Abstract
Background
Previous work in healthy non-human primates and humans has shown that social status correlates positively with dopamine 2/3 receptor (D2/3 R) availability imaged with antagonist radioligands and positron emission tomography (PET). Further work in non-human primates suggests that this relationship is disrupted by chronic cocaine administration. This exploratory study examined the relationship between social status and D2/3R availability in healthy (HH) and cocaine dependent (CD) humans using the D3-preferring, agonist radioligand, [11C](+)PHNO.
Methods
Sixteen HH and sixteen CD individuals completed the Barratt Simplified Measure of Social Status (BSMSS) and underwent [11C](+)PHNO scanning to measure regional brain D2/3R binding potentials (BPND). Correlations between BPND and BSMSS scores were then assessed within each group.
Results
Within HH and CD groups, inverse associations between BSMSS score and BPND were observed in the substantia nigra/ventral tegmental area (SN/VTA) and the ventral striatum, and for the CD group alone, the amygdala. After adjusting for body mass index and age, negative correlations remained significant in the SN/VTA for HH and in the amygdala for CD subjects.
Conclusion
These preliminary data utilizing a dopamine agonist tracer demonstrate, for the first time, an inverse association between social status and D2/3R availability in the D3R rich extrastriatal regions of HH and CD humans.
Keywords: dopamine, cocaine, social status, PET imaging, [11C](+)PHNO
1. INTRODUCTION
Social status is an important factor relating to health and behaviors in mammals, including humans (Adler et al., 1994; Tamashiro et al., 2005). Particularly, dopaminergic function in the brain has been found to relate to social status in different species (Grant et al., 1998; Hall et al., 1998; Morgan et al., 2000). In healthy humans, greater social status as measured by the Barratt Simplified Measure of Social Status (BSMSS; Barratt, 2006), a comprehensive instrument which quantifies social status based on educational and occupational environment, has been associated with higher striatal dopamine subtype 2/3 receptor (D2/3R) availability when imaged with the antagonist positron emission tomography (PET) radioligand, [11C]raclopride (Martinez et al., 2010). This finding is consistent with earlier PET work in nonhuman primates that utilized a social status corollary, social dominance, to show that social dominance was positively associated with striatal D2/3R availability in substance-naïve cynomolgus macaques when measured by [18F]fluoroclebopride (Morgan et al., 2002). Although novel, those previous studies were both done utilizing similar non-selective dopamine D2/3R antagonist tracers either shown to be unreliable outside of the striatum (Graff-Guerrero et al., 2008) or examined the basal ganglia as a whole (Morgan et al., 2002), thus important somatodendritic D2/3R regions within the basal ganglia, such as the substantia nigra / ventral tegmental area (SN/VTA), have not been examined. Additionally, the aforementioned antagonist tracers bind to both “high” affinity (i.e., the D3R and certain active forms of the D2R) and “low” affinity (i.e., the non-active forms of the D2R) dopamine D2/3R receptors (George et al., 1985; Leff, 1995) with equal preference. Consequently, this lack of preference limits the degree to which the relative distribution of dopamine receptor subtype (i.e., D2R vs. D3R) and isoform (i.e., “high” vs. “low” affinity D2R) can be examined in certain regions. However, with an agonist tracer it has been shown that D3R predominates in the SN/VTA (as opposed to the dorsal striatum where D2R is predominant)(Graff-Guerrero et al., 2008; Narendran et al., 2006; Searle et al., 2010; Tziortzi et al., 2011). Thus, to date, no studies have examined social status and D2/3R receptor availability in a non-substance abusing healthy human population with an agonist dopamine tracer, both within and outside the striatum.
Given the role of dopamine in mediating cocaine effects, investigators have sought to understand potential relationships between social status, cocaine, and brain dopamine function. Morgan and colleagues found that the vulnerability to self-administer cocaine was associated with lower social status in cynomolgus macaques (Morgan et al., 2002). Follow-up work from the same group showed that after an extended period (between 5 and 45 months) of cocaine self-administration the positive association between social dominance and striatal D2/3R availability in socially housed nonhuman primates was lost (Czoty et al., 2004; for a complete review of social status and cocaine administration paradigms in nonhuman primates see Nader and Banks, 2014). Thus, current nonhuman primate evidence suggests a potentially complex relationship between the vulnerability to abuse cocaine, social status, and striatal dopamine function - a relationship that has yet to be examined in brain regions of clinical populations.
In light of these prior works (Czoty et al., 2004; Martinez et al., 2010; Morgan et al., 2002), this preliminary study looks, for first time, to utilize the D3-preferring agonist tracer [11C](+)PHNO to examine the association between social status and 1) striatal and extrastriatal (e.g., SN/VTA) D2/3R availability in non-substance abusing healthy humans (HH) humans and 2) investigate these same relationships in cocaine dependent (CD) humans for the first time with a radioligand.
2. PARTICIPANTS AND METHODS
2.1 Participants
Sixteen HH participants without a clinical history of illicit substance use and sixteen medically healthy, non-treatment-seeking CD participants were studied. Subject eligibility was confirmed by comprehensive medical and psychiatric histories, physical examination, neurological and mental status exam, routine laboratory studies, electrocardiogram, and semi-structured (Sheehan et al., 1998) or structured clinical interview (American Psychiatric Association. and American Psychiatric Association Task Force on DSM-IV, 2000).
CD participants were between the ages of 18 and 50 years, met DSM-IV criteria for cocaine dependence, used cocaine as their primary drug of choice via a rapid-onset (e.g., intravenous or smoked) route, and had a history of regular and recent cocaine use (e.g., as evidenced by benzoylecgonine positivity on urine toxicology testing). Three of the CD participants have been previously reported (Matuskey et al., 2014). Demographic and clinical features of all participants are shown in Table 1.
Table 1.
Demographic and clinical characteristics of HH and CD participants. Mean values (and standard deviation) are shown. AA = African American, C = Caucasian, C-H = Caucasian-Hispanic, SM = Smoke, IN = Intranasal, IV = Intravenous
| HH | CD | |
|---|---|---|
|
Number of
participants |
16 | 16 |
| Age in years | 40.3 (9.3) | 41.9 (5.4) |
| Gender | 13 M, 3 F | 12 M, 4 F |
| Race | 11 AA, 5 C | 10 AA, 5 C 1 C-H |
|
Body mass index
(kg/m2) |
29.7 (6.7) | 30.6 (7.0) |
| Years of Education | 15.3 (2.0) | 12.6 (1.4) |
| Years of cocaine use | - | 19.9 (5.9) |
|
Weekly cocaine use
in $USD |
- | $409 ($321) |
|
Weekly alcohol use
(in standard drinks) |
3.1 (6.1) | 15.1 (28.6) |
|
Daily nicotine use
(in cigarettes) |
0.6 (2.5) | 9.1 (6.5) |
|
Weekly cannabis use
(in joints rolled) |
- | <1 |
|
Days from last
cocaine use to PET scan |
- | 7.8 (5.1) |
|
Route(s) of cocaine
administration |
- | 4 SM and IN 10 SM Only 2 SM, IN, and IV |
Participants were excluded based on evidence of a current or lifetime major psychiatric illness (e.g., schizophrenia or bipolar disorder), current or past serious medical or neurological illness (e.g., history of head injury with loss of consciousness), current pregnancy as documented by pregnancy testing at screening and on the day of the PET imaging study, breast feeding, or contraindications to magnetic resonance imaging (MRI).
Once determined eligible, participants completed the BSMSS (Barratt, 2006), a comprehensive measure of social status based on the Hollingshead index (Hollingshead, 1975). The BSMSS and the Hollingshead index are similar in that they both generate a single comprehensive score of social status taking into account education and occupation of the research participant, their parents, and their spouse. The comprehensive score of the BSMSS is weighted in favor of the scores of the research participant and significant others over that of the parents. Subject screening records (e.g., occupation and education) were reviewed to confirm an accurate BSMSS report. One potential CD subject was excluded for providing incongruent information.
Participants were recruited from the greater New Haven area by advertisement, word of mouth and referral. Informed consent was obtained from all participants after a thorough explanation of the study procedures by a research assistant, study coordinator, or study investigator. This study was performed under protocols approved by the Yale Human Investigation, Yale University Radiation Safety, Yale-New Haven Hospital (YNHH) Radiation Safety, and Yale MRI Safety Committees.
2.2 Radiochemistry, Scanning, and Imaging Procedures
Carbon 11-labeled (+)-4-propyl-9-hydroxynaphthoxazine [[11C](+)PHNO] is a D2/3R agonist radiotracer that has D3R preferring properties and was prepared as previously reported (Gallezot et al., 2012). Injection information and radioactivity data for both HH and CD participants are shown in Table 2.
Table 2.
Injection information and radioactivity data for HH and CD participants. Means and standard deviations (in parenthesis) are shown.
| HH | CD | |
|---|---|---|
|
Mass dose
(ug/kg) |
0.024 (0.007) | 0.024 (0.006) |
|
Specific activity
(MBq/nmol) |
57.9 (31.8) | 52.0 (16.5) |
|
Radioactive Dose
(MBq) |
408.8 (140.4) | 406.9 (156.8) |
All scans used a high-resolution research tomograph (Siemens/CTI, Knoxville, TN, USA), which acquired 207 slices (1.2mm slice separation) with a reconstructed image resolution of ~3mm over 120 minutes at rest. A transmission scan with a 137Cs point source was obtained before the emission scan.
Structural MRI was performed on a 3 Tesla Trio system (Siemens Medical Solutions, Malvern, Pennsylvania) with a circularly polarized head coil for purposes of excluding individuals with anatomical abnormalities and anatomically coregistering with PET scans. The dimension and voxel size of MR images were 256 × 256 × 176 voxels and 0.98 × 0.98 × 1.0 mm3, respectively.
Motion correction was based on an optical detector (Vicra, NDI Systems, Waterloo, Ontario, Canada). Dynamic PET scan data were reconstructed with all corrections (attenuation; normalization; scatter; randoms; deadtime and motion), using the MOLAR algorithm (Carson, 2003) with the following frame timing: 6 × 30 sec; 3 × 1 min; 2 × 2 min; 22 × 5 min.
PET data were used to produce a time–activity curve for the cerebellum, which has minimal D2/3R binding and was used as the reference region as in previous studies (Boileau et al., 2012; Ginovart et al., 2007; Matuskey et al., 2014; Mizrahi et al., 2011; Payer et al., 2014; Searle et al., 2010). A summed image (0–10 min after injection) was created from the motion-corrected PET data and registered to the subject’s MR image, which in turn was nonlinearly registered to a MR template in Montreal Neurological Institute space. All transformations were performed using Bioimagesuite (version 2.5; http://www.bioimagesuite.com). Parametric images of binding potentials (BPND), which are linearly proportional to the density of available D2/3Rs under conditions of comparable non-specific tracer binding, were computed using a simplified reference tissue model (2-parameter version: SRTM2). This method has been previously validated for [11C](+)PHNO (Gallezot et al., 2014b; Wu and Carson, 2002) and was used to optimize the statistical quality of the SRTM applied in prior studies by reducing noise of the functional images (Matuskey et al., 2014).
Regions of interest (ROI) included the amygdala, caudate, hypothalamus, pallidum, putamen, SN/VTA, thalamus, and ventral striatum (VST) and, with the exception of the SN/VTA and VST, were based on the Anatomical Automatic Labeling (AAL) template delineated on MR (Tzourio-Mazoyer et al., 2002). Specifically, the SN/VTA was manually delineated on BPND images in template space as noted in the supplement of work done by Gallezot and colleagues (Gallezot et al., 2014b), and the VST template was based on guidelines previously described (Mawlawi et al., 2001). ROIs were applied to the BPND images to extract individual values.
2.3 Statistical Considerations
Between-group differences in BSMSS scores, education, mass dose, and radioactivity parameters were analyzed using independent t-tests. Potential associations between BSMSS scores, clinical characteristics, and demographics with ROIs were estimated using Pearson correlation coefficients and subsequently adjusted for body mass index (BMI) and age due to known influences of these variables on D2/3R (Caravaggio et al., 2013; Correa, 2014; Ishibashi et al., 2009; Kessler et al., 2014; Kim et al., 2011; Volkow et al., 2000; Wang et al., 2001). Analysis of covariance (ANCOVA) models were used to test whether associations differed between groups. All analyses were considered significant at the two-tailed α<0.05 threshold and were conducted using SPSS, version 19 (Armonk, NY). Correlations were not adjusted for multiple tests given the exploratory nature of these analyses.
3. RESULTS
Means and standard deviations of demographic, clinical characteristic, and injection parameter data for both HH and CD participants are shown in Tables 1 and 2. There were no significant associations between daily cigarette intake or weekly alcohol consumption and regional measures of BPND in either group.
Mean BSMSS scores for HH (44.8±10.1, range 30–62) and CD participants (30.2±7.1, range 15–44) differed significantly (p<0.001). Years of education were not statistically correlated with regional measures of BPND in either group; however, a statistical trend was observed in the SN/VTA (r=−0.49, p=0.06) in CD participants.
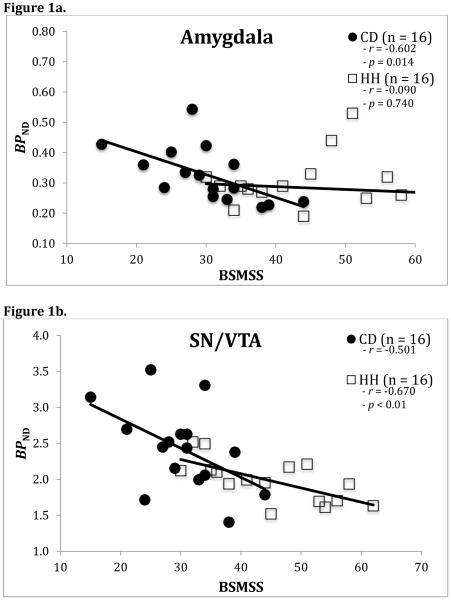
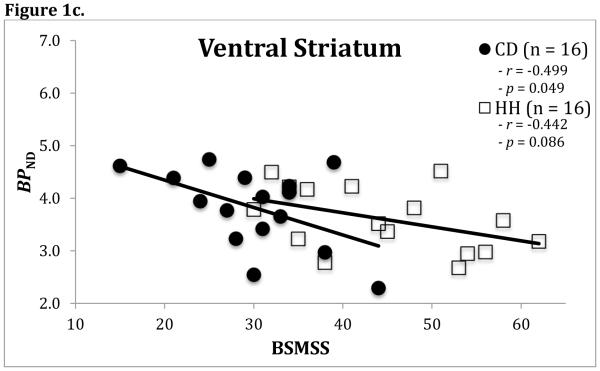
In HH participants, a significant inverse association was observed between BSMSS score and regional BPND in the SN/VTA (r=−0.67, p<0.01), as well as a statistical trend in the VST (r=−0.44, p=0.086) (Figure 1). In CD participants, significant negative correlations were observed in the amygdala (r=−0.60, p=0.014), SN/VTA (r=−0.50, p=0.048), and VST (r=−0.50, p=0.049)(Figure 1). No other regions emerged as statistically significant in either group and no region would have survived multiple comparison correction. Separate ANCOVA models revealed no differences in the above results between groups.
Figure 1.
Correlations between BSMSS and [11C](+)PHNO BPND in both CD and HH participants for the amygdala (Figure 1a), the substantia nigra/ventral tegmental (SN/VTA) area (Figure 1b), and the ventral striatum (Figure 1c). Correlations shown are not BMI and age-adjusted.
The association between BSMSS score and regional BPND in the SN/VTA retained significance after adjusting for BMI and age (corrected, r=−0.55, p=0.04) in HH participants. Similarly, the association remained significant in the amygdala among CD participants after adjustment for BMI and age (corrected, r=−0.55, p=0.04). Other associations lost significance after BMI and age adjustment. Additionally, injection parameters had no effects on correlations when examined with all participants.
A summary of both unadjusted and adjusted correlations between BSMSS score and all ROI BPND values for both HH and CD participants are shown in Tables 3 and 4. Between-group comparisons of BPND were outside the scope of this manuscript and thus not completed here, however, they will be reported as part of a larger cohort in a future publication.
Table 3.
Unadjusted and BMI and age-adjusted correlations between BSMSS and [11C](+)PHNO BPND in HH participants
| Amygdala | Caudate | Hypothalamus | Pallidum | Putamen | SN/VTA | Thalamus | Ventral Striatum |
|
|---|---|---|---|---|---|---|---|---|
|
BPND
Mean (S.D.) |
0.28 (0.10) |
1.90 (0.31) |
1.20 (0.28) |
3.55 (0.53) |
2.58 (0.38) |
1.98 (0.30) |
0.38 (0.10) |
3.60 (0.61) |
|
Unadjusted
Pearson r |
−0.09 | −0.38 | −0.26 | 0.02 | −0.30 | −0.67 | −0.34 | −0.44 |
|
Unadjusted
p Value |
0.74 | 0.15 | 0.34 | 0.94 | 0.27 | <0.01 | 0.20 | 0.09 |
|
Adjusted
Pearson r |
−0.08 | −0.29 | 0.07 | 0.31 | −0.14 | −0.55 | −0.27 | −0.25 |
|
Adjusted p
Value |
0.80 | 0.31 | 0.80 | 0.28 | 0.63 | 0.04 | 0.34 | 0.39 |
Table 4.
Unadjusted and BMI and age-adjusted correlations between BSMSS and [11C](+)PHNO BPND in CD participants
| Amygdala | Caudate | Hypothalamus | Pallidum | Putamen | SN/VTA | Thalamus | Ventral Striatum |
|
|---|---|---|---|---|---|---|---|---|
|
BPND
Mean (S.D.) |
0.33 (0.09) |
1.70 (0.45) |
1.42 (0.42) |
3.53 (0.51) |
2.32 (0.33) |
2.43 (0.58) |
0.36 (0.09) |
3.82 (0.75) |
|
Unadjusted
Pearson r |
−0.60 | −0.35 | −0.37 | −0.28 | −0.39 | −0.50 | −0.35 | −0.50 |
|
Unadjusted
p Value |
0.01 | 0.18 | 0.16 | 0.30 | 0.13 | 0.05 | 0.19 | 0.05 |
|
Adjusted
Pearson r |
−0.55 | −0.17 | −0.33 | −0.19 | −0.26 | −0.41 | −0.28 | −0.38 |
|
Adjusted p
Value |
0.04 | 0.57 | 0.25 | 0.53 | 0.36 | 0.15 | 0.34 | 0.18 |
4. DISCUSSION
To our knowledge, the current study is the first to examine the relationship between social status and extrastriatal D2/3R availability in humans using the D3-preferring agonist radioligand, [11C](+)PHNO. This work found two major findings. The first was an inverse association between social status and D2/3R availability in the SN/VTA of HH participants. This relationship retained significance after adjusting for the known effects of age (Correa, 2014; Ishibashi et al., 2009; Kim et al., 2011; Volkow et al., 2000) and BMI (Caravaggio et al., 2013; Kessler et al., 2014; Wang et al., 2001) on D2/3R availability. Secondly, this study is the first to examine social status and D2/3R availability in a human CD population utilizing PET. Interestingly, similar inverse associations were observed between social status and D2/3R availability in the amygdala, SN/VTA, and VST of CD participants, findings that remained significant in the amygdala alone after age and BMI correction.
Our findings in HH participants suggest a negative association between social status and extrastriatal D2/3R availability whereas prior work focusing on the striatum suggested D2/3R availability was positively correlated with social status and years of education in HHs (Martinez et al., 2010) and social dominance in non-human primates (Czoty et al., 2010; Morgan et al., 2002). Several factors may be responsible for these differences, but perhaps most notably, our study employed a different radiotracer with unique properties compared with prior work.
As discussed previously, [11C](+)PHNO has been shown to have a preference for binding to the D3R subtype as compared to the D2R subtype. This preference, along with excellent signal to noise properties, enables the visualization/quantification of dopamine receptors in a number of important extrastriatal regions such as the SN/VTA, where the D3R predominates (Graff-Guerrero et al., 2008; Narendran et al., 2006; Searle et al., 2010; Tziortzi et al., 2011). Thus, our findings here are the first to report on social status in this region, and the negative correlation observed may be accounted for by relatively different roles of D3R vs. D2R subtypes with respect to the trait. Moreover, from a neuroanatomic perspective, the SN/VTA contains dopamine cell bodies that project to the striatum and differences in the regulation of somatodendritic D2/3Rs as compared to those in nerve terminal regions, such the striatum, could also exist in relation to social status.
In addition, “high-affinity” forms of the D2/3R, for which [11C](+)PHNO has a specific preference, may be regulated differently in relation to social status compared to the total D2/3R binding as imaged by antagonist tracers in prior studies. In fact, prior studies in healthy and CD humans suggest differences in striatal D2/3R availability as imaged by antagonist vs. agonist tracers (Narendran et al., 2011; Payer et al., 2014). Another potential explanation for discrepancies between this and prior studies relates to ligand-related differences in vulnerability to competition from endogenous dopamine. As an agonist tracer, [11C](+)PHNO has been shown to be considerably more sensitive to endogenous dopamine fluctuations and PET tracer displacement than [11C]raclopride (Gallezot et al., 2014a; Ginovart et al., 2006; Shotbolt et al., 2012; Willeit et al., 2008). In fact, in the absence of other pharmacologic interventions such as the depletion of dopamine by the tyrosine-hydroxylase inhibitor, alpha-methly-para-tyrosine (AMPT; Abi-Dargham et al., 2000; Caravaggio et al., 2014; Laruelle et al., 1997; Martinez et al., 2009), BPND measures reflect tracer binding to available receptors (i.e., those not currently bound by endogenous transmitters (Innis et al., 2007)). As such, our receptor availability findings in the SN/VTA could be a reflection, in whole or in part, of higher levels of endogenous dopamine displacing the tracer (evidenced by lower BPND) and thus providing preliminary evidence that social status might influence dopamine neurotransmitter synthesis/release, a finding that [11C]raclopride within the striatum might not be able to detect due to the differences in tracers (i.e., antagonist vs. agonist) and anatomy (i.e., striatum vs. SN/VTA)
In addition to tracer effects, characteristics of the study cohorts may also be contributing to the observed differences. For example, average BSMSS scores were considerably higher in our HH participants (45 ± 10) as compared to those in prior work (33 ± 5; Martinez et al., 2010). Additionally, age-related differences between the present work and the work of Martinez and colleagues (40±9 years old vs. 30±4, respectively; Martinez et al., 2010)) could also have had an effect (Correa, 2014; Ishibashi et al., 2009; Kim et al., 2011; Volkow et al., 2000).
This work in CD, the first in humans, demonstrated an inverse association between social status and D2/3R availability in the VST, the SN/VTA, and the amygdala prior to adjusting for age and BMI. We corrected for these potential influences based on prior findings (Caravaggio et al., 2013; Correa, 2014; Ishibashi et al., 2009; Kessler et al., 2014; Kim et al., 2011; Volkow et al., 2000; Wang et al., 2001) and statistical significance was retained in the amygdala alone. No significant interactions were seen with HH and CD cohorts using ANCOVA models however, allowing the possibility that no differences exist between these groups in relation to social status and regional BPND values. While the amygdala finding is intriguing with respect to social status in CD, caution is warranted given the low BPND values (i.e., low signal to noise) and the exploratory context of this study.
Prior work in nonhuman primates indicated that chronic periods of cocaine self-administration might obscure the preexisting relationship between social status and D2/3R availability in striatal areas (Czoty et al., 2004). Even though we also found no relationship in the dorsal striatum in CD, it is difficult for direct comparisons between these works for two reasons, including 1) the previously discussed absence of a relationship between social status and striatal D2/3R availability in our human HH cohort and 2) the focus on a clinical population. The latter has important caveats such as modifications in experimental procedures and environmental control. Nonhuman studies have the ability to control cocaine access and social interactions as well as to obtain more direct measurements of social status, constraints not possible in clinical studies.
There are several potential limitations of our methods that merit discussion. The first is the use of the BSMSS as a measure of social status. The BSMSS was developed as a 1989 update to the earlier Hollingshead index (Hollingshead, 1975); as a result, several occupations queried by the BSMSS are obsolete (e.g., typist) or not reflective of current professions (e.g., webmaster or software engineer, etc.). Thus, updated versions of the scale and/or alternative measures/approaches to assessing social status may benefit future studies of the relationship between social status and D2/3R availability. The second limitation could be the confounding effects of nicotine and alcohol consumption. Although prior work utilizing [11C](+)PHNO has demonstrated that both alcohol (Erritzoe et al., 2014) and nicotine (Le Foll et al., 2014) influence [11C](+)PHNO binding, we failed to find any associations between daily nicotine intake (i.e., cigarettes smoked) or weekly alcohol consumption and regional measures of BPND in either cohort, suggesting the specificity of associations between BSMSS score and regional BPND. Notwithstanding, we did observe a correlation between weekly alcohol consumption and BSMSS scores within the CD but not HH cohort. However, this correlation was driven by three CD participants who reported drinking alcohol in excess of three standard deviations from the mean as compared to the rest of the cohort (i.e., those three subjects reported drinking between 32 and 109 drinks per week whereas the mean for the rest of the CD cohort was 4 ± 4 drinks per week). If these individuals are removed from the cohort this correlation is no longer observed, while the negative linear association between social status and D2/3R availability persists. This suggests alcohol consumption is not a crucial variable in our main study findings of BSMSS scores and BPND. That stated, future work would benefit from more rigorous matching of subjects for alcohol and nicotine to definitively rule-out such confounds. The third limitation involves the use in the current study of a single radiotracer, thus complicating direct comparisons with previous studies using different radioligands and leaving unresolved questions. Future studies employing multiple tracers in a within-subject design (e.g., where the same subjects are scanned with both [11C](+)PHNO and [11C]raclopride) will help improve our understanding of how D2/3R availability relates to social status. Finally, pharmacological interventions (e.g., AMPT) that eliminate the potentially confounding influence of endogenous dopamine on PET measures of radiotracer binding/receptor availability may also shed light on such relationships. In combination, such approaches would constitute a rigorous design for assessing how social status relates to endogenous dopamine brain levels in different clinical and non-clinical samples.
In summary, this study is the first to examine the relationship between social status and D2/3R availability in HH and CD humans with the D3-preferring, D2/3R agonist radioligand, [11C](+)PHNO. These data demonstrate that inverse associations exist between social status and extrastriatal D2/3R availability in both HH (SN/VTA) and CD individuals (amygdala) when adjusting for age and BMI. Although this work is exploratory in nature and warrants replication in larger, more statistically powered cohorts, these results suggest novel relationships not previously observed between social factors and brain reward pathways, a finding congruent with the notion of the human brain as a “social organ” (Cozolino, 2014). Furthermore, this finding implies that social status is an important variable that should be considered when investigating reward system deficiencies such as addiction.
Highlights.
This paper examines social status and D2/3R availability in humans
Healthy and cocaine dependent individuals were studied with the tracer [11C](+)PHNO
Inverse associations between social status and D2/3R availability were observed
These areas included D3R rich brain reward areas in both groups
These findings demonstrate a novel relationship between social status and D2/3R
Acknowledgments
This work was supported by a NARSAD Young Investigator Award Grant (M132018; DM), the National Institute on Drug Abuse (NIDA) (K24 DA017899; 1R03DA027456-01; RTM; K12DA00167; JH; P20 DA027844; RTM, MNP), the National Institute of Mental Health (NIMH; T32 MH019961; DM/RTM), Yale PET Center, and YCCI Pilot Projects Utilizing Core Technologies and the Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut. This work was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest with respect to the content of this manuscript. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Boehringer Ingelheim, Lundbeck, Ironwood, Shire and INSYS; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie, Glaxo-SmithKline, Pfizer, and Psyadon pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for gambling entities, law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Conflict of interest statement
The authors report no conflicts of interest with respect to the content of this manuscript
Contributors
All authors have read and approve of submission of this manuscript to Drug and Alcohol Dependence. We would like to thank the staff of the Clinical Neuroscience Research Unit (CNRU) at Connecticut Mental Health Center (CMHC), the Hospital Research Unit (HRU) of the Yale Center for Clinical Investigation (YCCI) at Yale-New Haven Hospital (YNHH), the Yale PET Center, the Yale Magnetic Resonance Research Center (MRRC), and especially Julie Holub, Nina Levine, Jane Wanyiri, and Lauren Kantrovitz.
REFERENCES
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health. The challenge of the gradient. Am. Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association,Task Force on DSM-IV. Diagnostic And Statistical Manual Of Mental Disorders : DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Barratt W. The Barratt Simplified Measure of Social Status (BSMSS) measuring SES. 2006 http://wbarratt.indstate.edu/socialclass/Barratt_Simplifed_Measure_of_Social_Status.pdf.
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J. Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Nakajima S, Borlido C, Remington G, Gerretsen P, Wilson A, Houle S, Menon M, Mamo D, Graff-Guerrero A. Estimating endogenous dopamine levels at D and D receptors in humans using the agonist radiotracer [C]-(+)-PHNO. Neuropsychopharmacology. 2014;39:2769–2776. doi: 10.1038/npp.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Raitsin S, Gerretsen P, Nakajima S, Wilson A, Graff-Guerrero A. Ventral striatum binding of a dopamine d receptor agonist but not antagonist predicts normal body mass index. Biol. Psychiatr. 2013;77:196–202. doi: 10.1016/j.biopsych.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RE, Barker WC, Liow J-S, Johnson CA. Design of a motion compensation OSEM list-mode algorithm for resolution-recovery reconstruction of the HRRT; Conference Record, IEEE Nuclear Science Symposium and Medical Imaging Conference; Portland, OR. 2003. pp. 3281–3285. [Google Scholar]
- Correa E, Gallezot J, Gaiser EC, Cosgrove K, Ding Y, Potenza MN, Malison RT, Carson RE, Matuskey D. Age-related decreases in binding of the D3-preferring radioligand [11C](+)PHNO in healthy male and female volunteers. Biol. Psychiatr. Suppl. 2014;75:403S–426S. 406S. [Google Scholar]
- Cozolino LJ. The Neuroscience Of Human Relationships : Attachment And The Developing Social Brain. W.W. Norton & Company; New York: 2014. [Google Scholar]
- Czoty PW, Gage HD, Nader MA. Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology. 2010;208:585–592. doi: 10.1007/s00213-009-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology. 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Tziortzi A, Bargiela D, Colasanti A, Searle GE, Gunn RN, Beaver JD, Waldman A, Nutt DJ, Bani M, Merlo-Pich E, Rabiner EA, Lingford-Hughes A. In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology. 2014;39:1703–1712. doi: 10.1038/npp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, Slifstein M, Fowles K, Ding YS, Huang Y, Laruelle M, Carson RE, Rabiner EA. Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66:489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Kloczynski T, Weinzimmer D, Labaree D, Zheng MQ, Lim K, Rabiner EA, Ridler K, Pittman B, Huang Y, Carson RE, Morris ED, Cosgrove KP. Imaging nicotine- and amphetamine-induced dopamine release in rhesus monkeys with [(11)C]PHNO vs [(11)C]raclopride PET. Neuropsychopharmacology. 2014a;39:866–874. doi: 10.1038/npp.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallezot JD, Zheng MQ, Lim K, Lin SF, Labaree D, Matuskey D, Huang Y, Ding YS, Carson RE, Malison RT. Parametric Imaging and Test-Retest Variability of 11C-(+)-PHNO Binding to D2/D3 Dopamine receptors in humans on the high-resolution research tomograph PET scanner. J. Nucl. Med. 2014b;55:960–966. doi: 10.2967/jnumed.113.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Watanabe M, Di Paolo T, Falardeau P, Labrie F, Seeman P. The functional state of the dopamine receptor in the anterior pituitary is in the high affinity form. Endocrinology. 1985;117:690–697. doi: 10.1210/endo-117-2-690. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, Wilson AA. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J. Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, Kapur S, Wilson AA. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J. Cereb. Blood Flow Metab. 2007;27:857–871. doi: 10.1038/sj.jcbfm.9600411. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum. Brain Mapp. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol. Biochem. Behav. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Available from the Department of Sociology, Yale University; New Haven, CT: 1975. (working paper published by the author) [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Ishii K, Oda K, Kawasaki K, Mizusawa H, Ishiwata K. Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse. 2009;63:282–290. doi: 10.1002/syn.20603. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Zald DH, Ansari MS, Li R, Cowan RL. Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity. Synapse. 2014;68:317–320. doi: 10.1002/syn.21738. [DOI] [PubMed] [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH. Effects of age on dopamine D2 receptor availability in striatal subdivisions: a high-resolution positron emission tomography study. Eur. Neuropsychopharmacol. 2011;21:885–891. doi: 10.1016/j.euroneuro.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Laruelle M, D'Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17:162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Guranda M, Wilson AA, Houle S, Rusjan PM, Wing VC, Zawertailo L, Busto U, Selby P, Brody AL, George TP, Boileau I. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-+-PHNO PET study in humans. Neuropsychopharmacology. 2014;39:415–424. doi: 10.1038/npp.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff P. The two-state model of receptor activation. Trends Pharmacol. Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am. J. Psychiatry. 2009;166:1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Broft A, Van Heertum R, Kleber HD. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol. Psychiatry. 2010;67:275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Gallezot JD, Pittman B, Williams W, Wanyiri J, Gaiser E, Lee DE, Hannestad J, Lim K, Zheng MQ, Lin SF, Labaree D, Potenza MN, Carson RE, Malison RT, Ding YS. Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug Alcohol Depend. 2014;139:100–105. doi: 10.1016/j.drugalcdep.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J. Cereb. Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Agid O, Borlido C, Suridjan I, Rusjan P, Houle S, Remington G, Wilson AA, Kapur S. Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr. Res. 2011;131:63–68. doi: 10.1016/j.schres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am. J. Primatol. 2000;52:115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nader MA, Banks ML. Environmental modulation of drug taking: Nonhuman primate models of cocaine abuse and PET neuroimaging. Neuropharmacology. 2014;76:510–517. doi: 10.1016/j.neuropharm.2013.05.044. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Martinez D, Mason NS, Lopresti BJ, Himes ML, Chen CM, May MA, Price JC, Mathis CA, Frankle WG. Imaging of dopamine D2/3 agonist binding in cocaine dependence: a [11C]NPA positron emission tomography study. Synapse. 2011;65:1344–1349. doi: 10.1002/syn.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, Boileau I. Heightened D(3) dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2014;39:311–318. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Mugnaini M, Griffante C, Wilson AA, Merlo-Pich E, Houle S, Gunn R, Rabiner EA, Laruelle M. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol. Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl .20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, Plisson C, Miller SR, Huiban M, Beaver JD, Gunn RN, Laruelle M, Rabiner EA. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J. Cereb. Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Front. Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, Felder C, Gatley SJ, Ding YS, Hitzemann R, Pappas N. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am. J. Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, Seeman P, Wilson AA, Kapur S. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J. Cereb. Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]