neurogenesis as a result of increased circulating levels of the adrenal stress hormone, corticosterone (Mirescu et al., 2006, Kopp et al., 2006, Halbower et al., 2006). Moreover, there is compelling evidence from high density EEG studies in youth measuring electrical activity of the brain showing that poor sleep can influence neural function, relating to impairments in memory formation and learning, executive functions, and emotional well-being (see Jan et al., 2010). Because sleep, and REM sleep in particular, facilitates memory consolidation, learning, and emotional development (Sejnowski and Destexhe, 2000, Tononi and Cirelli, 2006, Pugin et al., 2014, Huber and Born, 2014, Jan et al., 2010), poor sleep in adolescence may be associated with a host of negative outcomes via alterations in brain development and function (Dahl and Lewin, 2002). Yet, despite the important role of sleep in promoting brain development, we know little about how variations in sleep during adolescence are associated with alterations in the developing brain.
The adolescent years represent a key developmental period when the brain undergoes significant remodeling, which is accompanied by changes in the neurophysiological features of sleep, memory systems, socioemotional processing, and emotion regulation (Nelson et al., 2005, Spear, 2000). Myelination of frontocortical and frontostriatal white matter tracts continues through adolescence and into adulthood (Asato et al., 2010, Giorgio et al., 2008, Giorgio et al., 2010). Cellular maturation of white matter facilitates faster and more efficient neural transmission and cognitive processing (Giedd, 2008). Thus, relative delayed maturation and subtle alterations in the microstructure of white matter fibers during development can affect neurocognition and behavior, including behavioral problems (Li et al., 2005), substance use (Acheson et al., 2014, Bava et al., 2013), and poor cognitive performance (Chaddock-Heyman et al., 2013, Nagy et al., 2004). During periods of brain development, synaptic activity exhibits high levels of plasticity and connectivity and is therefore particularly influenced by environmental inputs (Jan et al., 2010). Moreover, stressors can significantly modify brain development (Kaufman et al., 2000). Sleep may therefore have an especially large impact on the developing adolescent brain. While sleep problems may not induce neural changes in white matter that are immediately evident, chronic or frequent sleep problems may have accumulating effects, impairing neuronal integrity, decreasing neurogenesis, and altering structural plasticity over time.
Adolescents demonstrate considerable variability in their sleep patterns, with intra-individual differences in sleep duration often exceeding between-person differences. Such variability in sleep is detrimental for health outcomes, perhaps more so than is chronically low sleep duration (Acebo and Carskadon, 2002). For example, adolescents with high day-to-day variability of sleep duration assessed by daily sleep logs over two weeks report greater depression, anxiety, fatigue, and lower subjective well-being controlling for average sleep duration (Fuligni and Hardway, 2006, Lemola et al., 2013). Daily diary measurements offer an ideal method for assessing sleep. Single-time surveys do not provide estimates of the daily variability in adolescents’ sleep that go beyond differences between weekdays and weekends (Fuligni and Hardway, 2006). Moreover, daily reporting minimizes the amount of error that occurs in retrospective reporting of events and allows for a direct estimation of the daily variability in adolescents’ sleep time.
In the current study, adolescents completed daily diary checklists two times, one year apart. In order to examine how sleep was associated with the developing brain, adolescents underwent a DTI scan a few months following the second wave of daily diaries. DTI offers the ability to indirectly study the microstructural components of white matter. Fractional anisotropy (FA), a measure of fiber bundle organization and microstructural integrity of white matter (WM), is a widely used general index of axonal integrity (Bora et al., 2012). By measuring sleep at two time points, once a few months prior to the DTI scan and once 1.5 years prior to the scan, we were able to test whether sleep problems have more immediate or long-term effects on brain development. Sleep problems may take time to impact the development of the brain, as alterations in myelination may not occur immediately. Indeed, early life stressors are associated with later reductions in FA (Paul et al., 2008, Teicher et al., 2010). Moreover, animal models have shown that stressors can differentially impact short-term and long-term neural processing. For instance, in rats, caffeine treatment is associated with short-term decreases in slow-wave activity (SWA), an electrophysiological signature of slow, synchronized, oscillatory neocortical activity that occurs during deep sleep, but the longer-term effects are reversed (i.e., increases in SWA) and persist over time (Olini et al., 2013). Thus, we expected to find more long-term associations between poor sleep and white matter integrity.
1. Methods
1.1. Participants
At the first and second waves of data collection, 48 adolescents (27 female) completed daily diary checklists (wave 1: Mage = 14.8 years; wave 2: Mage = 15.9 years). A few months after completing the second wave of daily diaries, adolescents completed a DTI scan (Mage = 16.3 years). All participants were right-handed, free of metal, reported no current medication except birth control, and did not report being diagnosed with any mood or sleep disorder. Parents provided written consent and adolescent participants completed written assent in accordance with UCLA's Institutional Review Board.
1.2. Procedures
At waves 1 and 2, interviewers visited the home of participants. Adolescents were provided with 14 days of diary checklists to complete every night before going to bed for two subsequent weeks. The three-page diary checklists took approximately 5–10 min to complete each night. Participants were instructed to fold and seal each completed diary checklist and to stamp the seal with an electronic time stamper each night. The time stamper imprinted the current date and time and was programmed with a security code such that adolescents could not alter the correct time and date. Participants were told that if inspection of the data indicated that they had completed the checklists correctly and on time, each family would also receive two movie passes. At the end of the two-week period, interviewers returned to the home to collect the diary checklists. Adolescents received $30 for participating. The time-stamper monitoring and incentives resulted in a high rate of compliance, with 96% of the potential diaries being completed. On average, adolescents completed 13.7 diaries at wave 1 and 13.0 diaries at wave 2. One adolescent provided fewer than 3 diaries at wave 2 and so is excluded from analyses examining wave 2 associations. At both waves, the diaries were collected during the fall semester of school across two weeks when school was in session. Waves 1 and 2 occurred during the same time of year, as close to 1 year apart as possible, in order to control for time of year effects. Within 1–3 months following the second wave of diaries, adolescents completed a DTI scan.
1.2.1. Daily sleep
Each day, adolescents reported the time they went to bed the prior night (“what time did you go to bed last night?”) and the time they woke up that morning (“what time did you get up from bed this morning?”). They then indicated the total time they slept (“how much time did you sleep last night?”). From these questions, we created four variables of interest, sleep duration, sleep duration variability, weekend–weekday sleep duration, and bedtime variability. Using the daily item on total sleep time, sleep duration was calculated by taking the average minutes of sleep attained each night across the 14 days and sleep duration variability was calculated by taking the standard deviation in minutes of sleep time attained each night across the 14 days. Weekend–weekday sleep duration was calculated by taking the difference in total sleep time between weekend and weekdays. Positive scores indicate greater sleep time on weekends whereas negative scores indicate greater sleep time on weekdays. Finally, bedtime variability was calculated by the standard deviation in bedtimes across the 14 days. Scores were calculated at wave 1 and wave 2.
1.3. DTI image acquisition and processing
Imaging data were collected using a 3 Tesla Siemens TrioMRI scanner. Diffusion weighted images were acquired using an echo-planar imaging (EPI) sequence (64 directions, TR/TE = 7200/93 ms, 50 slices; slice thickness = 2 mm, FOV = 190 mm, voxel size = 2 mm × 2 mm × 2 mm). This sequence also provides a T2 weighted volume (B0).
DTI processing and voxelwise statistical analysis were performed with FSL v4.1.6 (www.fmrib.ox.ac.uk/fsl). Datasets were corrected for head motion, eddy current distortion, and signal loss using the FMRIB Diffusion Toolbox. Four participants displayed >2 mm of translational motion. Excluding these individuals did not alter results, and therefore they were retained in analyses. Degree of diffusion was assessed with fractional anisotropy (FA).
Voxelwise statistical analysis of the FA data was carried out using TBSS [Tract-Based Spatial Statistics (Smith et al., 2006)], within the FSL toolbox (Smith et al., 2004). First, FA images were created by fitting a tensor model to the raw diffusion data using FDT, and then brain-extracted using BET (Smith, 2002). All subjects’ FA data were then aligned into a common space using the nonlinear registration tool FNIRT (Andersson et al., 2007a, Andersson et al., 2007b), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). Next, the mean FA image was created and thinned to create a mean FA skeleton, which represents the centers of all tracts common to the group. Each subject's aligned FA data were then projected onto this skeleton and the resulting data were used in voxelwise cross-subject analyses. To minimize partial-volume effects and areas of high inter-subject variability, values were thresholded at FA > 0.2. Data from each point on the skeleton formed the basis of voxelwise statistical comparisons.
DTI-based voxelwise statistics were carried out using FSL Randomize with 500 permutations and a standard GLM design. Randomize (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl/) uses a permutation based statistical inference that does not rely on a Gaussian distribution (Nichols and Holmes, 2002). A single-group average with covariate design was applied to assess voxelwise differences among individuals based on behavioral variables of interest (e.g., sleep variability), which were demeaned and individually entered as regressors in the GLM model. A statistical threshold of p < .05, corrected for multiple comparisons with familywise error correction (FWE) and Threshold-Free Cluster Enhancement (TFCE), was used for analyses. TFCE helps identify cluster-like structures in images without definition of an initial cluster-forming threshold or carrying out a large amount of data smoothing (Smith and Nichols, 2009). Identification of the significant white-matter tracts revealed by TBSS was based on the Johns Hopkins University (JHU) – ICBM-DTI-81 white-matter labels atlas and the white-matter tractography atlas (Wakana et al., 2004, Hua et al., 2008). For visualization purposes for the figures, the skeletonized results were thickened using the tbss_fill command. The images are displayed in radiological convention. When sex was included as a covariate, all the results below remain the same.
2. Results
2.1. Descriptives
At wave 1, adolescents went to bed at 11:00pm on average (range 8:20 pm to 1:45 am; bedtime variability = 1.44 h). Adolescents attained an average of 499 min (8.3 h) of sleep per night, ranging from 274 to 647 min. Adolescents’ sleep duration variability (i.e., one's own standard deviation in sleep time across 14 days) was 93 min on average, ranging from 36 to 152 min. Weekend–weekday sleep duration was 60 min (ranging from −133 to +198), indicating that adolescents attained 1 h more sleep on average on weekends than weekdays.
At wave 2, adolescents went to bed at 11:10 pm on average (range 8:50 pm to 2:10 am; bedtime variability = 1.33 h). Adolescents attained an average of 493 min (8.2 h) of sleep per night, ranging from 397 to 596 min. Adolescents’ sleep duration variability at wave 2 was 95 min on average, ranging from 21 to 199 min. Weekend–weekday sleep duration was 48 min on average (ranging from −135 to +228).
Within samples t-tests revealed no significant differences in any of the sleep variables between wave 1 and wave 2. In addition, we examined gender differences in sleep patterns. Males and females only differed in average sleep time at wave 2, such that females (M = 480 min, SD = 49) attained less sleep than males (M = 510 min, SD = 39) on average, t(46) = 2.3, p < .05. Next, we ran bivariate correlations between the sleep estimates at waves 1 and 2. As shown in Table 1, greater sleep duration at wave 1 was associated with greater sleep duration at wave 2. Greater variability in sleep time at wave 1 was associated with greater weekend–weekday sleep duration at wave 1 and less total sleep time at wave 2. Weekend–weekday sleep durations at waves 1 and 2 were correlated. Bedtime at wave 1 and wave 2 were highly correlated. Bedtime variability at wave 1 was associated with less sleep duration at wave 2. Bedtime variability at wave 2 was highly correlated with sleep duration variability at wave 2.
Table 1.
Correlations between sleep variables at wave 1 and wave 2.
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sleep duration W1 | 1 | |||||||||
| 2. Sleep variability W1 | −.06 | 1 | ||||||||
| 3. Weekend–weekday W1 | .20 | .43*** | 1 | |||||||
| 4. Bedtime W1 | −.26 | .00 | −.20 | 1 | ||||||
| 5. Bedtime variability W1 | −.15 | .10 | −.24 | −.23 | 1 | |||||
| 6. Sleep duration W2 | .37** | −.39** | −.07 | −.16 | −.40** | 1 | ||||
| 7. Sleep variability W2 | .12 | .18 | .21 | .14 | .02 | −.12 | 1 | |||
| 8. Weekend–weekday W2 | .16 | .08 | .41** | −.21 | −.03 | −.20 | .29* | 1 | ||
| 9. Bedtime W2 | −.17 | .06 | −.19 | .63*** | .12 | −.27 | .17 | −.28 | 1 | |
| 10. Bedtime variability W2 | .01 | .14 | −.05 | −.12 | −.04 | −.05 | .41*** | −.12 | .06 | 1 |
p < .05.
p < .01.
p < .005.
2.2. Association between sleep and FA
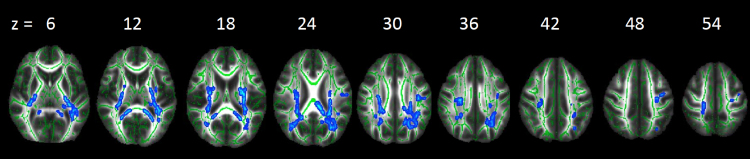
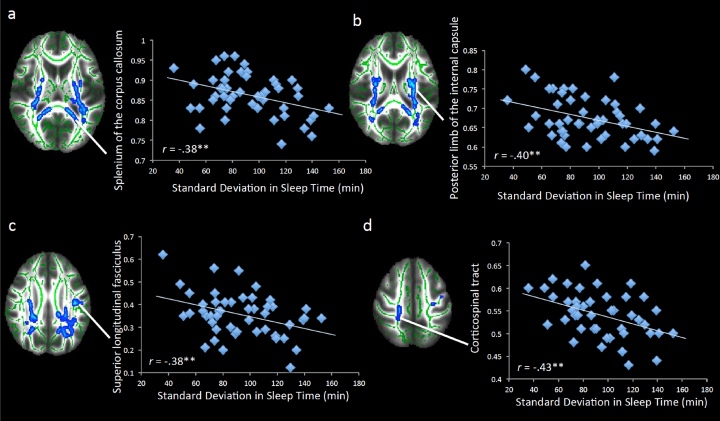
White matter microstructure was assessed using fractional anisotropy (FA), a measure of the directional coherence of brain tissue that provides an estimate of white matter integrity. Our first analysis tested how sleep duration variability and sleep duration at wave 1 correlated with FA. We simultaneously entered sleep duration variability and sleep duration as regressors to test how each was associated with white matter microstructure above and beyond the effect of the other. As shown in Table 2 and Fig. 1, greater variability in sleep duration across two weeks was associated with less FA in several white matter tracts. These clusters were located in association tracts (e.g., superior longitudinal fasciculus), projection tracts (e.g., anterior thalamatic radiata, anterior corona radiata, corticospinal tract, internal capsule), and the interhemispheric tract (corpus callosum). For descriptive purposes, we plotted several of these associations (Fig. 2).
Table 2.
Regions of FA that correlated negatively with sleep duration variability at wave 1, controlling for sleep duration at wave 1.
| Region | x | y | z | Voxels |
|---|---|---|---|---|
| L Posterior limb internal capsule | −27 | −26 | 17 | 3869a |
| L Retrolenticular part of the internal capsule | −30 | −37 | 17 | a |
| L Anterior thalamatic radiation | −25 | −52 | 26 | a |
| L Superior coronoa radiata | −27 | −7 | 21 | a |
| L Posterior corona radiata | −27 | −24 | 19 | a |
| L Cingulate gyrus | −16 | −55 | 31 | a |
| L Splenium of the corpus callosum | −16 | −44 | 10 | a |
| R Posterior limb internal capsule | 25 | −15 | 10 | 1476b |
| R Retrolenticular part of the internal capsule | 32 | −35 | 15 | b |
| R Superior coronoa radiata | 26 | −19 | 28 | b |
| R Posterior corona radiata | 27 | −40 | 24 | b |
| R Splenium of the corpus callosum | 14 | −41 | 10 | b |
| R Posterior thalamatic radiation | 34 | −59 | 16 | 295 |
| L Superior longitudinal fasciculus | −54 | −12 | 23 | 198 |
| L Superior longitudinal fasciculus | −26 | −21 | 44 | 157 |
| R Corticospinal tract | 19 | −31 | 52 | 84 |
| R Superior longitudinal fasciculus | 32 | −42 | 29 | 44 |
| R Anterior limb of the internal capsule | 12 | −2 | 13 | 24 |
Note: x, y, and z refer to MNI coordinates; Voxels refers to the number of voxels in each significant cluster; L and R refer to left and right hemispheres. Regions that share the same superscript are part of the same cluster. All regions are significant at p < .05, corrected.
Fig. 1.
Regions of FA that correlated negatively with sleep variability at wave 1. Significant results are displayed on the study-specific mean FA map and the study-specific mean FA skeleton. Note: Right = left.
Fig. 2.
Significant correlations between FA and sleep variability at wave 1. Scatterplots showing a visual depiction of the relation between sleep variability at wave 1 and FA in the (a) splenium of the corpus callosum, (b) posterior limb of the internal capsule, (c) superior longitudinal fasciculus, and (d) corticospinal tract. **p < .005. Note: Right = left.
Next, to examine whether the associations with sleep duration variability and FA were accounted for by weekend–weekday differences in sleep time, we entered weekend–weekday sleep duration as a regressor into the same model with sleep duration variability and sleep duration. Sleep duration variability continued to predict lower FA and weekend–weekday sleep duration was not associated with FA. Lastly, we tested whether the associations with sleep duration variability and FA were accounted for by variability in bedtime. We simultaneously entered bedtime variability, sleep duration variability, and sleep duration as regressors. Bedtime variability was not associated with FA, whereas sleep duration variability continued to predict lower FA.
Finally, we examined how sleep at wave 2 was associated with FA. We simultaneously entered sleep duration variability and sleep duration as regressors to test how each was associated with white matter microstructure above and beyond the effect of the other. Sleep duration variability and sleep duration at wave 2 were not associated with FA. In addition, we examined weekend–weekday sleep duration and bedtime variability in parallel models to those run at wave 1. None of these sleep indexes were associated with FA.
3. Discussion
Adolescence is a developmental period marked by increases in poor sleep coupled with significant reorganization of the brain, and extensive emotional, behavioral, and cognitive changes. The links between poor sleep and behavioral and psychological outcomes (e.g., academic difficulties, poor impulse control, problems with emotion regulation, negative affect) have been well established (Fuligni and Hardway, 2006, Anderson et al., 2008, O’Brien and Mindell, 2005, McKnight-Eily et al., 2011). Yet, despite the known importance of sleep for brain development (Roffwarg et al., 1996, Jan et al., 2010, Dahl and Lewin, 2002), we still know relatively little about how insufficient sleep during adolescence may alter the developing brain. This is the first study to our knowledge to examine how sleep during adolescence is longitudinally associated with white matter integrity. We find that greater variability in sleep duration 1.5 years prior to the DTI scan is associated with lower FA above and beyond the effects of sleep duration and variability in bedtime, whereas sleep variability a few months prior to the scan is not associated with FA. These effects suggest that variability in sleep duration, regardless of the duration in the total amount of sleep or large fluctuations in the time teenagers are going to bed, may have long-term effects on the developing brain.
The amount of daily variability in adolescents’ sleep time was striking, averaging over 1.5 h. Sleep variability measured 1.5 years prior to the scan was associated with significantly reduced FA in frontocortical and frontostriatal tracts, association tracts, projection tracts, and the interhemispheric tract. FA in these tracts should be increasing during adolescence (Asato et al., 2010, Giorgio et al., 2008, Giorgio et al., 2010). Thus, sleep variability is directly at odds with normative developmental trends. Notably, sleep variability predicted reduced FA above and beyond average sleep time, suggesting that variability in sleep duration may be more important for white matter development than does receiving chronically low levels of average sleep time. This finding is consistent with prior research showing that variability in sleep predicts adolescents’ psychological functioning more so than does average sleep time (Fuligni and Hardway, 2006, Lemola et al., 2013).
Our findings suggest that high levels of sleep variability may impair the development of white matter development. Developmental increases in myelination during adolescence facilitate faster and more efficient neural transmission and cognitive processing which, in turn, is related to better behavioral performance and learning (e.g., Jacobus et al., 2013). Here we find potentially long-term consequences of poor sleep, relating to lower FA in the parietocortical, frontocortical and frontostriatal tracts. Specifically, we found that greater sleep variability was associated with reduced FA in projection and association tracts including the internal capsule (IC), anterior thalamatic radiation (ATR), posterior thalamatic radiation (PTR), cingulum, superior corona radiata, posterior corona radiata, and superior longitudinal fasciculus (SLF). The IC is traversed by axonal fibers that connect the thalamus to the prefrontal cortex and forms part of a circuit linking the frontal lobe and basal ganglia. The ATR connects the dorsomedial and anterior thalamic nuclei with the prefrontal cortex (Schmahmann and Pandya, 2006). The cingulum connects the anterior cingulate cortex to other frontal sites as well as to the amygdala, nucleus accumbens, and thalamus (Goldman-Rakic et al., 1984). Deficits in the cingulate and ATR tracts result in the disintegration of the fronto-striato-thalamic circuitry and the disruption of functional connectivity between regions linked by these tracts. The PTR is a projection fiber from the posterior part of the thalamus to the occipital cortex, which includes axons connecting the cerebral cortex, basal ganglia, and thalamus. The PTR is part of the cortico-thalamo-cortical pathway, which is considered to play a key role in central information processing and overall cognitive control (Sherman and Guillery, 2002, Chaddock-Heyman et al., 2013). The posterior and superior corona radiata contain reciprocal prefrontal–striatal connections and projection fibers. These tracts are central to detection of salient rewarding cues, positive affect, and higher order processing necessary to effectively monitor conflict and resist impulsive actions (Jacobus et al., 2013). Finally, the superior longitudinal fasciculus is a long association fiber connecting the prefrontal to parietal cortex. The SLF plays an important role in higher-level cognitive processes, including attention and executive functions. Together, these tracts carry important connections between the prefrontal cortex and subcortical regions, and are therefore essential for cognitive and executive functioning and emotion regulation (Asato et al., 2010). Importantly, these tracts are still undergoing significant development during adolescence (Asato et al., 2010, Giorgio et al., 2008, Giorgio et al., 2010). Thus, variability in sleep duration may impair cognitive and socioemotional well-being by altering the development of white matter integrity in these tracts. However, we did not measure cognitive or emotional outcomes or the consolidation of memories and learning. Thus, future research should use longitudinal techniques to examine functional outcomes at both neural and behavioral levels in order to effectively examine how sleep and alterations in white matter are associated with developmental aspects of affect, cognition, and behavior.
Adolescents in this study averaged over 8 h of sleep per night, which is similar to what has been obtained in other studies with this age group (Wolfson and Carskadon, 1998, Fuligni and Hardway, 2006) and is close to the number of hours recommended for adolescents (Wolfson and Carskadon, 1998). Yet, some participants attained as few as 4.5 h of sleep on average. Interestingly, average sleep duration was not associated with white matter integrity. In addition, the effects of sleep variability on brain development were not due to large differences in sleep duration on weekends versus weekdays or to variability in bedtime. This suggests that day-to-day variability in the duration of sleep within the week may be particularly detrimental for youth.
3.1. Limitations
Our study is correlational and does not provide evidence about the direction or causality of the effects. Thus, it is possible that alterations in white matter integrity result in greater variability in sleep. However, because variability in sleep measured a few months prior to the scan was not associated with FA, whereas variability in sleep measured 1.5 years prior to the scan was associated with lower FA, our findings suggest that sleep problems may not induce neural changes in white matter that are immediately evident. Rather, sleep problems may have accumulating effects that impair neural development over time. Therefore, it may take time for sleep patterns to have an effect on structural changes in the brain. We have previously found that poor quality sleep has immediate consequences for functional brain activation in adolescence, such that insufficient sleep is related to lower activation in the PFC and heightened activation in the striatum, which, in turn, are associated with greater risk taking behavior (Telzer et al., 2013). Here we show that the consequences of poor sleep for structural brain development may have more accumulating and long-term effects. It is also possible that adolescents’ brains were more malleable and sensitive to environmental stressors at wave 1, and so poor sleep may have had immediate changes on neural circuitry at age 14.8 years that persisted, neural changes we did not find at wave 2 when the adolescents were older and potentially less sensitive to environmental stressors.
We utilized adolescents’ self-reported sleep. Although daily diaries generally avoid retrospective errors in recall and provide a means for estimating daily variability in adolescents’ sleep that cannot be captured with retrospective reports, daily self-reports are still subjective measures that can be prone to reporting biases. Thus, using a device such as an actigraph to monitor participants’ motor movements throughout the night could provide more objective measures of sleep as well as a wider variety of sleep measures. For instance, actigraphy provides measures of sleep quality, including sleep efficiency, sleep latency onset, and wake after sleep onset, measures which could provide a deeper understanding of the role of sleep on brain development. Thus, our study is limited in that we only assessed the time adolescents went to bed and the time spent sleeping across study days. While this allowed us to capture several important indexes of sleep (i.e., sleep duration, sleep variability, weekend–weekday sleep, and variability in bedtime), we were not able to assess other measures of the quality of adolescents’ sleep. Future studies should continue to carefully unpack the role of multiple sleep indexes on adolescents’ brain development and behavioral functioning in order to fully understand when and why chronically low sleep duration versus high variability in sleep duration impact youths’ development.
Finally, DTI is an effective measure for assessing white matter integrity. Fractional anisotropy (FA), the most widely used parameter of DTI (Assaf and Pasternak, 2008), is sensitive to a variety of parameters including fiber density, axonal diameter, and regional myelination (Beaulieu, 2002). However, changes in FA values during normal development largely reflect changes in axonal myelination and can therefore be used as an indirect measurement of myelin level (Mädler et al., 2008). Nonetheless, these other indexes included in FA also contribute to observed anisotropy (Assaf and Pasternak, 2008). Thus, future research should take advantage of methodological advances in estimating white matter integrity to fully understand the specific mechanisms that are altered as a function of poor sleep. In addition, future research should unpack how sleep may contribute to alterations in other structural brain measures, including gray matter development and MR elastography (Mariappan et al., 2010).
In conclusion, we utilized longitudinal reports of daily sleep during adolescence and find that variability in adolescents’ sleep patterns can have implications for their brain development. Attaining low-variability sleep therefore is essential not only for short-term functioning and well-being, but also for longer-term neurobiological development. Parents, practitioners, and policymakers should pay close attention to the daily changes in adolescents’ sleep patterns and identify ways to decrease variable amounts of sleep time, which can potentially have lasting implications for the developing teenage brain.
Conflict of interest
We have no biomedical, financial, or potential conflicts of interest.
Authors’ note
Support for this study was provided by the NICHD (R01HD057164-S, Fuligni), NSF (NSF 1023293, Telzer), and a University of California Institute for Mexico and the United States Dissertation Research Grant (Telzer).
References
- Acebo C., Carskadon M.A. Influence of irregular sleep patterns on waking behavior. In: Carskadon M.A., editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; Cambridge, UK: 2002. pp. 220–235. [Google Scholar]
- Acheson A., Wijtenburg S.A., Rowland L.M., Winkler A.M., Gaston F., Mathias C.W., Fox P.T., Lovallo W.R., Wright S.N., Hong L.E., Dougherty D.M., Kochunov P. Assessment of whole brain white matter integrity in youths and young adults with a family history of substance-use disorders. Hum. Brain Mapp. 2014;35(11):5401–5413. doi: 10.1002/hbm.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B., Storfer-Isser A., Taylor H.G., Rosen C.L., Redline S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2008;123:e701–e707. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. 2007. Non-linear optimisation FMRIB technical report TR07JA1. from www.fmrib.ox.ac.uk/analysis/techrep. [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. 2007. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. from www.fmrib.ox.ac.uk/analysis/techrep. [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y., Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J. Mol. Neurosci. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Bava S., Jacobus J., Thayer R.E., Tapert S.F. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol. Clin. Exp. Res. 2013;37:E181–E189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed. 2002;15(7/8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bora E., Yücel M., Fornito A., Pantelis C., Harrison B.J., Cocchi L., Lubman D.I. White matter microstructure in opiate addiction. Addiction Biology. 2012;17(1):141–148. doi: 10.1111/j.1369-1600.2010.00266.x. [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L., Erickson K.I., Voss M.W., Powers J.P., Knecht A.M., Pontifex M.B., Drollette E.S., Moore R.D., Raine L.B., Scudder M.R., Hillman C.H., Kramer A.F. White matter microstructure is associated with cognitive control in children. Biol. Psychol. 2013;94(1):109–115. doi: 10.1016/j.biopsycho.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain I.M., Baker F.C. Changes in sleep as a function of adolescent development. Neuropsychol. Rev. 2011;21(1):5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E., Lewin D.S. Pathways to adolescent health sleep regulation and behavior. J. Adolesc. Health. 2002;31(6):175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Fuligni A.J., Hardway C. Daily variation in adolescents’ sleep, activities, and psychological well-being. J. Res. Adolesc. 2006;16(3):353–378. [Google Scholar]
- Giedd J.N. The teen brain: insights from neuroimaging. J. Adolesc. Health. 2008;42(4):335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Giorgio A., Watkins K.E., Douaud G., James A.C., James S., De Stefano N., Matthews P.M., Smith S.M., Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Giorgio A., Watkins K.E., Chadwick M., James S., Winmill L., Douaud G., De Stefano N., Matthews P.M., Smith S.M., Johansen-Berg H., James A.C. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49(1):94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Selemon L.D., Schwartz M.L. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Halbower A.C., Degaonkar M., Barker P.B., Earley C.J., Marcus C.L., Smith P.L., Prahme M.C., Mahone E.M. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med. 2006;3(8):e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S., Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Born J. Sleep, synaptic connectivity, and hippocampal memory during early development. Trends Cogn. Sci. 2014;18(3):141–152. doi: 10.1016/j.tics.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Iglowstein I., Jenni O.G., Molinari L., Largo R.H. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L.M., Infante M.A., Bava S., Tapert S.F. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013;3(1):396–414. doi: 10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan J.E., Reiter R.J., Bax M.C., Ribary U., Freeman R.D., Wasdell M.B. Long-term sleep disturbances in children: a cause of neuronal loss. Eur. J. Paediatr. Neurol. 2010;14(5):380–390. doi: 10.1016/j.ejpn.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Plotsky P.M., Nemeroff C.B., Charney D.S. Effects of early adverse experiences on brain structure and function: clinical implications. Biol. Psychiatry. 2000;48(8):778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kopp C., Longordo F., Nicholson J.R., Lüthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J. Neurosci. 2006;26(48):12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemola S., Ledermann T., Friedman E.M. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLOS ONE. 2013;8(8):e71292. doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.Q., Mathews V.P., Wang Y., Dunn D., Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Ann. N. Y. Acad. Sci. 2005;1064(1):184–192. doi: 10.1196/annals.1340.034. [DOI] [PubMed] [Google Scholar]
- Mädler B., Drabycz S.A., Kolind S.H., Whittall K.P., MacKay A.L. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn. Reson. Imaging. 2008;26(7):874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Mariappan Y.K., Glaser K.J., Ehman R.L. Magnetic resonance elastography: a review. Clin. Anat. 2010;23(5):497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Eily L.R., Eaton D.K., Lowry R., Croft J.B., Presley-Cantrell L., Perry G.S. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev. Med. 2011;53(4):271–273. doi: 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Mirescu C., Peters J.D., Noiman L., Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H., Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien E.M., Mindell J.A. Sleep and risk-taking behavior in adolescents. Behav. Sleep Med. 2005;3(3):113–133. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- Olini N., Kurth S., Huber R. The effects of caffeine on sleep and maturational markers in the rat. PLOS ONE. 2013;8(9):e72539. doi: 10.1371/journal.pone.0072539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R., Henry L., Grieve S.M., Guilmette T.J., Niaura R., Bryant R., Bruce S., Williams L.M., Richard C.C., Cohen R.A., Gordon E. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr. Dis. Treat. 2008;4(1):193–201. doi: 10.2147/ndt.s1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin F., Metz A.J., Wolf M., Achermann P., Jenni O.G., Huber R. Local increase of sleep slow wave activity after three weeks of working memory training in children and adolescents. Sleep. 2014;38(4):607–614. doi: 10.5665/sleep.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffwarg H.P., Muzio J.N., Dement W.C. Ontogenetic development of the human sleep-dream cycle. Science. 1996;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Rueckert D., Sonoda L.I., Hayes C., Hill D.L.G., Leach M.O., Hawkes D.J. Non-rigid registration using free-form deformations: Application to breast MR images. IEEE Transactions on Medical Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. Oxford University Press; New York: 2006. Fiber Pathways of the Brain. [Google Scholar]
- Sejnowski T.J., Destexhe A. Why do we sleep? Brain Res. 2000;886(1):208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- Sherman S.M., Guillery R.W. The role of the thalamus in the flow of information to the cortex. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 2002;357(1428):1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Gálvan A. The effects of poor quality sleep on brain function during risk taking in adolescence. NeuroImage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Sheu Y.S., Polcari A., McGreenery C.E. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. Am. J. Psychiatry. 2010;167(12):1464–1471. doi: 10.1176/appi.ajp.2010.10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsdottir B., Bjornsson J.K., Benediktsdottir B., Gislason T., Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J. Psychosom. Res. 2002;5:529–537. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Wakana S., Jiang H., Nagae-Poetscher L.M., Van Zijl P.C., Mori S. Fiber tract–based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wolfson A.R., Carskadon M.A. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]