Abstract
Associations between white matter pathway abnormalities and antisocial personality disorder in adults are well replicated, and there is some evidence for an association of white matter abnormalities with conduct disorder (CD) in adolescents. In this study, white matter maturation using diffusion tensor imaging (DTI) was examined in 110 children aged 10.0 ± 0.8 years selected to vary widely in their numbers of CD symptoms. The results replicated age-related increases in fractional anisotropy (FA) found in previous studies. There was not a significant association between the number of CD symptoms and FA, but CD symptoms were found to be significantly associated with greater axial and radial diffusivity in a broad range of white matter tracts, particularly in girls. In complementary analyses, there were similar significant differences in axial and radial diffusivity between children who met diagnostic criteria for CD and healthy children with no symptoms of CD, particularly in girls. Brain structural abnormalities may contribute to the emergence of CD in childhood, perhaps playing a greater role in girls.
Keywords: Conduct disorder, Diffusion tensor imaging, MRI, Fractional anisotropy, White matter
1. Introduction
Conduct disorder (CD) is a disorder that often emerges in childhood, characterized by aggressive and antisocial behavior, which creates considerable societal cost (Romeo et al., 2006). It has been argued that amygdala and orbitofrontal cortex dysfunction in adolescents with CD and psychopathic traits disrupts emotion-based decision-making, including moral decision making (Viding, 2004; Kiehl, 2006; Decety et al., 2009; Lockwood et al., 2013; Decety and Cowell, 2014).
DTI can measure the microstructural integrity of white matter, quantified indirectly by fractional anisotropy (FA). Furthermore, distinctions in two aspects of white matter integrity can made by measuring diffusion that is parallel (axial diffusivity) and perpendicular (radial diffusivity) to axonal tracts. Radial diffusivity has been used to assess myelination levels because it correlates with demyelination (Song et al., 2002, 2005; Klawiter et al., 2011). Conversely, axial diffusivity indexes axonal integrity (Budde et al., 2009). White matter abnormalities have consistently been found in adults with psychopathy or antisocial personality disorder (Sundram et al., 2012). In particular, antisocial adults, when compared with healthy controls, exhibit reduced FA in the uncinate fasciculus (UF), which may indicate abnormally low structural connectivity of the amygdala and ventromedial prefrontal cortex, in forensic inpatients (Craig et al., 2009; Sundram et al., 2012) and incarcerated psychopaths (Motzkin et al., 2011; Hoppenbrouwers et al., 2013).
Studies of the UF in adolescents have yielded less consistent results. For instance, one study found no FA differences in the UF between healthy children and children with CD and other disruptive behavior disorder diagnoses (Finger et al., 2012). A difference study of 17–20 year olds found greater rather than lower FA in the UF in males with childhood-onset CD relative to healthy male controls (Passamonti et al., 2012). More recently, a study of male adolescents with aggressive CD and healthy male controls also found greater FA and lower radial diffusivity in the left UF in youth with CD (Sarkar et al., 2013). However, we are aware of no studies that have examined the association between CD and white matter abnormalities in childhood.
This gap in the literature is important for two reasons. First, CD that is present in childhood appears to be more impairing, persistent, and comorbid with attention-deficit/hyperactivity disorder (ADHD) (Moffitt et al., 1996). Second, previous studies may have ignored an important period of development. Normal development is characterized by increased total cortical white matter into early adulthood (Paus et al., 2001), and includes nonlinear increases in FA and nonlinear decreases in mean diffusivity from young childhood into an individual’s twenties (Lebel and Beaulieu, 2011). It is entirely possible that antisocial personality disorder is associated with decreased FA in adulthood because of a disrupted developmental trajectory, characterized by white matter overdevelopment early in life. Thus, the current study sought to characterize the association of CD with white matter development during childhood (9–11 year olds).
Furthermore, because there are marked sex differences in the prevalence of CD that must be understood to fully understand the nature of CD (Rutter et al., 2003; Moffitt et al., 2008), and because there are sex differences in brain development (Lenroot et al., 2007), special attention was paid in the current work to the interaction between CD and gender. The “gender paradox” hypothesis states that, to overcome gender-specific protective factors, persons of a given sex with a mental disorder that is less prevalent in that sex must exhibit greater dysfunction (Eme, 1992) and more comorbidity with other mental disorders (Loeber and Keenan, 1994). Thus, we will test for interactions with gender in all analyses.
2. Methods
2.1. Participants
A diverse sample of 110 children (10.0 ± 0.8 years; 53 males, 57 females; 49 White, 61 African American) were recruited using extreme groups sampling (Preacher et al., 2005). Families were recruited from both outpatient child mental health clinics (using flyers calling for children with behavior problems) and pediatric well-visit waiting rooms (using a flyer calling for well-behaved children). Based on a telephone screening interview, children were recruited into two strata at high or low risk for meeting DSM-IV diagnostic criteria for CD until approximately equal numbers were recruited in each stratum. In addition, within each of these two strata, children were preferentially recruited in approximately equal numbers of white girls, white boys, African American girls, and African American boys. The white stratum included both Hispanic and non-Hispanic white children. Parents and children were sequentially administered the DISC Predictive Scale (DPS) for CD, which predicts the full diagnosis of CD with high sensitivity and specificity (Lucas et al., 2001). The DPS consists of 8 “stem questions” from the reliable and valid Diagnostic Interview Schedule for Children (DISC-IV) CD module (Shaffer et al., 2000). Eight DPS questions refer to symptoms of CD and one to school expulsion. Children were selected for the high-risk stratum if the parent alone endorsed 2 or more DPS items, the child alone endorsed 3 or more items, or the parent and child collectively endorsed 3 or more separate items. Children were selected for the low-risk stratum if neither informant endorsed any DPS CD items. To spread the distribution of CD symptoms, children with intermediate scores of 1 on the DPS were not included in the study to allow the recruitment of children with more CD symptoms.
To disentangle severity of CD symptoms from the child’s sex and race-ethnicity, selection continued until equal numbers of high- and low-risk children of each sex and race-ethnic category agreed to participate. Exclusion criteria included presence of a pervasive developmental disorder, history of head trauma with loss of consciousness exceeding 15 minutes, and safety contraindications for neuroimaging. On the day of scanning, the full DISC-IV (Shaffer et al., 2000) was administered in separate rooms to the primary caregiver and to the child by trained interviewers, including the module covering CD symptoms during the last 12 months. DTI data were obtained for 53 children with no symptoms of CD (27 males; 33 African American) and 57 children with at least one symptom of CD (26 males; 28 African American). All participants gave assent, and informed written consent was obtained from the child’s parent or legal guardian. The study was approved by the Internal Review Board at the University of Chicago.
Scanning parameters
Images were acquired on a 3T Philips Achieva Quasar scanner equipped with an 8-channel SENSE head coil at the Brain Research Imaging Center at the University of Chicago. A single-shot echo planar imaging pulse sequence was used (TR/TE = 12,572 ms/55 ms; 80 slices; voxel size = 2 mm3; matrix size = 112 × 112) with one zero-weighted image (b = 0 s/mm2) and 32 diffusion sensitizing orientations (b = 1000 s/mm2).
Diffusion-weighted images were processed and analyzed using the FMRIB Software Library v5.0 (FSL) (Jenkinson et al., 2012). First, images were skull-stripped (Smith, 2002) and head movement and eddy current corrections were performed. Next, fractional anisotropy (FA) images were calculated by fitting tensors to each voxel using FDT. Statistical analysis was then performed on the FA data using Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006), using default parameters. Individual subjects’ FA images were coregistered to standard space using a nonlinear b-spline warp. These aligned images were then averaged and thresholded at 0.2 to create a mean FA skeleton onto which individual FA data were projected. Axial diffusivity (the primary diffusion eigenvariate) and radial diffusivity (average of secondary and tertiary eigenvariates) were also calculated and projected onto the same skeleton.
The association between CD and white matter integrity was tested using two complementary strategies within the same analytic framework. In the first strategy, we measured CD by counts of the number of CD symptoms. This was done because dichotomous classifications of mental disorders may be less biologically valid (Craddock and Owen, 2007) and dimensional analyses are often better suited to hypothesis testing (Kraemer et al., 2004). In the second strategy, we used DSM-IV criteria for the diagnosis of CD to conduct group comparisons.
2.2. Analyses of counts of CD symptoms
Three general linear models were used to estimate the influence of age, gender, race, number of CD symptoms, the gender-by-CD interaction, and race-by-CD symptoms interaction on FA, radial diffusivity, and axial diffusivity of the skeleton-projected data In addition to these predictor variables of interest, each model also included maternal completion of high school as a covariate of no interest. This was included because it is robustly associated with the child’s tested intelligence (Edwards and Roff, 2010; Bornstein et al., 2013; Ghassabian et al., 2014). The FSL function RANDOMISE (Winkler et al., 2014) was used to perform non-parametric statistical analyses, in which 5000 permutations were conducted to estimate the actual null distribution for comparison to obtained test statistics for significant positive and negative effects of each predictor variable. Threshold-Free Cluster Enhancement (TFCE) was used, rather than voxel-based thresholding, and corrected for multiple comparisons with family-wise error. All reported statistics are FWE-corrected p < 0.05. For viewing purposes, statistical images were “thickened” and are shown in radiological convention.
2.3. Analyses of the diagnosis of CD
To complement the analyses of symptom counts we also compared three nominal groups in planned comparisons: met DSM-IV criteria for CD (n=39), subthreshold CD (one or two symptoms of CD; n=18), and healthy control (HC) children with no symptoms of CD (n=53) groups. The same covariates and methods were used in these comparisons as in the analyses of symptom counts.
3. Results
Demographic characteristics of the scanned sample are shown in Table 1. Children who met diagnostic criteria for CD had 3 or more symptoms of CD in this table. Consistent with the oversampling strategy, there were not significant associations at p < .05 between the number of CD symptoms exhibited by the children and their age, sex, or race-ethnicity. There was not a linear difference association between the number of CD symptoms and the percent of mothers who graduated from high school (p = 0.70).
Table 1.
Demographic characteristics of and the percent prevalence of each conduct disorder symptom as a function of the total number of conduct disorder symptoms in the 110 scanned children included in the analyses.
| Number of CD symptoms | 0 | 1 | 2 | 3 | 4 | 5 | 6+ |
|---|---|---|---|---|---|---|---|
| N | 53 | 18 | 18 | 5 | 4 | 7 | 5 |
| Demographic characteristics | |||||||
| Age (years) | 9.9 | 10.3 | 9.9 | 9.8 | 10.0 | 10.3 | 9.8 |
| Sex (% female) | 50.9 | 44.4 | 66.7 | 20.0 | 100.0 | 42.9 | 40.0 |
| Race-ethnicity | |||||||
| %Afr-Am | 52.8 | 50.0 | 66.7 | 60.0 | 25.0 | 57.1 | 80.0 |
| %Hispanic | 5.7 | 5.6 | 11.1 | 20.0 | 0.0 | 14.3 | 20.0 |
| %Mother HS graduate | 90.6 | 66.7 | 72.2 | 60.0 | 100.0 | 100.0 | 100.0 |
|
| |||||||
| Child’s CD symptoms | |||||||
| Theft without confrontation | 0.0 | 50.0 | 72.2 | 80.0 | 75.0 | 85.7 | 60.0 |
| Vandalism | 0.0 | 22.2 | 38.9 | 60.0 | 75.0 | 85.7 | 100.0 |
| Bullying | 0.0 | 11.1 | 16.7 | 20.0 | 100.0 | 71.4 | 100.0 |
| Lying to con | 0.0 | 5.6 | 27.8 | 20.0 | 75.0 | 71.4 | 80.0 |
| Using a weapon | 0.0 | 0.0 | 16.7 | 0.0 | 25.0 | 57.1 | 100.0 |
| Starting physical fights | 0.0 | 0.0 | 22.2 | 20.0 | 25.0 | 14.3 | 100.0 |
| Cruel to people | 0.0 | 0.0 | 0.0 | 40.0 | 0.0 | 42.9 | 80.0 |
| Cruel to animals | 0.0 | 5.6 | 0.0 | 60.0 | 0.0 | 28.6 | 20.0 |
| Out late without permission | 0.0 | 5.6 | 5.6 | 0.0 | 0.0 | 0.0 | 20.0 |
| Theft with confrontation | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | 14.3 | 20.0 |
| Breaking and entering | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 14.3 | 0.0 |
| Truancy | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 14.3 | 0.0 |
Note: CD = conduct disorder symptoms reported by parent and youth; no children were reported to engage in running away from home overnight, firesetting, or forced sex; children with 3 or more CD symptoms were said to meet diagnostic criteria for CD; children with 1 or 2 symptoms were said to exhibit subthreshold CD.
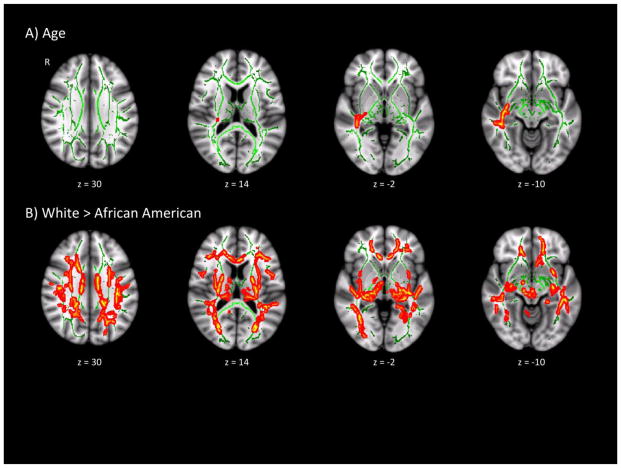
There were main effects of both age and race on FA (Table 2 and Figure 1). Age was associated with increased FA in the retrolenticular part of the right internal capsule, as well as the right medial lemniscus, corticospinal tract, and left superior cerebellar peduncle. Relative to African American participants, white participants had greater FA values across most of the bilateral internal capsules, superior longitudinal fasciculi, and cingulum. No regions showed less FA with increased age or greater FA in African Americans. There were no significant main effects of gender or CD (whether modeled as continuous or as three groups) on FA, and no significant race-by-CD or gender-by-CD interaction effects on FA.
Table 2.
Variations in FA associated with chronological age and race-ethnicity.
| Contrast | Region | MNI coordinates
|
Cluster size | Peak t | TFCE-corrected p | Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Increase with age | ||||||||
| Right inferior longitudinal fasiculus | 41 | −21 | −9 | 878 | 3.79 | 0.029 | 0.73 | |
| Right inferior cerebellar peduncle | 9 | −41 | −39 | 193 | 4.16 | 0.044 | 0.80 | |
| Left superior cerebellar peduncle | −7 | −40 | −27 | 46 | 3.79 | 0.048 | 0.73 | |
| Right corticospinal tract | 5 | −33 | −39 | 35 | 2.54 | 0.049 | 0.49 | |
| Right corticospinal tract | 6 | −26 | −31 | 10 | 3.80 | 0.050 | 0.73 | |
| White > African American | ||||||||
| Right superior longitudinal fasiculus | 32 | −2 | 19 | 32918 | 5.02 | 0.002 | 0.96 | |
| Right cerebellum | 31 | −53 | −27 | 262 | 4.97 | 0.029 | 0.95 | |
All reported effects significant at FWE p < .05.
Figure 1.
Significant demographic effects on fractional anisotropy. Regions showing main effects of age (A) or race (B) on fractional anisotropy (FA), overlaid on the mean FA skeleton (green). Significant regions (p < 0.05 FWE-corrected) have been thickened for viewing.
3.1. Results for counts of CD symptoms
The results of all statistical tests of association between measures of white matter integrity with numbers of CD symptoms and between diagnostic groups are reported in Table 3. No tests of associations or differences with FA were statistically significant. Controlling for all demographic factors, there was a significant main effect of the number of CD symptoms on axial diffusivity values, which was qualified by a gender-by-CD interaction and a race-by-CD interaction (Figure 2). More CD symptoms were associated with greater axial diffusivity in the left corticospinal tract, cerebellum, and right superior corona radiata, forceps minor, superior longitudinal fasciculus, and UF. These regions, as well as the posterior limb of the left internal capsule, especially left longitudinal fasciculus, also showed a significant gender-by-CD effect, reflecting a stronger association of more CD symptoms with greater axial diffusivity in females than in males (Figure 2B). The race-by-CD interaction indicated that the association of axial diffusivity with more CD symptoms was stronger in African American than in Hispanic and non-Hispanic white children (Figure 2C).
Table 3.
Significant associations between the number of CD symptoms and axial and radial diffusivity in white matter tracts, including tests of interactions with gender and race-ethicity.
| Measure | Contrast | Region | MNI coordinates
|
Cluster size | Peak t | TFCE-corrected p | Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Axial Diffusivity | |||||||||
| CD Symptoms | |||||||||
| Left corticospinal tract | −5 | −26 | −35 | 9137 | 5.71 | 0.029 | 1.09 | ||
| Right superior longitudinal fasciculus | 19 | −2 | 46 | 3002 | 3.97 | 0.038 | 0.76 | ||
| Right cerebellum (vermis) | 10 | −58 | −20 | 305 | 3.73 | 0.049 | 0.71 | ||
| Right cerebellum (vermis) | 8 | −66 | −24 | 105 | 3.78 | 0.049 | 0.72 | ||
| Right forceps minor | 18 | 44 | 26 | 69 | 4.16 | 0.049 | 0.80 | ||
| Right forceps major | 22 | −63 | 35 | 68 | 5.77 | 0.050 | 1.11 | ||
| CD Symptoms x Gender | |||||||||
| Left corticospinal tract | −8 | −25 | −29 | 21150 | 5.98 | 0.018 | 1.15 | ||
| Right inferior longitudinal fasciculus | 44 | −30 | 7 | 1249 | 3.16 | 0.044 | 0.61 | ||
| Cerebellum (vermis) | 5 | −51 | −17 | 965 | 5.37 | 0.043 | 1.03 | ||
| Right anterior thalamic radiation | 19 | −33 | 4 | 78 | 5.18 | 0.048 | 0.99 | ||
| CD Symptoms x Race-Ethnicity | |||||||||
| Left corticospinal tract | −16 | −16 | −11 | 18455 | 3.21 | 0.007 | 0.61 | ||
| Right superior longitudinal fasciculus | 21 | −8 | 45 | 11218 | 3.39 | 0.010 | 0.65 | ||
| Right inferior fronto-occipital fasciculus | 25 | −77 | 23 | 156 | 3.39 | 0.049 | 0.65 | ||
| Right forceps major | 13 | −83 | 27 | 40 | 3.55 | 0.050 | 0.68 | ||
| Right forceps major | 10 | −85 | 24 | 3 | 3.67 | 0.050 | 0.70 | ||
| Left inferior longitudinal fasciculus | −48 | −18 | −4 | 3 | 3.55 | 0.050 | 0.68 | ||
| Radial Diffusivity | |||||||||
| CD Symptoms x Gender | |||||||||
| Right superior longitudinal fasciculus | 35 | −18 | 26 | 54781 | 4.65 | 0.008 | 0.89 | ||
| Left uncinate fasciculus | −19 | 5 | −13 | 39 | 3.40 | 0.050 | 0.65 | ||
| Left corticospinal tract | −22 | −45 | 50 | 35 | 3.77 | 0.050 | 0.72 | ||
| Right anterior thalamic radiation | 1 | −5 | −11 | 7 | 4.39 | 0.050 | 0.84 | ||
| CD Symptoms x Race-Ethnicity | |||||||||
| Right corticospinal tract | 6 | −25 | −12 | 62055 | 3.47 | 0.007 | 0.66 | ||
All reported effects significant at FWE p < .05.
Figure 2.
Significant associations with axial diffusivity. Regions showing a positive main effect of CD symptoms (A) or a significant interaction between CD symptoms and gender (B) or a significant interaction between CD symptoms and race (C). Significant regions (p < 0.05 FWE-corrected) have been thickened for viewing and overlaid on the mean FA skeleton (green).
Although there was not a significant main effect of the number of CD symptoms on radial diffusivity, there were extensive regions showing a significant gender-by-number of CD symptoms interaction and a race-by-CD symptoms interaction on radial diffusivity (Figure 3). Again, there was a stronger association between the number of CD symptoms and increased radial diffusivity among females than males in the internal and external capsules, corpus callosum, corona radiata, and cerebellar peduncles (Figure 3A). Additionally, radial diffusivity in these areas was more strongly associated with the number of CD symptoms in African American compared to Hispanic and non-Hispanic white participants (Figure 3B).
Figure 3.

Significant associations with radial diffusivity. Regions showing a significant interaction between CD symptoms and gender (A) or CD symptoms and race (B). Significant regions (p < 0.05 FWE-corrected) have been thickened for viewing and overlaid on the mean FA skeleton (green).
3.2. Results for the Diagnosis of CD
Comparisons of nominal groups based on DSM-V diagnostic criteria for CD were conducted for FA, axial diffusivity, and radial diffusivity. No significant differences were found between children with 1 or 2 CD symptoms and either the group with zero CD symptoms or with the group that met diagnostic criteria for CD for any of these measures of white matter integrity. When children who met diagnostic criteria for CD were compared to children with zero symptoms of CD, no regions showed a significant main effect of CD on axial diffusivity, but the gender-by-group and race-by-group interactions were significant (Figure 4). Pair-wise comparisons indicated that the comparison of axial diffusivity between children with CD and children with zero CD symptoms was significant in females but not in males (Figure 4A) and significant in African American children but not in other children (Figure 4B).
Figure 4.
Significant diagnostic group differences in axial diffusivity. Planned comparisons revealed significant interactions between gender and diagnostic group (A) and between race and diagnostic group (B). Significant regions (p < 0.05 FWE-corrected) have been thickened for viewing and overlaid on the mean FA skeleton (green).
There was a significant difference between children who met criteria for CD and healthy children on radial diffusivity in a broad range of regions of the brain (Figure 5). The main effect was qualified by significant interactions with both gender and race-ethnicity. Like axial diffusivity, pairwise comparisons indicated that the comparison of radial diffusivity between children with CD and children with zero CD symptoms was significant in females but not in males (Figure 5B) and significant in African American children but not in other children (Figure 5C).
Figure 5.
Significant diagnostic group differences in radial diffusivity. Planned comparisons revealed that children who met diagnostic criteria for CD showed widespread increases in radial diffusivity compared to healthy controls (A). Further, there was a significant interaction between gender and diagnostic group (B) and between race and diagnostic group (C). Significant regions (p < 0.05 FWE-corrected) have been thickened for viewing and overlaid on the mean FA skeleton (green).
4. Discussion
Using a large, diverse sample of female and male children, this study examined variations in microstructural white matter integrity in children who were younger (9–11 years) than in previous studies. Some of the findings of the present study apply equally to girls and boys with high levels of CD symptoms. We found a significant association between axial diffusivity in the right and left superior longitudinal fasciculus and the number of CD symptoms. This association was not moderated by gender, indicating no evidence of a gender difference, but the association was significantly stronger in African American children than in children of other race-ethnicities. This finding replicates the findings of Haney-Caron et al. (2014). White matter abnormalities in the superior longitudinal fasciculus have been associated with deficits in selective attention in children (Klarborg et al., 2013) and to be more common in persons with schizophrenia (Karlsgodt et al., 2008). We also found a significant association between the number of CD symptoms and the axial diffusivity in the right forceps minor and forceps major. These associations were not moderated by gender, but were stronger in African American children. These tracts of the corpus callosum connect right and left medial and lateral surfaces of the frontal lobes and connect the right and left occipital lobes, respectively. Interestingly, while decreased axial diffusivity in the forceps minor has previously been linked to CD in adolescents (Haney-Caron et al., 2014), our study found the opposite effect. This discrepancy may have arisen from the use of a younger sample, since increased axial diffusivity in the forceps minor has also been found to be associated with ADHD in childhood (Lawrence et al., 2013), which is very highly correlated with CD during childhood (Lahey et al., 1998, 2008).
Because of its theoretically important role linking the amygdala with the ventromedial prefrontal cortex, previously findings of microstructural abnormalities in the UF in antisocial and psychopathic adults have been of great interest. As summarized in the introduction, findings in adolescents who engage in antisocial behavior (defined as CD or more broadly) have been inconsistent regarding the UF. We find that reduced radial diffusivity in the left UF was associated with more CD symptoms, but the interaction with gender indicated that the association was significantly stronger in girls than boys. This finding is consistent with the previous findings of Sarkar et al. (2013), with the important exception that their sample was all adolescent males. However, the current findings match several previous studies of adolescents which showed evidence of microstructural atypicalities in UF associated with CD (Haney-Caron et al., 2014; Passamonti et al., 2012; Sarkar et al., 2013), suggesting that more research is needed to clarify the relationship between CD and UF integrity across development.
Beyond UF, several other regions also showed association with CD that were moderated by gender for both axial and radial diffusivity. Greater axial diffusivity was associated with the number of CD symptoms more strongly in girls than boys in the left corticospinal tract in the region of the anterior limb of the internal capsule (ALIC), the right inferior longitudinal fasciculus, the right anterior thalamic radiation, and the vermis of the cerebellum. In addition, the number of CD symptoms was associated with greater radial diffusivity in the right superior longitudinal fasciculus, left corticospinal tract, and the right anterior thalamic radiation more strongly in girls. Atypical white matter in the inferior longitudinal fasciculus has been previously linked with CD (Haney-Caron et al., 2014), ADHD (Lawrence et al., 2013), and depression (Bessette et al., 2013). White matter abnormalities in the anterior thalamic radiation have not been previously shown in CD, but are consistently identified correlates of schizophrenia and bipolar disorder (Sussmann et al., 2009; Mamah et al., 2010). White matter abnormalities in the cerebrospinal tract/ALIC have not previously been linked to CD, but are associated with depression and schizophrenia (Rosenberger et al., 2012; Zhang et al., 2013). It is now clear that the cerebellum plays a role in the modulation of cognitive, emotional, and social processes, and an intact cerebellar vermis is essential for providing neocortical regulation of the limbic system (Villanueva, 2012). While structural abnormalities in cerebellum have not been previously linked to CD, disruption of the vermis has been linked to psychiatric disorders involving emotional and social behavioral deficits, including children with ADHD (Bledsoe et al., 2011), major depression (Yucel et al., 2013), and bipolar disorder (Villanueva, 2012).
We used counts of CD symptoms to assess relations between white matter atypicalities and CD to maximize statistical power, but findings based on this dimensional approach to CD were largely mirrored in group comparisons of 39 children who met DSM-5 criteria for CD and 53 children with no symptoms of CD. As shown in Table 4, these analyses also revealed robust moderation of associations between CD and axial and radial diffusivity in nearly all the same tracts. The present findings of more widespread axial and radial diffusivity in white matter tracts of the cerebrum and cerebellum in girls with CD than in boys is fully consistent with the gender paradox hypothesis. Importantly, the present findings indicate the presence of abnormalities of white matter in girls with CD that are commonly found in a range of other serious mental disorders, including ADHD, depression, bipolar disorder, and schizophrenia. Thus, it is possible that girls only exhibit higher numbers of CD when they exhibit far more severe pathophysiology than do boys, for whom there may be weaker protective factors against the development of CD.
Table 4.
Tests of group differences axial and radial diffusivity between 39 children with CD and 53 children with no symptoms of CD within genders and race-ethnic categories.
| Measure | Contrast | Region | MNI coordinates
|
Cluster size | Peak t | TFCE-corrected p | Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Axial diffusivity | |||||||||
| CD-HC in Females | |||||||||
| Left superior corona radiata | −16 | 7 | 33 | 6654 | 3.76 | 0.016 | 1.25 | ||
| Right superior longitudinal fasciculus | 19 | −1 | 46 | 1487 | 3.29 | 0.038 | 1.10 | ||
| Left corticospinal tract | −8 | −24 | −28 | 400 | 3.81 | 0.039 | 1.27 | ||
| Right forceps major | 22 | −63 | 35 | 115 | 3.81 | 0.049 | 1.27 | ||
| Left anterior thalamic radiation | −13 | −26 | 0 | 81 | 3.45 | 0.047 | 1.15 | ||
| Right anterior thalamic radiation | 33 | 26 | 20 | 34 | 2.53 | 0.049 | 0.84 | ||
| Left cingulum | −15 | −47 | 27 | 7 | 3.06 | 0.050 | 1.02 | ||
| Left corticospinal tract | −22 | −21 | −4 | 3 | 3.02 | 0.050 | 1.01 | ||
| CD-HC in African Americans | |||||||||
| Body of corpus callosum | −9 | −12 | 28 | 27049 | 3.48 | 0.015 | 1.45 | ||
| L superior longitudinal fasciculus | −32 | −13 | 49 | 217 | 3.11 | 0.015 | 1.30 | ||
| Radial diffusivity | |||||||||
| CD-HC | |||||||||
| Cerebellum (vermis) | −12 | −57 | −47 | 55357 | 2.67 | 0.013 | 0.62 | ||
| CD-HC in Females | |||||||||
| Cerebellum (vermis) | −14 | −56 | −40 | 69731 | 4.83 | 0.015 | 1.61 | ||
| CD-HC in African Americans | |||||||||
| Cerebellum (vermis) | −8 | −41 | −38 | 70836 | 3.05 | 0.003 | 1.27 | ||
CD: CD group (three or more symptoms of CD); HC: Healthy control group (zero symptoms of CD); TFCE = threshold-free cluster enhancement; all reported effects significant at FWE p < .05.
Though this study focused on identifying associations between white matter integrity and CD, significant race-ethnicity effects were also observed. In particular, African American children exhibited reduced FA in a wide range of regions, including the cingulum, superior longitudinal fasciculi, and internal capsules (Figure 1B). This is in contrast with one previous study in adults showing that African Americans had higher white matter grade (Yue et al., 1997). This could indicate an influence of race-ethnicity of the shape of developmental trajectories. However, little is known at this time about race-ethnicity differences in white matter development in the typical population, with many studies either treating race-ethnicity as a covariate of no interest, or analyzing Caucasians separately from minorities (Stavitsky et al., 2010). In addition, the present study identified a number of significant interactions between CD and race-ethnicity for both axial and radial diffusivity in a number of white matter tracts. These include the superior and inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, forceps minor and major, cerebellar vermis, and the anterior thalamic radiation, and the corticospinal tract. It will be important for future studies of more representative samples to attempt to confirm these interactions and identify cultural and environmental factors associated with these differences.
One of the most important findings of this study is that CD is already associated with greater axial and radial diffusivity in 9–11 year old children in a broad range of white matter tracts, both when CD is measured by the count of CD symptoms or treated as a categorical diagnosis. These associations of CD with axial and radial diffusivity were particularly strong in females and among African American children. Although the interpretation of increased radial and axial diffusivity is complicated (Wheeler-Kingshott and Cercignani, 2009), these findings suggest that greater numbers of childhood CD symptoms may be associated with decreased axonal and myelin integrity (Kumar et al., 2013), particularly in girls. These findings are generally consistent with the hypothesis that the origins of the replicated white matter abnormalities in the brains of adults with antisocial personality disorder have their origins early in life. The gender-by-CD interactions may be related to the fact that females show earlier peaks in white matter development (Lenroot et al., 2007) and tend to approach adult levels of radial diffusivity faster than males in the majority of fiber tracts (Asato et al., 2010).
Hilights.
Diffusion tensor imaging in 110 children found changes in fractional anisotropy with age
No effect of conduct disorder symptoms on fractional anisotropy
Conduct disorder symptoms predict axial and radial diffusivity, but only in girls
Anatomical abnormalities have a greater impact in girls
Acknowledgments
The study was supported by a grant from NIH (R01 MH084934) to Dr. Jean Decety.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cerebral Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette KL, Nave AM, Caprihan A, Stevens MC. White matter abnormalities in adolescents with major depressive disorder. Brain Imaging and Behavior. 2013;8:531–541. doi: 10.1007/s11682-013-9274-8. [DOI] [PubMed] [Google Scholar]
- Bledsoe JC, Semrud-Clikeman M, Pliszka SR. Neuroanatomical and neuropsychological correlates of the cerebellum in children with attention-deficit/hyperactivity disorder-combined type. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:593–601. doi: 10.1016/j.jaac.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Hahn CS, Wolke D. Systems and cascades in cognitive development and academic achievement. Child Development. 2013;84:154–162. doi: 10.1111/j.1467-8624.2012.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. Journal of Neuroscience. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Owen MJ. Rethinking psychosis: The disadvantages of a dichotomous classification now outweigh the advantages. World Psychiatry. 2007;6:84–91. [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, Picchioni M, McGuire PK, Fahy T, Murphy DGM. Altered connections on the road to psychopathy. Molecular psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Decety J, Cowell JM. The complex relation between morality and empathy. Trends in cognitive sciences. 2014;18:337–339. doi: 10.1016/j.tics.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RD, Roff J. Negative effects of paternal age on children’s neurocognitive outcomes can be explained by maternal education and number of siblings. PloS One. 2010;5:e12157. doi: 10.1371/journal.pone.0012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eme RF. Selective female affliction in the developmental disorders of childhood: A literature review. Journal of Clinical Child Psychology. 1992;21:354–364. [Google Scholar]
- Ghassabian A, Rescorla L, Henrichs J, Jaddoe VW, Verhulst FC, Tiemeier H. Early lexical development and risk of verbal and nonverbal cognitive delay at school age. Acta Paediatrica. 2014;103:70–80. doi: 10.1111/apa.12449. [DOI] [PubMed] [Google Scholar]
- Haney-Caron E, Caprihan A, Stevens MC. DTI-measured white matter abnormalities in adolescents with Conduct Disorder. Journal of Psychiatric Research. 2014;48:111–120. doi: 10.1016/j.jpsychires.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, Nazeri A, de Jesus DR, Stirpe T, Felsky D, Schutter DJLG, Daskalakis ZJ, Voineskos AN. White matter deficits in psychopathic offenders and correlation with factor structure. PloS One. 2013;8:e72375. doi: 10.1371/journal.pone.0072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TGM, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarborg B, Skak Madsen K, Vestergaard M, Skimminge A, Jernigan TL, Baaré WFC. Sustained attention is associated with right superior longitudinal fasciculus and superior parietal white matter microstructure in children. Human Brain Mapping. 2013;34:3216–3232. doi: 10.1002/hbm.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. NeuroImage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Noda A, O’Hara R. Categorical versus dimensional approaches to diagnosis: Methodological challenges. Journal of Psychiatric Research. 2004;38:17–25. doi: 10.1016/s0022-3956(03)00097-9. [DOI] [PubMed] [Google Scholar]
- Kumar R, Chavez AS, Macey PM, Woo MA, Harper RM. Brain axial and radial diffusivity changes with age and gender in healthy adults. Brain Research. 2013;1512:22–36. doi: 10.1016/j.brainres.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Quay HC, Applegate B, Shaffer D, Waldman I, Hart EL, McBurnett K, Frick PJ, Jensen PS, Dulcan MK, Canino G, Bird HR. Validity of DSM-IV subtypes of conduct disorder based on age of onset. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:435–442. doi: 10.1097/00004583-199804000-00022. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Van Hulle C, Urbano RC, Krueger RF, Applegate B, Garriock HA, Chapman DA, Waldman ID. Testing structural models of DSM-IV symptoms of common forms of child and adolescent psychopathology. Journal of Abnormal Child Psychology. 2008;36:187–206. doi: 10.1007/s10802-007-9169-5. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Levitt JG, Loo SK, Ly R, Yee V, O’Neill J, Alger J, Narr KL. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:431–440. e4. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of Neuroscience. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Sebastian CL, McCrory EJ, Hyde ZH, Gu X, De Brito SA, Viding E. Association of callous traits with reduced neural response to others’ pain in children with conduct problems. Current Biology. 2013;23:901–905. doi: 10.1016/j.cub.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Keenan K. Interaction between conduct disorder and its comorbid conditions: effects of age and gender. Clinical Psychology Review. 1994;14:497–523. [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, Gado MH, Barch DM, Czernansky JG. Anterior thalamic radiation integrity in schizophrenia: A diffusion-tensor imaging study. Psychiatry Research. 2010;183:144–150. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Jaffee SR, Kim-Cohen J, Koenen KC, Odgers CL, Slutske WS, Viding E. Research review: DSM-V conduct disorder: research needs for an evidence base. Journal of child psychology and psychiatry, and allied disciplines. 2008;49:3–33. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Dickson N, Silva P, Stanton W. Childhood-onset versus adolescent-onset antisocial conduct problems in males: Natural history from ages 3 to 18 years. Development and Psychopathology. 1996;8:399–424. [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. The Journal of Neuroscience. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, Hagan CC, Calder AJ. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PloS one. 2012;7:e48789. doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, MacCallum RC, Nicewander WA. Use of the extreme groups approach: A critical reexamination and new recommendations. Psychological Methods. 2005;10:178–192. doi: 10.1037/1082-989X.10.2.178. [DOI] [PubMed] [Google Scholar]
- Romeo R, Knapp M, Scott S. Economic cost of severe antisocial behaviour in children--and who pays it. The British Journal of Psychiatry. 2006;188:547–553. doi: 10.1192/bjp.bp.104.007625. [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Nestor PG, Oh JS, Levitt JJ, Kindleman G, Bouix S, Fitzsimmons J, Niznikiewicz M, Westin CF, Kikinis R, McCarley RW, Shenton ME, Kubicki M. Anterior limb of the internal capsule in schizophrenia: A diffusion tensor tractography study. Brain Imaging and Behavior. 2012;6:417–425. doi: 10.1007/s11682-012-9152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. Journal of Child Psychology and Psychiatry. 2003;44:1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Catani M, Dell’acqua F, Fahy T, Deeley Q, Murphy DGM. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: A diffusion tensor imaging study. Psychological Medicine. 2013;43:401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stavitsky K, Du Y, Seichepine D, Laudate TM, Beiser A, Seshadri S, Decarli C, Wolf PA, Au R. White matter hyperintensity and cognitive functioning in the racial and ethnic minority cohort of the Framingham heart study. Neuroepidemiology. 2010;35:117–122. doi: 10.1159/000313443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundram F, Deeley Q, Sarkar S, Daly E, Latham R, Craig M, Raczek M, Fahy T, Picchioni M, Barker GJ, Murphy DGM. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex. 2012;48:216–229. doi: 10.1016/j.cortex.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Munoz Maniega S, Job D, Hall J, Bastin ME, Johnstone EC, Lawrie SM, McIntosh AM. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disorders. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Viding E. Annotation: Understanding the development of psychopathy. Journal of Child Psychology and Psychiatry. 2004;45:1329–1337. doi: 10.1111/j.1469-7610.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- Villanueva R. The cerebellum and neuropsychiatric disorders. Psychiatry Research. 2012;198:527–532. doi: 10.1016/j.psychres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magnetic Resonance in Medicine. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel K, Nazarov A, Taylor VH, Macdonald K, Hall GB, MacQueen GM. Cerebellar vermis volume in major depressive disorder. Brain Structure and Function. 2013;218:851–858. doi: 10.1007/s00429-012-0433-2. [DOI] [PubMed] [Google Scholar]
- Yue NC, Arnold AM, Longstreth T, Elster D, Jungreis A, Leary HO, Poirier C, Bryan RN. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: Data from the Cardiovascular Health Study. Neuroradiology. 1997;202:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- Zhang A, Ajilore O, Zhan L, Gadelkarim J, Korthauer L, Yang S, Leow A, Kumar A. White matter tract integrity of anterior limb of internal capsule in major depression and type 2 diabetes. Neuropsychopharmacology. 2013;38:1451–1459. doi: 10.1038/npp.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]