Introduction
Electrographic seizures and status epilepticus are common in critically ill children with acute encephalopathy, leading to increasing use of continuous EEG monitoring. Many children with electrographic status epilepticus have no associated clinical signs, so EEG monitoring is required for seizure identification. Further, there is increasing evidence that high seizure burdens, often classified as electrographic status epilepticus, are associated with worse outcomes. This review discusses the incidence of electrographic status epilepticus, risk factors for electrographic status epilepticus, and associations between electrographic status epilepticus and outcomes, and it summarizes recent guidelines and consensus statements addressing EEG monitoring in critically ill children.
Electrographic Seizure and Status Epilepticus Incidence
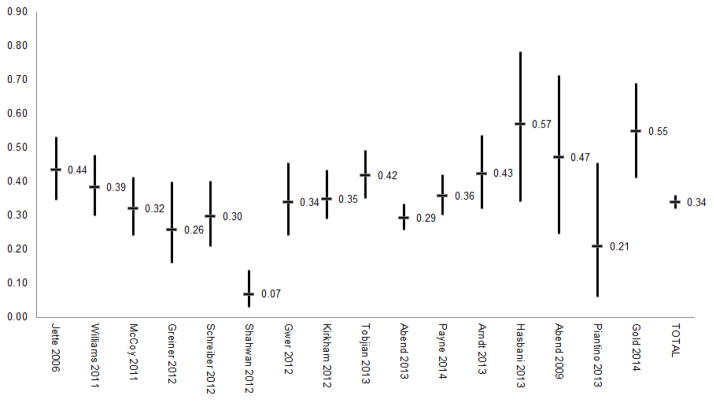
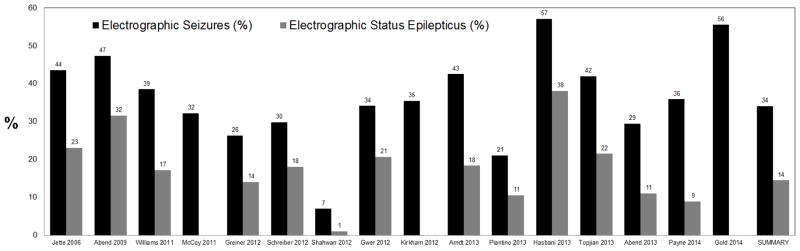
Single and multi-center investigations have reported that about 10–40% of critically ill children who undergo continuous EEG monitoring (cEEG) experience electrographic seizures. Further, about one-third of children with electrographic seizures have a sufficiently high seizure burden to be categorized as experiencing electrographic status epilepticus, although the exact definitions of status epilepticus have varied across studies.[1–19] The largest study of continuous EEG monitoring in the pediatric intensive care unit was a retrospective study of 550 critically ill children from 11 tertiary care institutions from the United States and Canada who underwent clinically indicated EEG monitoring. Electrographic seizures occurred in 30% of monitored children. Among children with electrographic seizures, 33% had electrographic status epilepticus which was defined as either a seizure lasting more than 30 minutes or briefer recurrent seizures totalling 30 minutes within a one hour period. About one-third of children with electrographic seizures had exclusively EEG-only (non-convulsive) seizures which would not have been identified by clinical observation alone.[13] These multi-center findings are consistent with single center studies, as summarized in Figures 1 and 2.[3,6,8–10,12,14–16,18]
Figure 1.
Proportions of subjects with electrographic seizures and electrographic status epilepticus from studies in which critically ill children underwent continuous EEG monitoring.
Figure 2.
Incidence of electrographic seizures reported in studies of continuous EEG monitoring of critically ill children. The 95% confidence interval bars were generally not reported by the publications and were calculated based on the number of subjects with seizures and the total number of subjects in the study (Stata-12, immediate confidence interval command).
Much of the variability in electrographic seizure incidence reported in the literature may have two explanations. First, many of the studies are relatively small, leading to the wide 95% confidence intervals (Figure 2). Second, most of the reported studies involve clinically indicated continuous EEG monitoring, and the exact clinical indications varied across institutions and clinicians. Table 1 summarizes a number of studies of continuous EEG monitoring which have included varying cohorts of critically ill children. The studies towards the top of the table included only children with known acute brain disorders such as traumatic brain injury or hypoxic ischemic encephalopathy. These studies reported higher electrographic seizure incidences. In contrast, the studies towards the bottom of the table included many patients who were critically ill and encephalopathic but had no known neurologic disorder. These studies reported lower electrographic seizure incidences.
Table 1.
Studies of critically ill children undergoing continuous EEG monitoring based on varying indications. Studies in which the continuous EEG monitoring was performed for children with known acute neurologic problems report higher incidences of electrographic seizure and/or electrographic status epilepticus than studies with broader continuous EEG monitoring criteria in which some children do not have a known acute neurologic problem.
| Study | N Age | EEG Indication | % with Acute CNS Disorder | ES or ESE |
|---|---|---|---|---|
| Hasbani 2013 | 21 Ped | Abusive TBI | 100% | 57% |
| Gold 2014 | 54 Ped | Encephalitis | 100% | 56% |
| Abend 2009 | 19 Ped | s/p Cardiac Arrest with HIE | 100% | 48% |
| Abend 2011 | 100 Ped | ΔMS & acute CNS condition | 100% | 46% |
| Arndt 2013 | 87 Ped | TBI (mild-severe) requiring PICU | 100% | 43% |
| Greiner 2012 | 57 Ped | ICU with cEEG for possible NCSE | Most | 40% |
| Kirkham 2012 | 140 Ped | Comatose in ICU | Most | 35% |
| Gwer 2012 | 82 Ped | Non-traumatic coma in ICU. | Most | 34% |
| Piantino 2013 | 19 Ped | ECMO | Most | 21% |
| Jette 2006 | 117 Neo+Ped | Critically ill and underwent cEEG | >68% | 39% |
| Williams 2011 | 122 Neo+Ped | Critically ill and underwent cEEG | >62% | 38% |
| Payne 2014 | 259 Ped | Critically ill and underwent cEEG | 58% | 36% |
| Schreiber 2012 | 94 Ped | Acute non=pharmacologic encephalopathy | 57% | 30% |
| McCoy 2011 | 121 Neo+Ped | Critically ill and underwent cEEG | 52% | 29% |
| Shahwan 2010 | 100 Ped | Sustained depressed consciousness | 50% | 7% |
Several studies have demonstrated that EEG-only seizures occur in children who have not received any or recent paralytics.[15,18] Thus, clinicians cannot be reassured that seizures would be clinically observed in non-paralyzed patients. Mechanistically, this finding also indicates that critically ill children may experience electromechanical uncoupling (dissociation of electrical brain activity and outward mechanical signs), as has been described in critically ill neonates.[20,21]
To help standardize clinical care and research, a working definition for non-convulsive status epilepticus has been proposed. In patients without pre-existing epileptic encephalopathy, non-convulsive status epilepticus is diagnosed based on two electroencephalographic characteristics. First, there can be epileptiform discharges occur at >2.5 Hz. Second, there can be epileptiform discharges occurring at ≤2.5 Hz or rhythmic delta/theta activity at >0.5 Hz along with any one of three characteristics. The three characteristics include (1) electroencephalographic and clinical improvement after intravenous anti-seizure medications are administered, (2) subtle clinical ictal phenomena during the electroencephalographic patterns, and/or (3) typical spatial/temporal evolution.[22] These electrographic characteristics help differentiate between non-convulsive status epilepticus and non-ictal rhythmic or periodic patterns.[23] Based on the clinical and electroencephalographic features, the proposed definition classifies non-convulsive status epilepticus by “type” (electroclinical classification) and “etiology.” The types of non-convulsive status epilepticus are (1) with coma/stupor and (2) without coma/stupor. Those without coma/stupor may be subdivided into (1) generalized onset, (2) focal onset, or (3) unknown onset. The etiology of non-convulsive status epilepticus may be classified as cryptogenic (unknown) or symptomatic (known). The symptomatic etiology category may be subdivided as (1) acute, (2) remote, (3) progressive, or (4) occurring in an age-related electroclinical syndrome. Based on these definitions, the majority of critically ill children with an acute encephalopathy would be classified as non-convulsive status epilepticus with stupor/coma with an acute etiology.[22]
Electrographic Seizure Risk Factors
Several risk factors for electrographic seizures have been reported including younger age (infants as compared to older children), [8,11,13,16,18] the occurrence of convulsive seizures[9,13,14] or status epilepticus[8] prior to initiation of continuous EEG monitoring, the presence of acute structural brain injury, [7–9,11,12,14,16] and the presence of inter-ictal epileptiform discharges[8,12–14] or periodic epileptiform discharges.[3] In many of the studies, the differences in seizure occurrence are statistically significant based on the presence of absence of a risk factor but the absolute differences in the proportion of children with and without seizures are only 10–20%. Thus, these risk factors may have limited utility to clinicians aiming to select which patients should undergo continuous EEG monitoring.
Seizure prediction models accounting for multiple risk factors might allow clinicians to target EEG monitoring resources to children at the highest seizure risk, while accounting for the resources available at their institution. A recent study derived a seizure prediction model from the retrospectively acquired multi-center dataset described above and subsequently validated the model using a separate single center dataset. Both the generation and validation datasets were derived from clinically indicated continuous EEG monitoring with heterogeneous acute encephalopathy etiologies. The model had fair to good discrimination between patients with and without electrographic seizures. It could be used by clinicians performing three steps. First, the clinician would obtain two clinical variables (patient age and whether there were clinically evident seizures) and two routine EEG variables (background category and inter-ictal epileptiform discharge presence). Second, the clinician could determine a model score based on these four variables. Third, the clinician would select patients with model scores above their institutional cut-off to undergo continuous EEG monitoring. Individual institutions could select different model score cut-offs based on their available continuous EEG monitoring resources. A center with substantial EEG monitoring resources might perform monitoring for any patient with a low model score. For example, at a cutoff score of 0.10, 58% of patients without electrographic seizures would be identified as not needing EEG monitoring, so limited resources would not be expended. However, 14% of patients with electrographic seizures would not undergo continuous EEG monitoring, so their seizures would not be identified and managed. Given a seizure prevalence of 30%, this cutoff score would yield a positive predictive value of 47% and negative predictive value of 91%.[24] Centers with more limited continuous EEG monitoring resources might select higher model cut-off scores. Higher cut-off scores might fail to identify more patients experiencing electrographic seizures, but would also direct limited monitoring resources to patients much more likely to experience electrographic seizures.
Further development of models incorporating additional variables and aiming to predict electrographic seizure risk among more etiologically homogeneous cohorts is needed. Additionally, to date, studies have aimed to identify risk factors and develop prediction models for electrographic seizures, and not specifically for electrographic status epilepticus. Studies evaluating outcome (described below) have indicated that high electrographic seizure burdens are associated with worse patient outcomes while low seizure burdens are not. Additional outcome studies are needed, but if our clinical practice evolves such that we are aiming to identify and manage patients with electrographic status epilepticus, but not necessarily aiming to identify patients experiencing a small number of brief electrographic seizures, then it will be increasingly important to identify risk factors and develop prediction models for electrographic status epilepticus.
EEG Monitoring Duration
Decisions regarding the duration of continuous EEG monitoring must balance the goal of identifying seizures with practical concerns regarding limited continuous EEG monitoring resources. Observational studies of critically ill children undergoing clinically indicated continuous EEG monitoring have reported that about 50% and 90% of patients with electrographic seizures are identified with 1 hour and 24–48 hours of EEG monitoring, respectively (Figure 3).[3,6,8,9,12,14,15,18]
Figure 3.
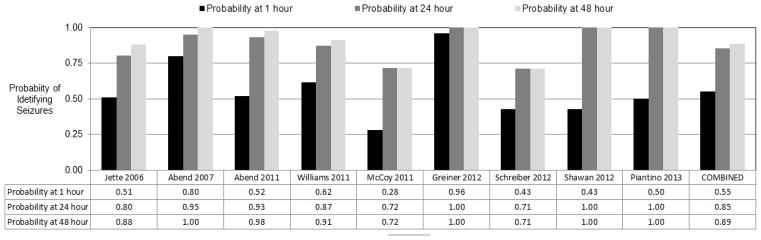
Proportion of subjects in whom electrographic seizures were identified by continuous EEG monitoring for 1 hour, 24 hours, and 48 hours.
Importantly, most of these studies define seizure onset timing based on the onset of EEG monitoring, and not based on the onset of the acute brain insult. This may be problematic since in clinical practice patients may present or be transferred to a given institution at varying durations after the onset of acute brain insult and patients may experience additional brain injury while in the intensive care unit. Thus, it is unclear how to interpret continuous EEG monitoring onset timing in these situations. Additionally, most of the studies describe clinical practice in which critically ill children underwent 1–3 days of clinically indicated continuous EEG monitoring, and some patients may have experienced seizures after EEG monitoring was discontinued.
The Neurocritical Care Society’s Guideline for the Evaluation and Management of Status Epilepticus strongly recommends performing 48 hours of continuous EEG monitoring to identify electrographic status epilepticus in comatose children following an acute brain insult.[25] As discussed further below, the American Clinical Neurophysiology Society’s Consensus Statement on EEG Monitoring of Critically Ill Adults and Children recommends that EEG monitoring be initiated as quickly as possible and continued for at least 24 hours in patients at risk for electrographic seizures.
Outcome
The extent to which electrographic seizures produce secondary brain injury, as compared to simply signifying the presence more severe acute brain injury remains unknown. Additionally, the extent to which electrographic seizures produce secondary brain injury likely varies based on the acute brain injury etiology, seizure burden, and varying approaches to manage seizure. Studies are just beginning to elucidate these relationships. To date, a number of studies have reported an association between high electrographic seizure burdens and worse outcomes, even after adjustment for potential confounders related to acute encephalopathy etiology and critical illness severity.[10,13,26,27]
There have been several studies of short-term outcome. A prospective observational study of 1–3 channel EEG in 204 critically ill neonates and children reported that electrographic seizures were associated with a higher risk of unfavorable neurologic outcome in a multivariate analysis that included age, etiology, pediatric index of mortality score, Adelaide coma score, and EEG background category.[10] A prospective observational study of full array EEG monitoring in 200 children in the pediatric intensive care unit that assessed discharge outcome reported electrographic status epilepticus was associated with higher mortality and worsened pediatric cerebral performance category scores in multivariate analyses that included seizure category, age, acute neurologic disorder, prior neurodevelopmental status, and EEG background category. In contrast, electrographic seizures were not associated with worse outcomes.[26] A retrospective multi-center study of 550 children in the pediatric intensive care unit assessed discharge mortality and reported an association between electrographic status epilepticus and mortality in a multivariate analysis that included seizure category, acute encephalopathy etiology, and EEG background. In contrast, electrographic seizures were not associated with worse outcomes.[13] A prospective observational study of 259 critically ill children measured the maximum hourly seizure burden. In a multivariate analysis that adjusted for diagnosis and illness severity, every 1% increase in the maximum hourly seizure burden was associated with a 1.3 odds of neurological decline.[17]
There have been fewer studies of long-term outcome. A study assessing outcome among 60 children a median of 2.7 years following pediatric intensive care unit admission reported that electrographic status epilepticus was associated unfavorable Glasgow Outcome Scale (Extended Pediatric Version) category, lower Pediatric Quality of Life scores, and an increased risk of subsequently diagnosed epilepsy in multivariate analyses including age, acute neurologic diagnosis category, EEG background category, and several other clinical variables. In contrast, electrographic seizures were not associated with worse outcomes.[27]
Clinical Practice, Guidelines, and Consensus Statements
A survey of continuous EEG monitoring use in the pediatric intensive care units of 61 large hospitals in the United States and Canada reported a 30% increase in the median number of patients who underwent continuous EEG monitoring per month between 2010 and 2011.[28] Common indications for continuous EEG monitoring included determining whether events of unclear etiology were seizures and identifying electrographic seizures in patients considered at-risk for seizures, such as children with altered mental status following a convulsion, altered mental status with a known acute brain injury, and altered mental status of unknown etiology. About one-third to one-half of institutions performed EEG monitoring as part of clinical pathways for specific acute encephalopathy conditions such as hypoxic-ischemic encephalopathy following cardiac arrest or severe traumatic brain injury.[28]
The Neurocritical Care Society’s Guidelines for the Evaluation and Management of Status Epilepticus define status epilepticus as “five minutes or more of (i) continuous clinical and/or electrographic seizure activity or (ii) recurrent seizure activity without recovery (returning to baseline) between seizures” and recommend that “the treatment of status epilepticus should occur rapidly and continue sequentially until electrographic seizures are halted.” Consistent with these statements, the guideline advocates for the use of continuous EEG monitoring to identify electrographic seizures in at-risk patients including patients with persisting altered mental status for more than 10 minutes after convulsive seizures or status epilepticus or encephalopathic children after resuscitation from cardiac arrest, with traumatic brain injury, with intracranial hemorrhage, or with unexplained encephalopathy. The guideline strongly recommends the continuous EEG monitoring be continued for 48 hours in comatose patients.[25]
The Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society published a consensus statement addressing indications and technical specifications for continuous EEG monitoring in non-neonatal children and adults. The statement recognizes that some techniques are only available in specialized centers and aims to lay out an “idealized” system useful for program development and improvement.[29,30] The main indication for continuous EEG monitoring is to identify non-convulsive seizures or status epilepticus, and several specific cohorts of patients are described. First, continuous EEG monitoring is recommended in patients with persistently abnormal mental status following generalized convulsive status epilepticus or other clinically-evident seizures. Children with convulsive seizures [9,13,14] or convulsive status epilepticus [8] prior to EEG monitoring initiation are at higher risk for experiencing electrographic seizures. In a retrospective study of 98 children in pediatric intensive care units undergoing clinically-indicated continuous EEG monitoring after termination of convulsive status epilepticus, 33% had ongoing electrographic seizures.[31] Second, continuous EEG monitoring is recommended in patients with acute supratentorial brain injury with altered mental status. Conditions associated with electrographic seizures include intraparenchymal hemorrhage, arterial ischemic stroke, moderate to severe traumatic brain injury, central nervous system infections, hypoxic-ischemic brain injury, sepsis associated encephalopathy, use of extracorporeal membrane oxygenation, recent neurosurgical procedures, brain tumors, and prior epilepsy diagnosis. Third, continuous EEG monitoring is recommended in patients with fluctuating mental status of unexplained alteration of mental status.[29]
The consensus statement describes that a second indication for continuous EEG monitoring is to assess the efficacy of therapy for seizures and status epilepticus. Electrographic seizures or status epilepticus may occur after convulsive seizures terminate, and many of the breakthrough seizures during status epilepticus management with continuous intravenous medications are non-convulsive. Thus, continuous EEG monitoring is recommended after management of convulsive status epilepticus if persisting non-convulsive seizures are suspected, during the entire course of intravenous anaesthetic administration, and for about 24 hours after anaesthetics are tapered (or longer following administration of long-lasting medications).[29]
In addition to discussion of continuous EEG monitoring indications, the consensus statement also comments on staffing, equipment, networks, data storage, and overall workflow.[30]
Conclusions
Electrographic status epilepticus is common in critically ill children, and identification often requires continuous EEG monitoring. Recent studies indicate that electrographic status epilepticus is associated with worse clinical outcomes even in adjusted analyses including variables related to critical illness and brain injury severity, suggesting that the seizures may be contributing to secondary brain injury. Based on these data, continuous EEG monitoring use is increasing and advocated for by recent guideline and consensus statements. Further study is needed to identify optimal management strategies, and determine whether these management strategies improve patient outcomes.
Highlights.
Electrographic status epilepticus is common in critically ill children with acute encephalopathy.
Electrographic status epilepticus is associated with worse outcome.
Recent guideline and consensus statement advocate for EEG monitoring to identify and manage electrographic status epilepticus.
Acknowledgments
Dr. Abend is supported by NIH (K23NS076550).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abend NS, Wusthoff CJ, Goldberg EM, Dlugos DJ. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol. 2013:1170–9. doi: 10.1016/S1474-4422(13)70246-1. [DOI] [PubMed] [Google Scholar]
- 2.Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol. 2005:162–5. doi: 10.1016/j.pediatrneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006:1750–5. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- 4.Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007:165–70. doi: 10.1016/j.pediatrneurol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia. 2006:1504–9. doi: 10.1111/j.1528-1167.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 6.Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia. 2010:1198–204. doi: 10.1111/j.1528-1167.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- 7.Abend NS, Topjian A, Ichord R, Herman ST, Helfaer M, Donnelly M, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009:1931–40. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011:1130–6. doi: 10.1111/j.1528-1167.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- 9.Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012:e748–55. doi: 10.1542/peds.2011-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkham FJ, Wade AM, McElduff F, Boyd SG, Tasker RC, Edwards M, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012:853–62. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arango JI, Deibert CP, Brown D, Bell M, Dvorchik I, Adelson PD. Posttraumatic seizures in children with severe traumatic brain injury. Childs Nerv Syst. 2012:1925–9. doi: 10.1007/s00381-012-1863-0. [DOI] [PubMed] [Google Scholar]
- 12.Piantino JA, Wainwright MS, Grimason M, Smith CM, Hussain E, Byron D, et al. Nonconvulsive Seizures Are Common in Children Treated With Extracorporeal Cardiac Life Support. Pediatr Crit Care Med. 2013:601–9. doi: 10.1097/PCC.0b013e318291755a. [DOI] [PubMed] [Google Scholar]
- 13.Abend NS, Arndt DH, Carpenter JL, Chapman KE, Cornett KM, Gallentine WB, et al. Electrographic seizures in pediatric ICU patients: Cohort study of risk factors and mortality. Neurology. 2013:383–91. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy B, Sharma R, Ochi A, Go C, Otsubo H, Hutchison JS, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011:1973–8. doi: 10.1111/j.1528-1167.2011.03291.x. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber JM, Zelleke T, Gaillard WD, Kaulas H, Dean N, Carpenter JL. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012:31–8. doi: 10.1007/s12028-012-9715-z. [DOI] [PubMed] [Google Scholar]
- 16.Arndt DH, Lerner JT, Matsumoto JH, Madikians A, Yudovin S, Valino H, et al. Subclinical early posttraumatic seizures detected by continuous EEG monitoring in a consecutive pediatric cohort. Epilepsia. 2013:1780–8. doi: 10.1111/epi.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payne ET, Zhao XY, Frndova H, McBain K, Sharma R, Hutchison JS, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014:1429–38. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abend NS, Gutierrez-Colina AM, Topjian AA, Zhao H, Guo R, Donnelly M, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011:1071–7. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold JJ, Crawford JR, Glaser C, Sheriff H, Wang S, Nespeca M. The role of continuous electroencephalography in childhood encephalitis. Pediatr Neurol. 2014:318–23. doi: 10.1016/j.pediatrneurol.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003:277–80. doi: 10.1016/s0887-8994(02)00621-5. [DOI] [PubMed] [Google Scholar]
- 21.Glykys J, Dzhala VI, Kuchibhotla KV, Feng G, Kuner T, Augustine G, et al. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009:657–72. doi: 10.1016/j.neuron.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beniczky S, Hirsch LJ, Kaplan PW, Pressler R, Bauer G, Aurlien H, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013:28–9. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 24.Yang A, Arndt DH, Berg RA, Carpenter JL, Chapman KE, Dlugos DJ, et al. Development and validation of a seizure prediction model in critically ill children. Seizure. 2015:104–11. doi: 10.1016/j.seizure.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 26.Topjian AA, Gutierrez-Colina AM, Sanchez SM, Berg RA, Friess SH, Dlugos DJ, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Critical Care Medicine. 2013:215–23. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagenman KL, Blake TP, Sanchez SM, Schultheis MT, Radcliffe J, Berg RA, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014:396–404. doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez SM, Carpenter J, Chapman KE, Dlugos DJ, Gallentine W, Giza CC, et al. Pediatric ICU EEG Monitoring: Current Resources and Practice in the United States and Canada. Journal of Clinical Neurophysiology. 2013:156–60. doi: 10.1097/WNP.0b013e31827eda27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part I: Indications. J Clin Neurophysiol. 2015 doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part II: Personnel, Technical Specifications and Clinical Practice. J Clin Neurophysiol. 2015 doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez IS, Abend NS, Arndt DH, Carpenter JL, Chapman KE, Cornett KM, et al. Electrographic Seizures after Convulsive Status Epilepticus in Children and Young Adults: A Retrospective Multicenter Study. Journal of Pediatrics. 2014:339–46. doi: 10.1016/j.jpeds.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]