Abstract
Interventions used to treat patellofemoral pain in runners are often designed to alter patellofemoral mechanics. This study used a computational model to investigate the influence of two interventions, step rate manipulation and quadriceps strengthening, on patellofemoral contact pressures during running. Running mechanics were analyzed using a lower extremity musculoskeletal model that included a knee with six degree-of-freedom tibiofemoral and patellofemoral joints. An elastic foundation model was used to compute articular contact pressures. The lower extremity model was scaled to anthropometric dimensions of 22 healthy adults, who ran on an instrumented treadmill at 90%, 100% and 110% of their preferred step rate. Numerical optimization was then used to predict the muscle forces, secondary tibiofemoral kinematics and all patellofemoral kinematics that would generate the measured hip, knee and ankle joint accelerations. Mean and peak patella contact pressures reached 5.0 and 9.7 MPa during the midstance phase of running. Increasing step rate by 10% significantly reduced mean contact pressures by 10.4% and contact area by 7.4%, but had small effects on lateral patella translation and tilt. Enhancing vastus medialis strength did not substantially affect pressure magnitudes or lateral patella translation, but did shift contact pressure medially toward the patellar median ridge. Thus, the model suggests that step rate tends to primarily modulate the magnitude of contact pressure and contact area, while vastus medialis strengthening has the potential to alter mediolateral pressure locations. These results are relevant to consider in the design of interventions used to prevent or treat patellofemoral pain in runners.
Keywords: patellofemoral pain, vastus medialis strengthening, step length, patellofemoral kinematics
Introduction
Patellofemoral pain (PFP) is an extremely common injury among recreational runners (Taunton et al., 2002) and remains challenging to treat clinically (Brody and Thein, 1998; Juhn, 1999). Part of the difficulty arises from a limited understanding of the effects that specific interventions have on mechanical precipitators of pain. Recent imaging studies suggest that PFP may arise from localized cartilage pressure or subchondral bone damage (Draper et al., 2012; Farrokhi et al., 2011; Ho et al., 2014; Näslund et al., 2005). Moreover, elevated cartilage stress can contribute to cartilage degeneration (Segal et al., 2012; Segal et al., 2009). Hence it is important to characterize the influence of current interventions on cartilage stress to better understand their potential efficacy in mitigating PFP and long-term tissue damage.
Some treatment strategies for PFP focus on modifying running form (Bonacci et al., 2014; Cheung and Davis, 2011; Noehren et al., 2011; Wille et al., 2013; Willy and Davis, 2013). Step rate modification is a particularly attractive option given that it is easily trainable via auditory cues and can induce notable changes in limb loading patterns (Heiderscheit et al., 2011; Wille et al., 2013). For example, we previously showed that a 10% increase in step rate resulted in a 14% decrease in patellofemoral joint reaction force (Lenhart et al., 2014). However, it remains unclear how this net change in loading is reflected in cartilage pressure patterns given that limb kinematics (Heiderscheit et al., 2011) and muscle coordination patterns (Chumanov et al., 2012) are also adapting with step rate. Other treatment strategies for PFP have focused more locally on facilitating balanced quadriceps loading (Choi et al., 2011; Clijsen et al., 2014; van der Heijden et al., 2015). The underlying idea is that weakness or diminished activity in the vastus medialis may result in the patella tracking and tilting laterally, inducing localized stress concentrations (Chester et al., 2008; Goh et al., 1995; Lorenz et al., 2012b). Diminished activation of the vastus medialis has been observed in patients exhibiting patellar maltracking (Lin et al., 2010; Owings and Grabiner, 2002; Pal et al., 2012), though others have questioned the influence of quadriceps load distributions on joint loading patterns and pain (Cavazzuti et al., 2010; MacIntyre and Robertson, 1992; Sawatsky et al., 2012). A simulation study of running found that vastus medialis weakness can increase mediolateral patellofemoral joint loads (Neptune et al., 2000), though the knee model used could not resolve cartilage tissue pressure. Thus, additional work remains to understand how alterations in quadriceps loading affect patellofemoral cartilage pressure during running.
The relevance of cartilage loading to PFP requires an estimation of tissue stress patterns during functional tasks such as running. One viable technique involves the use of inverse musculoskeletal modeling to estimate net patellofemoral joint force, which can then be divided by the cartilage contact area as measured in cadavers (Bonacci et al., 2014; Teng and Powers, 2014) or medical images (Brechter and Powers, 2002; Vannatta and Kernozek, 2014; Ward and Powers, 2004; Willson et al., 2014; Wirtz et al., 2012). However, this approach would ignore variations in contact area that can arise with increased loading and tissue deformation (Besier et al., 2005). In this study, we used a validated multibody knee model (Lenhart et al., 2015) and co-simulation approach (Thelen et al., 2014) to simultaneously predict muscle forces, ligament loads and cartilage contact pressure in running. The objective was to investigate the effects of running step rate and quadriceps strength balance on the patellofemoral loading, kinematics, and cartilage contact pressures. We hypothesized that an increase in step rate would induce a decrease in both patellofemoral contact pressures and area. Further, we hypothesized that patellar position and contact pressure would migrate laterally with weakening of the vastus medialis.
Methods
Running Motion Analysis
Twenty-two healthy adults (15 M, 7 F; Table 1) who self-identified as recreational runners (at least 3 months of ≥ 24 km per week) participated in a protocol approved by the UW-Madison Health Sciences Institutional Review Board. Participants provided appropriate written informed consent before testing began. Participants reported no current pain during running, had not experienced lower extremity injury in the last 3 months, and had never undergone lower extremity surgery.
Table 1.
Subject characteristics
| Number of Subjects | 22 |
| Mass (kg) | 71.0 (8.8) |
| Height (m) | 1.80 (0.09) |
| Running Volume (km/week) | 45.5 (24.1) |
| Preferred Running Speed (m/s) | 2.83 (0.52) |
| Preferred Step Rate (steps/minute) | 173.1 (9.5) |
An eight-camera passive motion capture system (Motion Analysis Corporation, Santa Rosa, CA) was used to track whole body kinematics during treadmill running. Twenty one markers were placed on anatomical landmarks and another 14 markers were attached to rigid plates strapped to the thighs and shanks. Participants were asked to run on an instrumented treadmill (Bertec Corporation, Columbus, OH) at their preferred speed (Table 1). During a five minute warm-up run, each participant’s preferred step rate was determined. Participants were then asked to run at their preferred speed for 1–2 minutes at 90%, 100%, or 110% of their preferred step rate, with trial order randomized. An audible metronome was used to establish and maintain the desired step rate. Fifteen seconds of data were recorded and five right-footed strides were chosen from each condition for analysis. Kinematic data were collected at 200 Hz and then low-pass filtered at 12 Hz. Foot-floor reaction forces were collected at 2000 Hz and low-passed filtered at 50 Hz.
Musculoskeletal Model
Muscle and knee loads were estimated using a 3D musculoskeletal model (Arnold et al., 2010) that was adapted to include a knee with 6 degree of freedom (DOF) tibiofemoral and patellofemoral joints (Fig. 1). The pelvis was the base segment with 3 translational and 3 rotational DOF. The hip was modeled as a ball-in-socket with 3 DOF. The ankle was 1 DOF allowing for plantar- and dorsiflexion. The lower extremity model included geometric descriptions of 44 musculotendon units crossing the hip, knee, and ankle of the right limb (Arnold et al., 2010).
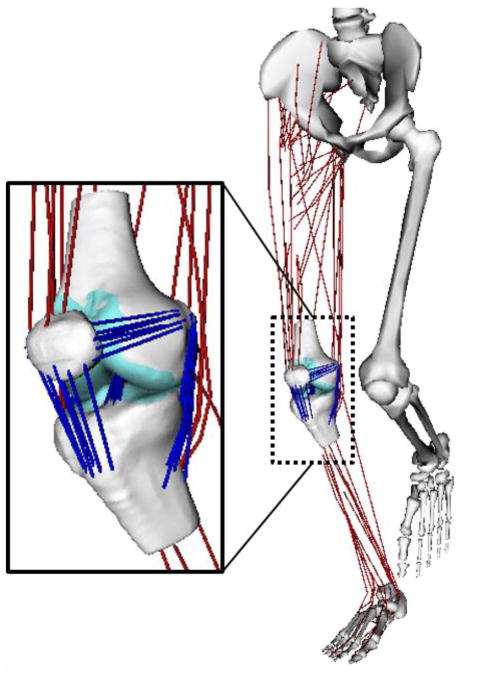
Figure 1.
A three body knee model with 6 degree of freedom tibiofemoral (TF) and patellofemoral (PF) joints was incorporated into a musculsoskeletal model of the lower extremity. The knee model included femur, tibia and patella cartilage surface geometries and bundles of nonlinear elastic ligaments. TF and PF cartilage contact pressure was computed using an elastic foundation model. The ligaments represented including the superficial and deep medial collateral ligament, posteromedial capsule, lateral collateral ligament, iliotibial band, anteriomedial and posteriolateral anterior cruciate ligament, anteriolateral and posteriomedial posterior cruciate ligament, patellar tendon, medial and lateral patellofemoral ligaments, popliteofibular ligament and the posterior capsule.
The development and validation of the multibody knee model is described in detail elsewhere (Lenhart et al., 2015). Briefly, we first segmented the major ligaments and the femoral, tibial, and patellar cartilage surfaces from high resolution MR images collected on a young adult female (age 23 yrs, height 1.65 m, mass 61 kg). Eleven ligaments were represented by bundles of nonlinear springs spanning from origin to insertion, with wrapping objects to prevent ligaments from passing through underlying bone segments. Ligament force-strain relationship was assumed to be quadratic at low loads and linear at higher loads (Blankevoort et al., 1991), with linear stiffness estimated from the ligament cross-sectional areas as measured on MRI. Reference strains were adapted from the literature (Shelburne et al., 2006; Shin et al., 2007).
Cartilage pressure was computed using an elastic foundation formulation, in which pressure is assumed to be a nonlinear function of the depth of penetration between overlapping surfaces (Bei and Fregly, 2004). Surfaces were represented by triangulated meshes with 4000, 2000, and 2000 triangles for the femoral, tibial, and patellar cartilages, respectively. Cartilage thickness was assumed to be uniform for both joints, with a combined thickness of 6 mm for the tibiofemoral joint and 7 mm for the patellofemoral joint (Cohen et al., 1999; Eckstein et al., 2001; Li et al., 2005; Sittek et al., 1996). Cartilage elastic modulus was set to 5 MPa and Poisson’s ratio set to 0.45 (Blankevoort et al., 1991; Caruntu and Hefzy, 2004). The lower extremity model was implemented in SIMM (Delp and Loan, 2000), with the Dynamics Pipeline (Musculographics Inc., Santa Rosa, CA) and SD/Fast (Parametric Technology Corp., Needham, MA) used to generate code describing the multibody equations of motion. Simulation of Knee Mechanics during Running
The lower extremity model was scaled to subject-specific segment lengths as determined in an upright calibration trial. At each time step in a running trial, the generalized coordinates of the model were then computed using an inverse kinematics algorithm that minimized the weighted sum of squared differences between the experimental and simulated marker positions. In the inverse kinematics stage, the generalized coordinates representing secondary tibiofemoral kinematics and all patellofemoral kinematics were defined as constrained functions of knee flexion. These kinematic constraint functions were derived by simulating passive knee flexion of the knee model with relaxed muscles and no external loading. Experimentally determined generalized coordinates (qexp ) were low-pass filtered at 12 Hz and numerically differentiated to obtain the generalized speeds (q̇exp) and accelerations (q̈exp). Numerical optimization was then used to compute the muscle forces and secondary knee kinematics that generated the primary lower extremity joint angular accelerations while minimizing a cost-function J:
| (1) |
defined as the sum of weighted (by muscle volume V) squared muscle activations (a), with a weighted (W) regularization term added on to minimize frame-to-frame adjustments in secondary knee kinematics (δqs). In the above equation, m (=44) is the number of muscles and n (=11) is the number of secondary knee kinematics, which includes tibiofemoral translations, adduction, and internal rotation, and all patellofemoral translations and rotations. The variations in secondary kinematics were added to the secondary knee kinematics as determined in the solution obtained at the prior time step, i.e. . Note that these secondary knee adjustments alter both the ligament forces and cartilage contact pressures acting across the tibiofemoral and patellofemoral joints. The primary generalized coordinates were assumed to simply be those measured experimentally, i.e. . We assumed that individual muscle forces were the product of activation level (a, which can range from 0 to 1) and the isometric force capacity of the muscle (F0). The optimization constraints were that the muscle forces and adjusted secondary coordinates must induce the experimentally estimated hip flexion, hip adduction, hip rotation, knee flexion, and ankle joint angular accelerations ( ) of the primary degrees of freedom:
| (2) |
while generating zero accelerations in the secondary tibiofemoral and patellofemoral DOF.
| (3) |
In the above equations, and are the accelerations of the primary and secondary DOF generated by a unit force applied by muscle i, q̇ represents the measured generalized speeds and q̈other represents the accelerations due to non-muscular effects including centripetal and Coriolis effects, gravity, ligament forces, contact forces, and external ground reaction forces. The accelerations of the pelvis degrees of freedom were prescribed in the solution, such that the upper body dynamic effects were accounted for. This optimization problem was solved iteratively using a sequential quadratic programming algorithm (Lawrence et al., 1997). The result was a prediction of the muscle activations, secondary knee kinematics, and patellofemoral contact pressures over a running cycle (Fig. 2).
Figure 2.
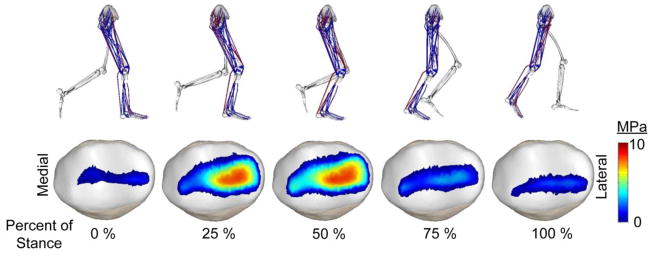
Lower extremity posture, activated muscles (red), and patellar contact pressures of a representative subject over the stance phase of running.
Statistical Analysis
For each running trial, we determined the frame during stance that resulted in the largest net patellofemoral contact force. At this frame, we then extracted the area of the patella cartilage surface in contact, the peak patella contact pressure, the average patella pressure over the contact area, and the mediolateral location of the center of contact pressure relative to the median ridge of the patella. Repeated measures ANOVA, with step rate condition being a repeated factor, were then used to compare these metrics between the different step rate conditions. Post-hoc analyses were performed using Tukey’s Honest Significance Tests. Significance level was set to p=0.05. All statistical analyses were performed using STATISTICA 12 (Statsoft, Inc, Tulsa, OK).
Sensitivity Study of the Influence of Vastii Strength Distribution
To test the influence of altered strength ratios between the vastus lateralis (VL) and vastus medialis (VM), we varied the VL/VM maximum isometric strength ratio in the musculoskeletal model from 0.75 to 2.25 (Farahmand et al., 1998), while holding the sum of the VL and VM strengths constant. For each VL/VM strength ratio, we re-solved the optimization problem for the muscle forces and secondary knee kinematics that would generate the experimental hip, knee, and ankle accelerations. These sensitivity analyses were performed on the preferred step rate running trials of all subjects. We then determined the average changes in quadriceps load distribution, patellofemoral joint load, patellofemoral kinematics, and cartilage pressure that arise with changes in VL/VM strength ratio.
Results
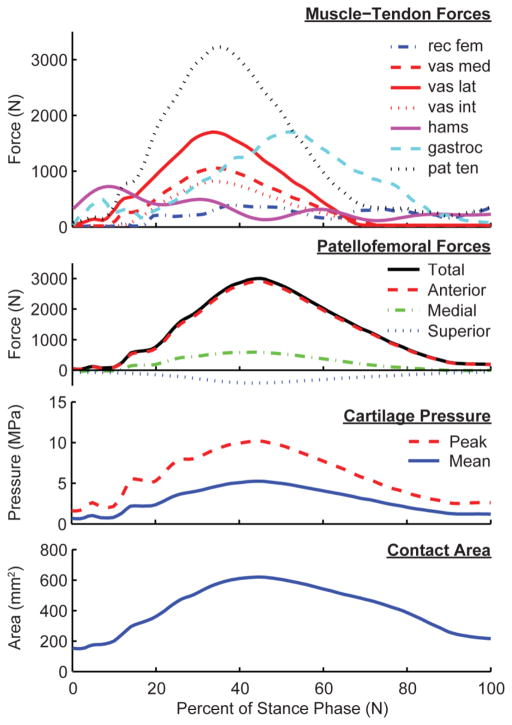
Patellofemoral contact forces, contact area, and contact pressure magnitudes exhibited similar temporal variations over stance, all peaking in mid-stance (Fig. 3). Patella contact forces generally included a medially directed component, which resulted in the patella pressure being more concentrated on the lateral facet. Patella contact areas increased approximately three-fold from heel contact to mid-stance, reaching average contact areas of 637±75 mm2 at the preferred step rate. Mean and peak patellar contact pressures at the preferred step rate were 5.1±0.7 MPa and 9.8±1.4 MPa, respectively.
Figure 3.
Model predictions of muscle and patella loads over stance phase of a sample preferred rate running stride. Patellofemoral force, pressure magnitudes and contact area all reach a maximum magnitude at approximately 45% of stance, slightly after peak quadriceps and patellar tendon loading.
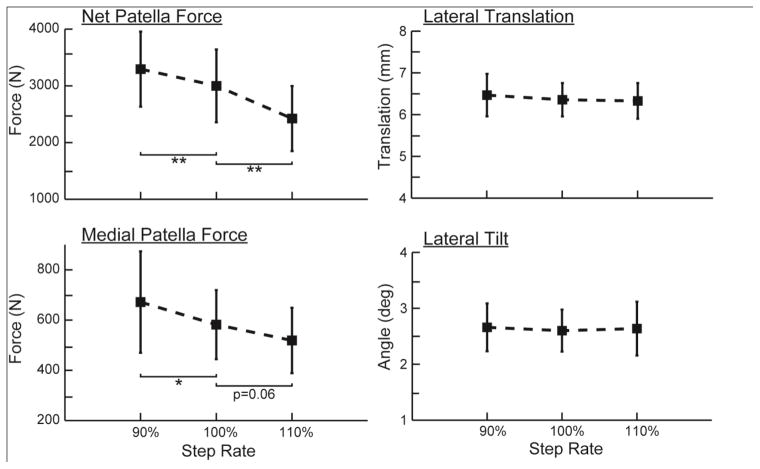
Step rate significantly affected peak contact force magnitudes (Fig. 4). Relative to preferred, the increased step rate induced a 16.6% lower net patellofemoral force, while the decreased step rate resulted in a 15.4% higher patellofemoral force. There were also significant effects of step rate on the medial component of the patellofemoral force, with −10.6% and +14.0% changes induced by +10% and −10% step rate changes, respectively. There were no significant changes in either lateral patellar translation or lateral patellar tilt with step rate (Fig. 4).
Figure 4.
Increasing step rate induced significant reductions in both the magnitude and medial component of the patellofemoral force. However, there were no corresponding effects of step rate on the lateral patella position or lateral patella tilt.
Step-rate induced significant changes in both the pressure magnitude and area of contact (Fig. 5). The increased step rate induced an average 7.4% lower contact area and 10.4% lower mean pressure (Fig. 6). In contrast, decreasing step rate induced a 5.2% higher contact area and 9.6% higher mean pressure. There was a significant (p<0.05), but relatively small lateral shift (0.4 mm) in the patellar center of pressure with a 10% increase in step rate.
Figure 5.
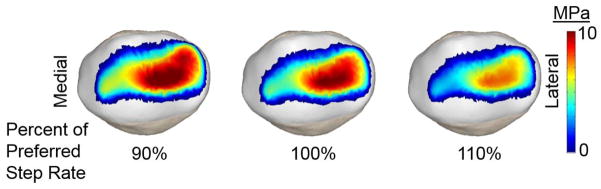
Sample images of the patella facet contact pressures at the time of peak patellofemoral loading when running at 90%, 100% and 110% of the preferred step rate. A reduction in both contact pressure magnitudes and area are observed with an increase in step rate.
Figure 6.
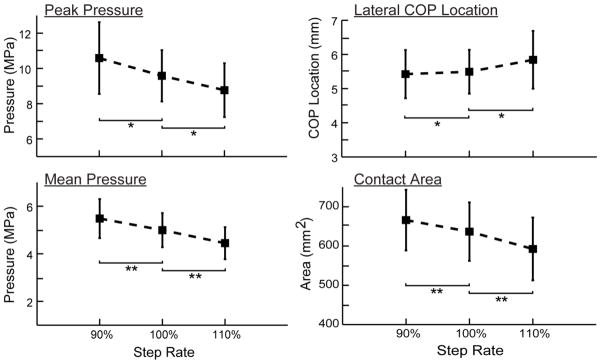
Increasing step rate induced significant reductions in peak contact pressure, mean pressure and contact area. There was also a significant, but small, lateral shift in the center of pressure when increasing step rate 10% over the preferred condition.
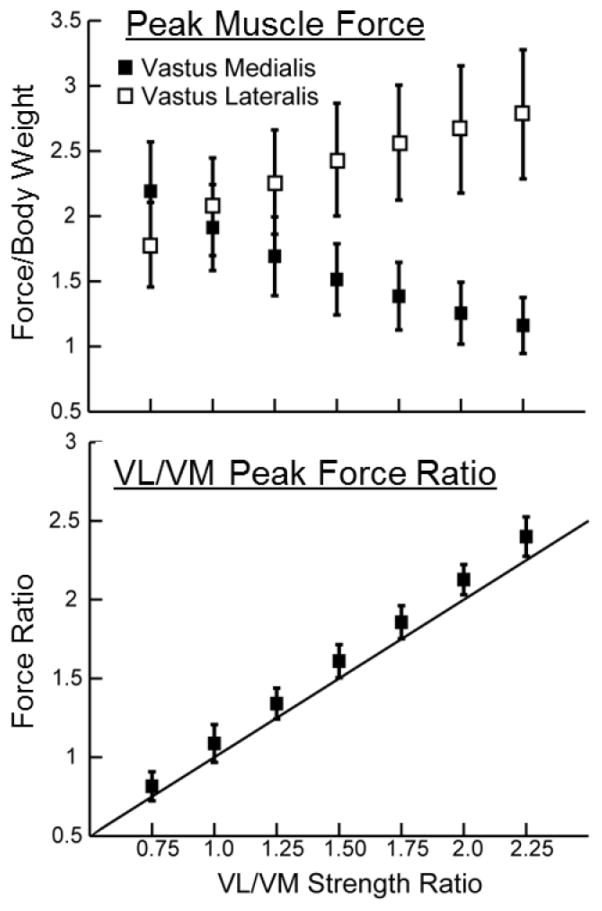
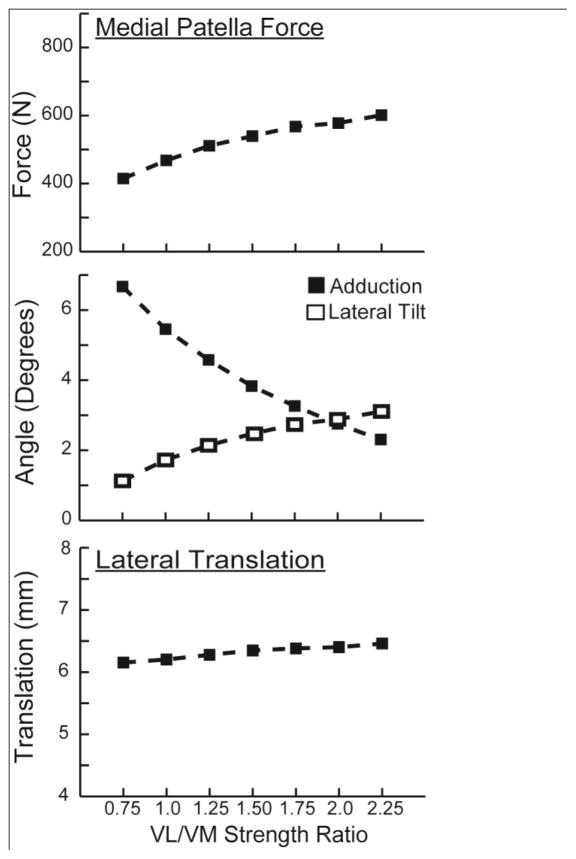
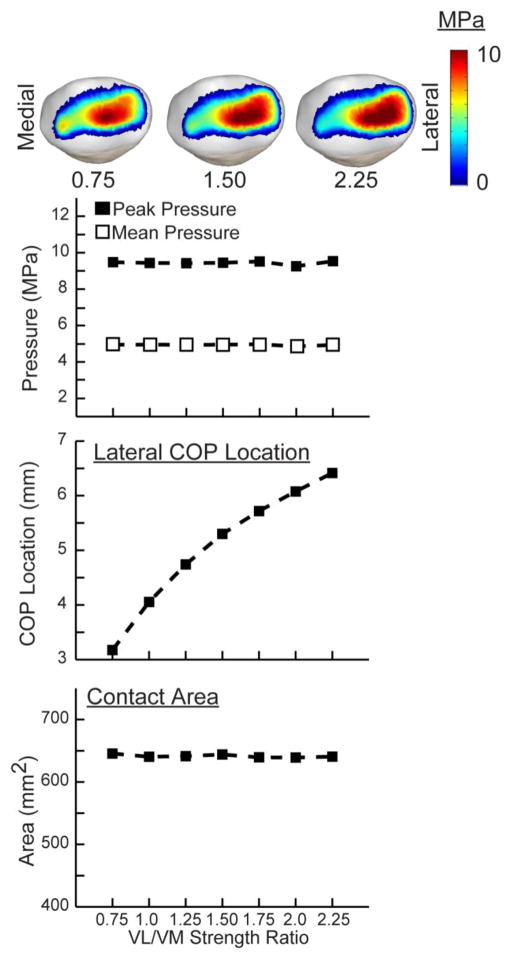
Modifying the VL/VM strength ratio resulted in proportional changes in the VL/VM force ratio at the time of peak loading (Fig. 7). Thus, the VL/VM strength ratio effects are a result of the variation in contributions of the VL and VM to the net quadriceps load. Increasing the VL/VM strength ratio from 0.75 to 2.25 produced a greater medial patella load (average increase of 185 N), while also inducing increased patella abduction (+4.4 deg) and patella lateral tilt (+2.0 deg) (Fig. 8). The effect of VL/VM strength on lateral patella translation was small, averaging less than 0.5 mm. However, increasing VL/VM strength did induce a significant shift in the mediolateral center of pressure, which migrated 3.5 mm over the strength ratios considered (Fig. 9). The patella contact area, and the peak and mean patella contact pressure magnitudes were all relatively insensitive to the vastii load distribution.
Figure 7.
Modulating the strength ratio of vastus lateral (VL) to vastus medialis (VM) altered the relative loading incurred by the two muscles in mid-stance of running. The corresponding VL/VM activation ratios are proportional to the strength ratios assumed in the model, with a slope slightly greater than unity (solid line).
Figure 8.
Increasing the VL/VM strength ratio induced a greater medial contact force acting on the patella. At the kinematic level, there were corresponding increases in patella abduction and lateral patella tilt, but relatively little effect on lateral patella translation.
Figure 9.
The primary effect of the VL/VM strength ratio was on the mediolateral location of the contact pressure on the patellar facets. A decrease in the strength ratio medialized the location of contact pressure, but had relatively little effect on the pressure magnitudes or area of contact.
Discussion
The primary purpose of this study was to investigate whether step rate modification or vastus medialis strengthening could alter patellofemoral cartilage contact pressures in running. Our results suggest that increasing step rate can effectively decrease cartilage contact area, and the peak and average cartilage contact pressures that arise in mid-stance phase. Enhancing vastus medialis strength did not affect pressure magnitudes, but did shift the location of contact pressure medially on the patella. These results are relevant to consider in the design of interventions used to prevent or treat patellofemoral pain in runners.
We estimate mean patella contact pressures of ~5 MPa in normal running, with peak pressures of ~10 MPa. These patella contact pressure magnitudes are considerably lower than prior mean pressure estimates for running, which range from 8.5 to 21.5 MPa (Bonacci et al., 2014; Kulmala et al., 2013; Teng and Powers, 2014; Vannatta and Kernozek, 2014; Willson et al., 2014; Wirtz et al., 2012). This discrepancy seems to primarily arise from our contact area predictions (>600 mm2 at time of peak loading) being considerably larger than what has previously been measured in static low load scenarios (Connolly et al., 2009; Powers et al., 1998) and then extrapolated to running. Besier et al. showed that patellar contact areas increase when supporting partial body in standing, reaching 500–600 mm2 at knee flexion angles seen in stance during running (Besier et al., 2005). Thus, the much larger vertical loads seen in running (~2.5 body weight) may well induce increased cartilage tissue deformations, and hence the larger contact areas, estimated in this study.
Clinical interventions used to treat patellofemoral pain in runners often attempt to alter patellofemoral biomechanics. For example, two common goals are to reduce patellofemoral joint loads and/or normalize patellofemoral kinematics. We have previously shown that a 10% increase in running step rate (i.e. shortening step length) can significantly reduce knee energy absorption (Heiderscheit et al., 2011) and patellofemoral joint force (Lenhart et al., 2014), with the latter effect being primarily due to running with a more extended limb. Willson et al. (2014) previously estimated that patellofemoral joint stress can be reduced 21% by running with shorter step lengths. In this study, we showed that the lower patellofemoral joint force translates into diminished contact pressure as would be expected, though the mean pressure reduction (−10.4%) is considerably less than the reduction in patellofemoral force. The discrepancy between the magnitude of force and pressure changes arises from a simultaneous decrease in cartilage contact area (−7.4%), which is primarily due to lower loads inducing less cartilage deformation. We also estimate a lateral shift of the center of pressure with increased step rate, though this effect is small (<0.5 mm) and may have limited clinical relevance.
Vastus medialis strengthening and/or neuromuscular stimulation have classically been used to enhance VM output, which could conceivably shift patellofemoral motion medially. Indeed, vastus medialis weakness and atrophy (Botanlioglu et al., 2013; Pattyn et al., 2011) and patellar maltracking (Pal et al., 2013; Pal et al., 2011) have been observed in patients exhibiting patellofemoral pain. Additionally, in vitro studies suggest that VM weakness can laterally shift the patella (Lorenz et al., 2012b; Sakai et al., 2000). A prior biomechanical running simulation found that weakness or an onset delay in the VM could increase the medial force acting on the patella, though the effects were relatively modest (Neptune et al., 2000). However, the latter study used a simpler 1 degree of freedom knee joint, which did not explicitly model the ligamentous and cartilage contact constraints. Our study using a more detailed knee model also found a reduction in the medial patella force with VM strengthening, and also predicted a notable medial shift in the patella center of pressure. It is interesting to note that altered quadriceps load distributions induced relatively minor changes in lateral patella translation (<0.5 mm) and tilt (~2 deg), a result that has also been observed in vitro (Lorenz et al., 2012a). Thus, it would seem challenging to assess patella kinematics with sufficient resolution to infer underlying contact pressure patterns.
The range of VL/VM strength ratios we considered reflect the range of VL/VM physiological cross-sectional area ratios (0.90–2.18) reported in a cadaver study by Farahmand et al. (1998). Average physiological cross-sectional area estimates from two other studies would put the average VL/VM ratio at 1.0 and 1.6 (Handsfield et al., 2014; Ward et al., 2009). The latter study by Ward et al. (2009) was the basis of the nominal musculoskeletal model of Arnold et al. (2010), which we used in this study. Thus, it remains unclear how much variability exists in the VL/VM strength ratio and how modifiable this might be. However, it is important to note that our predicted VL/VM force ratios were proportional to the strength ratio assumed in the model (Fig. 7). An alternative approach would be to adapt the neuromuscular control, which alters relative activation of VL and VM, such that the VL/VM force ratio would also be altered. To test, this, we varied the relative weighting of VL and VM in the muscle force distribution cost function (Eq. 1), and found that it altered the VL/VM force patterns (Fig. 7) and patella contact pressures (Fig. 9) in a manner similar to those obtained by altering the VL/VM strength ratio. Hence, changes in either the strength or activation of VL relative to VM can influence the patellofemoral pressure patterns (Fig. 9).
There are some limitations that should be noted in interpreting the results of this study. While the use of a 12 DOF knee model is a major advancement in dynamic analysis of running, there are limitations in the model creation and use. First, we have validated the knee model via comparison with in vivo knee kinematics measured via dynamic MRI (Lenhart et al., 2015). However due to the constraints of MR imaging, in vivo validation tasks were done in supine postures at loads considerably lower than those seen in running. Second, we used a single generic knee model that was scaled to represent the anthropometry of the subjects in this study. While we confirmed that the trochlear groove and the patella geometry of the knee model fall within one standard deviation of the average geometry measurements reported in the literature (Dargel et al., 2009; Laprade and Culham, 2003; Yoo et al., 2007), it would be desirable to consider subject-specific articular geometries. Third, we used numerical optimization to estimate internal loads and secondary knee kinematics at a point in time, rather than dynamic simulation to predict the multibody behavior over time. This simplification required us to assume the inertial effects of secondary knee accelerations are negligible in stance. To test this assumption, we re-solved the optimization problem with secondary knee accelerations obtained from the inverse kinematics solution and found no substantial change in results. Fourth, we used a single performance criterion to resolve muscle force distribution. While absolute results would vary with a different performance criterion, our primary interest was in step rate and strengthening effects without any concomitant change in other factors. Fifth, all of our analyses are based on motion analysis of runners who are injury free. Further work is needed to see if the results extend to runners who exhibit PFP. Finally, we recognize that patellar maltracking and PFP may arise from sources other than quadriceps load imbalance (Witvrouw et al., 2014), and therefore may benefit from treatment strategies different than those investigated in this paper
In conclusion, both step rate modification and vastus medialis strengthening induce changes in patellofemoral contact pressures in running. Step rate tends to primarily modulate the magnitude of contact pressure and contact area, while vastus medialis strengthening has the potential to alter mediolateral pressure locations. These observations are relevant to consider in the context of clinical interventions that are used to mitigate PFP in symptomatic runners.
Acknowledgments
The authors gratefully acknowledge the support of NIH grants EB015410 and F30AR065838, NSF grant 0966535, the UW Institute for Clinical and Translational Research (UL1TR000427), and the UW Medical Scientist Training Program (T32GM008692). We also thank Christa Wille and Elizabeth Chumanov, DPT, PhD, for their assistance in experimental data collections.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rachel L. Lenhart, Department of Biomedical Engineering, University of Wisconsin-Madison
Colin R. Smith, Department of Mechanical Engineering, University of Wisconsin-Madison
Michael F. Vignos, Department of Mechanical Engineering, University of Wisconsin-Madison
Jarred Kaiser, Department of Mechanical Engineering, University of Wisconsin-Madison.
Bryan C. Heiderscheit, Department of Biomedical Engineering, University of Wisconsin-Madison. Department of Orthopedics and Rehabilitation, University of Wisconsin-Madison. Badger Athletic Performance, University of Wisconsin-Madison, Madison, WI
Darryl G. Thelen, Department of Biomedical Engineering, University of Wisconsin-Madison. Department of Mechanical Engineering, University of Wisconsin-Madison. Department of Orthopedics and Rehabilitation, University of Wisconsin-Madison
References
- Arnold EM, Ward SR, Lieber RL, Delp SL. A model of the lower limb for analysis of human movement. Annals of Biomedical Engineering. 2010;38:269–279. doi: 10.1007/s10439-009-9852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei Y, Fregly BJ. Multibody dynamic simulation of knee contact mechanics. Medical Engineering & Physics. 2004;26:777–789. doi: 10.1016/j.medengphy.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besier TF, Draper CE, Gold GE, Beaupré GS, Delp SL. Patellofemoral joint contact area increases with knee flexion and weight-bearing. Journal of Orthopaedic Research. 2005;23:345–350. doi: 10.1016/j.orthres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Blankevoort L, Kuiper J, Huiskes R, Grootenboer H. Articular contact in a three-dimensional model of the knee. Journal of Biomechanics. 1991;24:1019–1031. doi: 10.1016/0021-9290(91)90019-j. [DOI] [PubMed] [Google Scholar]
- Bonacci J, Vicenzino B, Spratford W, Collins P. Take your shoes off to reduce patellofemoral joint stress during running. British Journal of Sports Medicine. 2014;48:425–428. doi: 10.1136/bjsports-2013-092160. [DOI] [PubMed] [Google Scholar]
- Botanlioglu H, Kantarci F, Kaynak G, Unal Y, Ertan S, Aydingoz O, Erginer R, Unlu MC, Mihmanli I, Babacan M. Shear wave elastography properties of vastus lateralis and vastus medialis obliquus muscles in normal subjects and female patients with patellofemoral pain syndrome. Skeletal Radiology. 2013;42:659–666. doi: 10.1007/s00256-012-1520-4. [DOI] [PubMed] [Google Scholar]
- Brechter JH, Powers CM. Patellofemoral joint stress during stair ascent and descent in persons with and without patellofemoral pain. Gait & Posture. 2002;16:115–123. doi: 10.1016/s0966-6362(02)00090-5. [DOI] [PubMed] [Google Scholar]
- Brody LT, Thein JM. Nonoperative treatment for patellofemoral pain. Journal of Orthopaedic & Sports Physical Therapy. 1998;28:336–344. doi: 10.2519/jospt.1998.28.5.336. [DOI] [PubMed] [Google Scholar]
- Caruntu DI, Hefzy MS. 3-D anatomically based dynamic modeling of the human knee to include tibio-femoral and patello-femoral joints. Journal of Biomechanical Engineering. 2004;126:44–53. doi: 10.1115/1.1644565. [DOI] [PubMed] [Google Scholar]
- Cavazzuti L, Merlo A, Orlandi F, Campanini I. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Gait & posture. 2010;32:290–295. doi: 10.1016/j.gaitpost.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Chester R, Smith TO, Sweeting D, Dixon J, Wood S, Song F. The relative timing of VMO and VL in the aetiology of anterior knee pain: a systematic review and meta-analysis. BMC Musculoskeletal Disorders. 2008;9:64. doi: 10.1186/1471-2474-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung R, Davis IS. Landing pattern modification to improve patellofemoral pain in runners: a case series. Journal of Orthopaedic and Sports Physical Therapy. 2011;41:914–919. doi: 10.2519/jospt.2011.3771. [DOI] [PubMed] [Google Scholar]
- Choi B, Kim M, Jeon HS. The effects of an isometric knee extension with hip adduction (KEWHA) exercise on selective VMO muscle strengthening. J Electromyogr Kinesiol. 2011;21:1011–1016. doi: 10.1016/j.jelekin.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Chumanov ES, Wille CM, Michalski MP, Heiderscheit BC. Changes in muscle activation patterns when running step rate is increased. Gait & Posture. 2012;36:231–235. doi: 10.1016/j.gaitpost.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clijsen R, Fuchs J, Taeymans J. Effectiveness of exercise therapy in treatment of patients with patellofemoral pain syndrome: systematic review and meta-analysis. Phys Ther. 2014;94:1697–1708. doi: 10.2522/ptj.20130310. [DOI] [PubMed] [Google Scholar]
- Cohen ZA, McCarthy DM, Kwak SD, Legrand P, Fogarasi F, Ciaccio EJ, Ateshian GA. Knee cartilage topography, thickness, and contact areas from MRI: in-vitro calibration and in-vivo measurements. Osteoarthritis and Cartilage. 1999;7:95–109. doi: 10.1053/joca.1998.0165. [DOI] [PubMed] [Google Scholar]
- Connolly K, Ronsky J, Westover L, Küpper J, Frayne R. Differences in patellofemoral contact mechanics associated with patellofemoral pain syndrome. Journal of Biomechanics. 2009;42:2802–2807. doi: 10.1016/j.jbiomech.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Dargel J, Feiser J, Gotter M, Pennig D, Koebke J. Side differences in the anatomy of human knee joints. Knee Surg Sports Traumatol Arthrosc. 2009;17:1368–1376. doi: 10.1007/s00167-009-0870-5. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP. A computational framework for simulation and analysis of human and animal movement. IEEE Computing in Science and Engineering. 2000;2:46–55. [Google Scholar]
- Draper CE, Fredericson M, Gold GE, Besier TF, Delp SL, Beaupre GS, Quon A. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. Journal of Orthopaedic Research. 2012;30:209–213. doi: 10.1002/jor.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F, Winzheimer M, Hohe J, Englmeier K-H, Reiser M. Interindividual variability and correlation among morphological parameters of knee joint cartilage plates: analysis with three-dimensional MR imaging. Osteoarthritis and Cartilage. 2001;9:101–111. doi: 10.1053/joca.2000.0365. [DOI] [PubMed] [Google Scholar]
- Farahmand F, Sejiavongse W, Amis AA. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. Journal of Orthopaedic Research. 1998;16:136–143. doi: 10.1002/jor.1100160123. [DOI] [PubMed] [Google Scholar]
- Farrokhi S, Keyak JH, Powers CM. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthritis and Cartilage. 2011;19:287–294. doi: 10.1016/j.joca.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J, Lee P, Bose K. A cadaver study of the function of the oblique part of vastus medialis. Journal of Bone & Joint Surgery, British Volume. 1995;77:225–231. [PubMed] [Google Scholar]
- Handsfield GG, Meyer CH, Hart JM, Abel MF, Blemker SS. Relationships of 35 lower limb muscles to height and body mass quantified using MRI. Journal of Biomechanics. 2014;47:631–638. doi: 10.1016/j.jbiomech.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Heiderscheit BC, Chumanov ES, Michalski MP, Wille CM, Ryan MB. Effects of step rate manipulation on joint mechanics during running. Medicine and Science in Sports and Exercise. 2011;43:296–302. doi: 10.1249/MSS.0b013e3181ebedf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K-Y, Keyak JH, Powers CM. Comparison of patella bone strain between females with and without patellofemoral pain: A finite element analysis study. Journal of Biomechanics. 2014;47:230–236. doi: 10.1016/j.jbiomech.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Juhn M. Patellofemoral pain syndrome: a review and guidelines for treatment. American Family Physician. 1999;60:2012–2018. [PubMed] [Google Scholar]
- Kulmala J-P, Avela J, Pasanen K, Parkkari J. Forefoot strikers exhibit lower running-induced knee loading than rearfoot strikers. Medicine and Science in Sports and Exercise. 2013;45:2306–2313. doi: 10.1249/MSS.0b013e31829efcf7. [DOI] [PubMed] [Google Scholar]
- Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clinical orthopaedics and related research. 2003;414:172–182. doi: 10.1097/01.blo.0000079269.91782.f5. [DOI] [PubMed] [Google Scholar]
- Lawrence CT, Zhou JL, Tits AL. User’s Guide for CFSQP Version 2.5: A C Code for Solving (Large Scale) Constrained Nonlinear (minimax) Optimization Problems, Generating Iterates Satisfying All Inequality Constraints. University of Maryland; College Park: 1997. [Google Scholar]
- Lenhart RL, Kaiser J, Smith CR, Thelen DG. Prediction and validation of load-dependent behavior of the tibiofemoral and patellofemoral joints during movement. Ann Biomed Eng. 2015 doi: 10.1007/s10439-015-1326-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart RL, Thelen DG, Wille CM, Chumanov ES, Heiderscheit BC. Increasing Running Step Rate Reduces Patellofemoral Joint Forces. Medicine and Science in Sports and Exercise. 2014;46:557–564. doi: 10.1249/MSS.0b013e3182a78c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Park SE, DeFrate LE, Schutzer ME, Ji L, Gill TJ, Rubash HE. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clinical Biomechanics. 2005;20:736–744. doi: 10.1016/j.clinbiomech.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Lin F, Wilson NA, Makhsous M, Press JM, Koh JL, Nuber GW, Zhang L-Q. In vivo patellar tracking induced by individual quadriceps components in individuals with patellofemoral pain. Journal of Biomechanics. 2010;43:235–241. doi: 10.1016/j.jbiomech.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz A, Muller O, Kohler P, Wunschel M, Wulker N, Leichtle UG. The influence of asymmetric quadriceps loading on patellar tracking--an in vitro study. The Knee. 2012a;19:818–822. doi: 10.1016/j.knee.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Lorenz A, Müller O, Kohler P, Wünschel M, Wülker N, Leichtle UG. The influence of asymmetric quadriceps loading on patellar tracking—An in vitro study. The Knee. 2012b;19:818–822. doi: 10.1016/j.knee.2012.04.011. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Robertson DG. Quadriceps muscle activity in women runners with and without patellofemoral pain syndrome. Arch Phys Med Rehabil. 1992;73:10–14. [PubMed] [Google Scholar]
- Näslund J, Odenbring S, Näslund U, Lundeberg T. Diffusely increased bone scintigraphic uptake in patellofemoral pain syndrome. British Journal of Sports Medicine. 2005;39:162–165. doi: 10.1136/bjsm.2004.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune R, Wright I, van den Bogert AJ. The influence of orthotic devices and vastus medialis strength and timing on patellofemoral loads during running. Clinical Biomechanics. 2000;15:611–618. doi: 10.1016/s0268-0033(00)00028-0. [DOI] [PubMed] [Google Scholar]
- Noehren B, Scholz J, Davis I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. British Journal of Sports Medicine. 2011;45:691–696. doi: 10.1136/bjsm.2009.069112. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Motor control of the vastus medialis oblique and vastus lateralis muscles is disrupted during eccentric contractions in subjects with patellofemoral pain. The American Journal of Sports Medicine. 2002;30:483–487. doi: 10.1177/03635465020300040601. [DOI] [PubMed] [Google Scholar]
- Pal S, Besier TF, Beaupre GS, Fredericson M, Delp SL, Gold GE. Patellar maltracking is prevalent among patellofemoral pain subjects with patella alta: An upright, weightbearing MRI study. Journal of Orthopaedic Research. 2013;31:448–457. doi: 10.1002/jor.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Besier TF, Draper CE, Fredericson M, Gold GE, Beaupre GS, Delp SL. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. Journal of Orthopaedic Research. 2012;30:927–933. doi: 10.1002/jor.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Draper CE, Fredericson M, Gold GE, Delp SL, Beaupre GS, Besier TF. Patellar maltracking correlates with vastus medialis activation delay in patellofemoral pain patients. American Journal of Sports Medicine. 2011;39:590–598. doi: 10.1177/0363546510384233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn E, Verdonk P, Steyaert A, Bossche LV, Van den Broecke W, Thijs Y, Witvrouw E. Vastus medialis obliquus atrophy does it exist in Patellofemoral Pain Syndrome? The American Journal of Sports Medicine. 2011;39:1450–1455. doi: 10.1177/0363546511401183. [DOI] [PubMed] [Google Scholar]
- Powers CM, Lilley JC, Lee TQ. The effects of axial and multi-plane loading of the extensor mechanism on the patellofemoral joint. Clinical Biomechanics. 1998;13:616–624. doi: 10.1016/s0268-0033(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Sakai N, Luo Z-P, Rand JA, An K-N. The influence of weakness in the vastus medialis oblique muscle on the patellofemoral joint: an in vitro biomechanical study. Clinical Biomechanics. 2000;15:335–339. doi: 10.1016/s0268-0033(99)00089-3. [DOI] [PubMed] [Google Scholar]
- Sawatsky A, Bourne D, Horisberger M, Jinha A, Herzog W. Changes in patellofemoral joint contact pressures caused by vastus medialis muscle weakness. Clinical Biomechanics. 2012;27:595–601. doi: 10.1016/j.clinbiomech.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Segal N, Kern A, Anderson D, Niu J, Lynch J, Guermazi A, Torner J, Brown T, Nevitt M Multicenter Knee Osteoarthritis Study Group. Elevated tibiofemoral articular contact stress predicts risk for bone marrow lesions and cartilage damage at 30 months. Osteoarthritis and Cartilage. 2012;20:1120. doi: 10.1016/j.joca.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal NA, Anderson DD, Iyer KS, Baker J, Torner JC, Lynch JA, Felson DT, Lewis CE, Brown TD. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. Journal of Orthopaedic Research. 2009;27:1562–1568. doi: 10.1002/jor.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne KB, Torry MR, Pandy MG. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. Journal of Orthopaedic Research. 2006;24:1983–1990. doi: 10.1002/jor.20255. [DOI] [PubMed] [Google Scholar]
- Shin CS, Chaudhari AM, Andriacchi TP. The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. Journal of Biomechanics. 2007;40:1145–1152. doi: 10.1016/j.jbiomech.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Sittek H, Eckstein F, Gavazzeni A, Milz S, Kiefer B, Schulte E, Reiser M. Assessment of normal patellar cartilage volume and thickness using MRI: an analysis of currently available pulse sequences. Skeletal Radiology. 1996;25:55–62. doi: 10.1007/s002560050032. [DOI] [PubMed] [Google Scholar]
- Taunton J, Ryan M, Clement D, McKenzie D, Lloyd-Smith D, Zumbo B. A retrospective case-control analysis of 2002 running injuries. British Journal of Sports Medicine. 2002;36:95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H-L, Powers CM. Sagittal plane trunk posture influences patellofemoral joint stress during running. Journal of Orthopaedic and Sports Physical Therapy. 2014;44:785–792. doi: 10.2519/jospt.2014.5249. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Choi KW, Schmitz AM. Co-Simulation of Neuromuscular Dynamics and Knee Mechanics During Human Walking. Journal of Biomechanical Engineering. 2014;136:021033. doi: 10.1115/1.4026358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden RA, Lankhorst NE, van Linschoten R, Bierma-Zeinstra SM, van Middelkoop M. Exercise for treating patellofemoral pain syndrome. The Cochrane database of systematic reviews. 2015; 1:CD010387. doi: 10.1002/14651858.CD010387.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannatta CN, Kernozek TW. Patellofemoral Joint Stress during Running with Alterations in Foot Strike Pattern. Medicine and Science in Sports and Exercise. 2014 doi: 10.1249/MSS.0000000000000503. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clinical Orthopaedics and Related Research. 2009;467:1074–1082. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SR, Powers CM. The influence of patella alta on patellofemoral joint stress during normal and fast walking. Clinical Biomechanics. 2004;19:1040–1047. doi: 10.1016/j.clinbiomech.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Wille CM, Chumanov ES, Schubert A, Kempf J, Heiderscheit BC. Running step rate modification to reduce anterior knee pain in runners. Journal of Orthopaedic and Sports Physical Therapy. 2013;43:A119. [Google Scholar]
- Willson JD, Sharpee R, Meardon SA, Kernozek TW. Effects of step length on patellofemoral joint stress in female runners with and without patellofemoral pain. Clinical Biomechanics. 2014;29:243–247. doi: 10.1016/j.clinbiomech.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Willy RW, Davis IS. Varied Response to Mirror Gait Retraining of Gluteus Medius Control, Hip Kinematics, Pain, and Function in 2 Female Runners With Patellofemoral Pain. Journal of Orthopaedic and Sports Physical Therapy. 2013;43:864–874. doi: 10.2519/jospt.2013.4516. [DOI] [PubMed] [Google Scholar]
- Wirtz AD, Willson JD, Kernozek TW, Hong D-A. Patellofemoral joint stress during running in females with and without patellofemoral pain. The Knee. 2012;19:703–708. doi: 10.1016/j.knee.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett-Jones DM, Willson JD, Earl-Boehm JE, Davis IS, Powers CM, McConnell J, Crossley KM. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. Br J Sports Med. 2014;48:411–414. doi: 10.1136/bjsports-2014-093450. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Yi SR, Kim JH. The geometry of patella and patellar tendon measured on knee MRI. Surg Radiol Anat. 2007;29:623–628. doi: 10.1007/s00276-007-0261-x. [DOI] [PubMed] [Google Scholar]