Abstract
Objective
To examine the independent and combined influences of late-life cognitive activity (CA) and physical activity (PA) on risk of incident mild cognitive impairment (MCI).
Methods
We used interval censored survival modeling to examine risk of incident MCI (Clinical Dementia Rating (CDR)=0.5) as a function of CA (high vs. low) and at least moderate intensity PA (any vs. none) among 864 cognitively normal (CDR=0) older adults.
Results
During three annual follow-up waves, 72 participants developed MCI. Compared to low CA with no PA, significant reductions in risk for MCI were observed for high CA with any PA (HR=0.20, 95% CI 0.07–0.52) and low CA with any PA (HR=0.52, 95% CI 0.29–0.93), but not for high CA without PA (HR=0.94, 95% CI 0.45–1.95).
Conclusions
These findings suggest that a combination of CA and PA may be most efficacious at reducing risk for cognitive impairment.
Keywords: Mild cognitive impairment, cognitive activity, physical activity, cohort study, epidemiology, observational study
1. INTRODUCTION
Mild cognitive impairment (MCI) is an intermediate cognitive state between normal aging and dementia [1]. Importantly, individuals with MCI are more likely to progress to dementia than their cognitively normal counterparts [1]. Developing and testing lifestyle interventions is a priority action of the National Alzheimer’s Project Act (NAPA) to potentially prevent or treat Alzheimer’s disease and related dementias. Two of the most promising lifestyle behaviors are: 1) engaging in cognitively stimulating activities, and 2) above average levels of physical activity [2]. Indeed, if it were possible to reduce the prevalence of cognitive or physical inactivity by 10–25%, this could potentially lower the number of AD cases by up to nearly 1 million worldwide [3]. Observational studies suggest that engaging in activities requiring mental effort confers a reduced risk of both MCI [4, 5] and dementia [6–8], and more physically active older adults have a lower risk of MCI [9] and dementia [10–13] than those who are not as active. Because intervention studies have yielded mixed results when examining the separate effects of cognitive [14] and physical [15] activity, there is now growing interest in combined intervention strategies [16, 17].
There is sparse observational research examining the independent value of cognitive and physical activity, or whether these activities when combined have larger and/or broader benefits to risk of cognitive impairment than either alone. The purpose of this observational study was to examine the independent and combined effects of cognitive and physical activity on risk for incident MCI in a population-based cohort study.
2. METHODS
2.1 Participants
Participants were members of a prospective cohort study designed to investigate MCI in the community, locally known as the Monongahela-Youghiogheny Healthy Aging Team (MYHAT). Details of the study design and methods are published elsewhere [18]. Briefly, community-dwelling elders aged 65 years or older were randomly drawn from voter registration lists of select towns in a geographically defined region of Southwestern Pennsylvania between 2006 and 2008. A total of 2,036 participants were initially recruited; based on an age-education adjusted Mini-Mental Status Examination (MMSE) score < 21/30 [19], 54 were considered too cognitively impaired for a study of MCI. The remaining 1,982 had a mean age of 77.6 years, were 61% female, 94.8% White, and had a median education level of high school graduate; 41.1% had more than high school education. These participants were assessed in detail on a range of demographic, psychosocial (e.g., lifestyle activities, social support, depressive symptoms) and health-related (e.g., health history, function, medication use, health services utilization, and neuropsychological performance) measures and followed annually for 6 years. The MYHAT study protocol was reviewed and approved by the Institutional Review Board of the University of Pittsburgh and written informed consent was obtained from all participants.
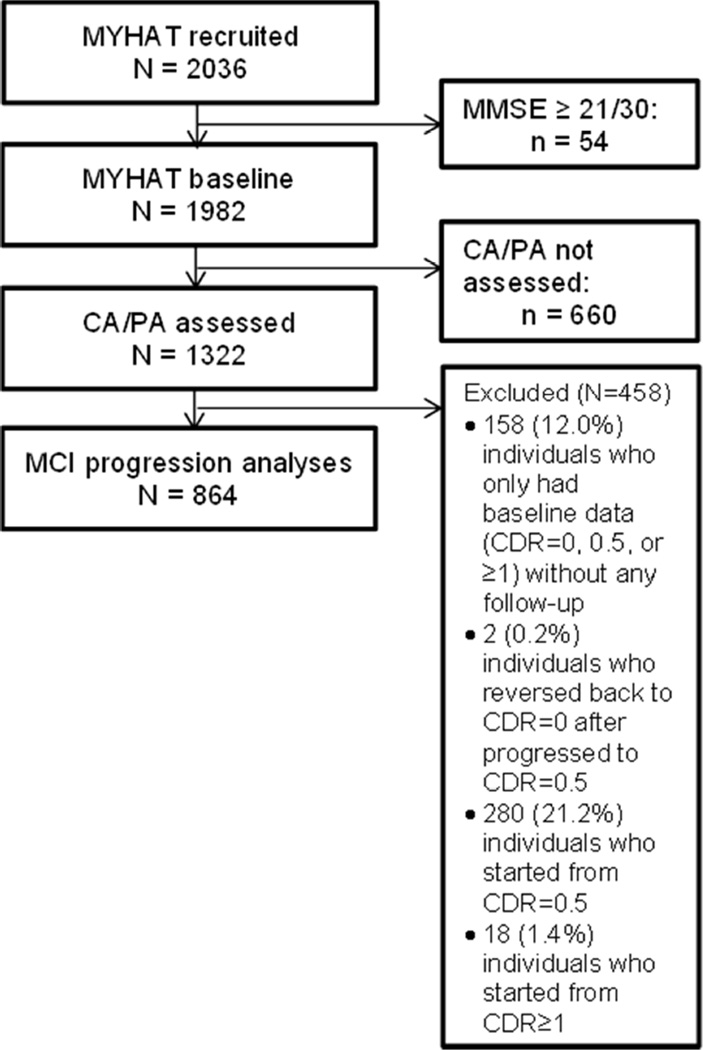
Because a key study measure, the Florida Cognitive Activities Scale (FCAS; described below), was added after the initial assessment, the wave at which a given participant was first assessed on the FCAS (second, third, or fourth wave) was considered his/her “baseline”; reducing the maximum follow-up to three years. Figure 1 shows the flow of participants into the present analysis. Participants with complete data on the measure of cognitive and physical activity (n=1,322) were initially eligible. We then excluded a total of 458 (34.6%) individuals: 158 (12.0%) who only had “baseline” data without any follow-up, 2 (0.2%) who reverted back to CDR=0 after progression to CDR=0.5 during subsequent follow-up, and 298 (22.5%) with prevalent cognitive impairment (CDR ≥0.5) at “baseline.” The remaining 864 were younger, had more education, more likely to be White, had a higher MMSE score, had fewer depressive symptoms, rated their health as very good or excellent, took fewer prescription medications, and engaged in high levels of CA and PA relative to the excluded subjects (Supplemental Table 1).
Figure 1.
Flow of MYHAT participants into the present analysis
2.2 Measures
2.2.1.Clinical Dementia Rating (CDR)
The Clinical Dementia Rating scale [20] stages dementia from none to severe. A summary CDR rating of 0 (no dementia), 0.5 (very mild dementia), and 1.0 through 3.0 (mild, moderate, and severe dementia) is generated using an algorithm that is weighted towards memory. For the present study, a CDR stage of 0.5, representing “very mild dementia”, was equated to MCI [21].
2.2.2. Cognitive Activity (CA)
The FCAS was used to assess participants’ engagement in cognitive activities. The FCAS is a validated, 25-item self-report scale designed to assess the frequency of engagement in a broad range of activities with differing cognitive demands [22] (e.g., “watching television”, “taking a course”). Response choices range from “never did, or used to, but not in the past year” (score=0) to “every day” (score=4) for a total possible score range of 0–100. For the present study, we examined the total scale score that we dichotomized at the median value of 44, as low (≤ 44) vs. high (> 44) CA.
2.2.3. Physical Activity (PA)
Our measure of PA was based on participants’ self- report (yes (score=1) vs. no (score =0)) of obtaining at least moderate intensity exercise from their normal physical activities, such as walking, climbing stairs, doing housework, performing child care duties, dancing, or gardening or yard work.
2.2.4. Covariates
We considered the following variables as potentially confounding factors: age (continuous, centered at median age 78), gender, race (White vs. non-White), and education (≤ high school vs. > high school). General mental status was measured with the Mini Mental Status Exam (MMSE; [23], continuous). Depressive symptoms were measured with a modified version of the mCES-D [24] (possible range 0–20) with the 90th percentile score of 1 used as the threshold for depressive symptoms. We also included measures of central obesity (waist-to-hip ratio, continuous), subjective rating of health (poor/fair/good vs. very good/excellent), and number of prescription medications dichotomized at the sample median value of 4 (0–3 vs. ≥ 4).
2.3 Statistical Analyses
We used Chi-square or Fisher’s Exact tests for categorical variables and Wilcoxon Rank Sum for continuous variables to compare the descriptive characteristics of those who did not and did progress from normal cognitive status to MCI, those with high and low engagement in cognitive activity, and those that did and did not report engaging in any physical activity.
We used interval-censored survival analyses [25] to estimate the associations of the cognitive and physical activity with the risk of incident MCI. Since participants were examined annually, an incident event can only be known to have occurred between two measurement occasions, i.e., during that interval. Our first model examined the association of CA with MCI without adjustment for PA. The second model examined the association of PA with MCI without adjustment for CA. Third, we included both cognitive and physical activity in the same model to examine their unique, i.e., independent, contributions to risk for incident MCI.
In our fourth model, we examined the combined effect of cognitive and physical activity on risk of incident MCI by creating 4 categories of participation according to their level of engagement in cognitive activity (low vs. high) and physical activity (none vs. any). We then tested the three combined CA and PA groups relative to the reference group of low CA and no PA.
All interval-censored survival models were adjusted for age, gender, and education. Additional covariates were included in the model if they met the definition of confounding, i.e., if they were associated with the exposure (either cognitive or physical activity) and with the outcome (progression to MCI) (p < 0.05). To improve efficiency of the interval-censored survival models, we imputed missing values for depressive symptoms, self-rated health, and waist-to-hip ratio 5 times to create 5 complete data sets. We then ran the survival models on each of the 5 created data sets, and calculated the average estimates of the risk effects from these 5 fitted models. Statistical analysis software (SAS) version 9.3 [26] was used for all analyses.
3. RESULTS
During the follow-up period (mean 1.8 years, SD=0.74, range 1–3 years), 72 individuals (8.3%) progressed from normal cognition to MCI. Table 1 shows the characteristics of all study participants at their own “baselines” and by progression status. CA was normally distributed with a mean and median score of 44.0 (SD=9.2, range 17–75). PA was more skewed in distribution because the majority of participants (69%) reported engaging in PA. Those who progressed to MCI were more likely to be older, have less education, score lower on the MMSE, describe their health as fair or poor, and engage in low cognitive activity and no physical activity.
Table 1.
Characteristics of the study sample at baseline by incident MCI status (N = 864)
| Total sample (N = 864) |
Cognitively Normal (N = 792) |
Incident MCI (N = 72) |
Test statistic§, p-value |
|
|---|---|---|---|---|
| Age, mean (SD) | 78.3 (6.8) | 77.9 (6.7) | 82.0 (6.8) | 4.74, <0.0001 |
| Gender, n (%) Female | 547 (63.3) | 497 (62.8) | 50 (69.4) | 1.27, 0.2593 |
| Education, n (%) > HS | 403 (46.6) | 381 (48.1) | 22 (30.6) | 8.17, 0.0043 |
| Race, n (%) Non-white | 35 (4.1) | 31 (3.9) | 4 (5.6) | 0.5252 |
| MMSE Score, mean (SD) | 27.9 (1.9) | 28.0 (1.8) | 26.8 (2.3) | −5.06, <0.0001 |
| Depression Symptoms (N = 863), n (%) ≥ 1 symptoms | 125 (14.5) | 113 (14.3) | 12 (16.7) | 0.30, 0.5826 |
| Self-rated Health (N = 863), n (%) very good/excellent | 370 (42.9) | 349 (44.1) | 21 (29.2) | 6.03, 0.0141 |
| Prescription Medications, n (%) ≥ 4 medications | 479 (55.4) | 432 (54.6) | 47 (65.3) | 3.08, 0.0794 |
| Waist-to-hip Ratio, mean (SD) (N = 828) | 0.899 (0.087) | 0.898 (0.086) | 0.900 (0.100) | −0.25, 0.7999 |
| Cognitive Activity, mean (SD) | 44.0 (9.2) | 44.4 (9.1) | 39.2 (8.8) | −4.57, <0.0001 |
| Cognitive Activity, n (%) High | 422 (48.8) | 404 (51.0) | 18 (25.0) | 17.87, <0.0001 |
| Physical Activity, n (%) Yes | 597 (69.1) | 564 (71.2) | 33 (45.8) | 19.91, <0.0001 |
The continuous variables were examined by the Wilcoxon rank sum test and the categorical variables were examined by the Chi-squared test or Fisher's exact test when appropriate.
We then compared the characteristics of those with low and high CA (Table 2) and those who did and did not engage in any PA (Table 3). Fifty-four participants (12.2%) of those with low CA and 18 (4.3%) of those with high CA became incident cases of MCI, respectively. High CA was associated with all covariates of interest; these participants were more likely to be younger, female, White, have more than high school education, score higher on the MMSE, have fewer depressive symptoms, self-rate their health as very good or excellent, take fewer prescription medications, have lower waist-to-hip ratio, and engage in PA. Those who reported being physically active were more likely to be male, self-rate their health as very good or excellent, take fewer prescription medications, and engage in a high level of CA. A greater proportion of incident MCI cases was observed for those with no PA (n=39, 14.6%) compared to those engaging in any PA (n=33, 5.5%).
Table 2.
Characteristics of those with low and high Cognitive Activity (CA)
| Low CA (N = 442; n=54 [12.2%] MCI incidence) |
High CA (N = 422; n=18 [4.3%] MCI incidence) |
Test-statistic§, P-value |
|
|---|---|---|---|
| Age, mean (SD) | 79.8 (6.9) | 76.7 (6.2) | −6.63, <0.0001 |
| Gender, n (%) Female | 252 (57.0) | 295 (69.9) | 15.45, <0.0001 |
| Education, n (%) > HS | 170 (38.5) | 233 (55.2) | 24.34, <0.0001 |
| Race, n (%) Non-white | 25 (5.7) | 10 (2.4) | 6.00, 0.0143 |
| MMSE Score, mean (SD) | 27.5 (2.0) | 28.4 (1.6) | 7.75, <0.0001 |
| Depression Symptoms (N = 863), n (%) ≥ 1 symptoms | 78 (17.7) | 47 (11.1) | 7.47, 0.0063 |
| Self-rated Health (N = 863), n (%) very good/excellent | 157 (35.6) | 213 (50.5) | 19.48, <0.0001 |
| Prescription Medications, n (%) ≥ 4 medications | 273 (61.8) | 206 (48.8) | 14.65, 0.0001 |
| Waist-to-hip Ratio, mean (SD) (N = 828) | 0.909 (0.086) | 0.888 (0.088) | −3.34, 0.0008 |
| Physical Activity, n (%) Yes | 279 (63.1) | 318 (75.4) | 15.13, 0.0001 |
The continuous variables were examined by the Wilcoxon rank sum test and the categorical variables were examined by the Chi-squared test or Fisher's exact test when appropriate.
Table 3.
Characteristics of those who do and do not engage in Physical Activity (PA)
| No PA (N = 267; n=39 [14.6%] MCI incidence) |
Any PA (N = 597; n=33 [5.5%] MCI incidence) |
Test-statistic§, P-value |
|
|---|---|---|---|
| Age, mean (SD) | 78.8 (6.8) | 78.1 (6.7) | 1.56, 0.1196 |
| Gender, n (%) Female | 186 (69.7) | 361 (60.5) | 6.71, 0.0096 |
| Education, n (%) > HS | 121 (45.3) | 282 (47.2) | 0.27, 0.6016 |
| Race, n (%) Non-white | 12 (4.5) | 23 (3.9) | 0.20, 0.6584 |
| MMSE Score, mean (SD) | 28.1 (1.8) | 27.9 (1.9) | 1.59, 0.1127 |
| Depression Symptoms (N = 863), n (%) ≥ 1 symptoms | 37 (13.9) | 88 (14.7) | 0.10, 0.7489 |
| Self-rated Health (N = 863), n (%) very good/excellent | 93 (34.8) | 277 (46.5) | 10.21, 0.0014 |
| Prescription Medications, n (%) ≥ 4 medications | 163 (61.1) | 316 (52.9) | 4.92, 0.0265 |
| Waist-to-hip Ratio, mean (SD) (N = 828) | 0.898 (0.091) | 0.899 (0.085) | −0.44, 0.6564 |
| Cognitive Activity, mean (SD) | 42.1 (10.3) | 44.8 (8.6) | −4.03, <0.0001 |
| Cognitive Activity, n (%) High | 104 (39.0) | 318 (53.3) | 15.13, 0.0001 |
The continuous variables were examined by the Wilcoxon rank sum test and the categorical variables were examined by the Chi-squared test or Fisher's exact test when appropriate.
Our main analyses (Table 4) examined the separate associations of CA and PA with risk of incident MCI. The risk of incident MCI was reduced by 46% for those with high CA (HR = 0.54, 95% CI 0.29–0.99; model 1)), and by 61% for those who reported any PA (HR = 0.39, 95% CI 0.22–0.67; model 2). However, when their independent effects were examined (Model 3), the protective influence of CA was attenuated (HR = 0.62, 95% CI 0.34–1.12), while engaging in PA remained significantly associated with lower risk of MCI (HR = 0.41, 95% CI 0.24–0.71).
Table 4.
The Associations of Cognitive and Physical Activity with Risk of Incident Cognitive Impairment
| Variable | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| CA | 0.54 (0.29–0.99)b | 0.62 (0.34–1.12) | ||
| PA | 0.39 (0.22–0.67)a | 0.41 (0.24–0.71)a | ||
| Low CA, No PA | 1.00 (Ref.) | |||
| Low CA, Any PA | 0.52 (0.29–0.93)b | |||
| High CA, No PA | 0.94 (0.45–1.95) | |||
| High CA, Any PA | 0.20 (0.07–0.52)a | |||
| Age1 | 1.06 (1.02–1.11)a | 1.07 (1.02–1.11)a | 1.06 (1.02–1.10)a | 1.06 (1.02–1.10)a |
| Gender (Female vs. Male) | 1.64 (0.89–3.02) | 1.35 (0.81–2.26) | 1.51 (0.81–2.78) | 1.53 (0.83–2.82) |
| Education (≤ HS vs. > HS) | 0.87 (0.51–1.50) | 0.82 (0.48–1.40) | 0.88 (0.51–1.52) | 0.86 (0.50–1.49) |
| Race (Non-white vs. White) | 1.09 (0.38–3.10) | – | 1.06 (0.37–3.03) | 1.06 (0.37–3.02) |
| MMSE Score | 0.81 (0.72–0.92)a | 0.77 (0.68–0.88)a | 0.78 (0.69–0.89)a | 0.79 (0.69–0.90)a |
| Depression Symptoms (0 vs. ≥ 1) | 0.94 (0.50–1.77) | – | 0.95 (0.50–1.80) | 0.95 (0.50–1.79) |
| Self-rated Health (poor/fair/good vs. very good/excellent) | 0.67 (0.39–1.14) | 0.68 (0.40–1.15) | 0.70 (0.41–1.20) | 0.72 (0.42–1.23) |
| Prescription Medications (0–3 vs. ≥ 4) | 1.22 (0.74–2.01) | 1.22 (0.74–2.01) | 1.15 (0.69–1.89) | 1.17 (0.71–1.93) |
| Waist-to-hip Ratio | 2.67 (0.10–68.16) | - | 1.87 (0.07–46.67) | 1.97 (0.08–49.69) |
Centered at mean age (= 78);
p < 0.01;
p < 0.05
Note: All of the models were fitted by interval-censored survival models which were adjusted for age, gender, education, and significant covariates.
Among the 864 participants, combined engagement in CA and PA was as follows: 163 (18.9%) for low CA and no PA, 279 (32.3%) for low CA and any PA, 104 (12.0%) for high CA and no PA, and 318 (36.8%) for high CA and any PA. The combined effects of CA and PA (Model 4) show that, relative to low CA with no PA, any PA with low CA is protective against MCI (HR = 0.52, 95% CI 0.29–0.93), but that high CA with no PA is not (HR = 0.94, 95% CI 0.45–1.95). Participants who engaged in any PA and a high level of CA have the lowest risk of MCI (HR = 0.20, 95% CI = 0.07–0.52).
4. DISCUSSION
In or sample, we found CA and PA are separately associated with a lower risk of incident MCI. However, when their independent effects were examined (i.e. modeled simultaneously), only PA was significantly associated with MCI risk. We also observed that compared to engaging in low CA and no PA, the combination of high CA with any PA showed the greatest risk reduction followed by low CA with any PA. Engaging in high CA with no PA did not reduce risk relative to the reference group. Taken together, these findings suggest that PA may be more influential than CA in terms of reducing risk for cognitive impairment.
Our results are consistent with other observational reports that CA and PA both lower the risk of MCI and dementia [4–13], but add new information suggesting that the relative contribution of PA to risk of MCI is greater than CA, and that PA in combination with higher CA is most efficacious. Only one other observational study has directly examined the combined influence of CA and PA on risk for MCI, and they found decreased odds of having MCI for those engaging in both PA and computer use compared to neither activity [27]. There has been a recent push for combined intervention approaches [16, 17], although thus far, the results from trials have not been consistent. One combination intervention trial of cognitive training and PA reported that cognitive training to be the driving force behind the combined effects with aerobic activity [28], while another reported that combined mental and physical engagement did not improve cognitive performance more than the control conditions [29]. In contrast, our findings suggest that interventions to remediate cognitive decline or impairment in older adults may be most effective if physical activity is the primary focus and cognitive activity is used to enhance the effect. This contradiction may be related to difference in study designs (observational vs. randomized), sample characteristics (voter registration vs. volunteer), and measurement of physical and cognitive activity (self-report vs. intervention).
Progression from normal cognitive status to MCI over a short follow-up period is often reflective of an underlying brain disease (e.g., degenerative, vascular or both), which in and of itself could lead to decreased cognitive and physical activity. Since our followup was as short as one year in some participants, the findings could be interpreted as showing that lower engagement in CA and PA are epiphenomena or markers of prodromal cognitive impairment. The alternative explanation, that being physically active and engaging in a higher level of CA delays progression to cognitive impairment is also plausible. This is supported by observational studies with longer follow-up that have found these effects remain even after excluding participants who progress to dementia within a few years of assessment of CA and PA [4]. There is also growing support from separate CA [14, 30] and PA [31, 32] interventions showing improvements in cognitive functioning.
We did not investigate potential mechanisms of action, but engaging in activities that require mental and physical effort likely influence the brain’s ability to compensate for neural deficits related to aging or disease pathology; the now well-known concept of brain or cognitive reserve [33, 34]. Animal models of environmental enrichment, which provide the opportunity for both cognitive and physical activity, suggest that these activities affect brain reserve through cellular and molecular mechanisms. These enhance network plasticity and functional compensation, efficiency of information processing and storage, and connectivity and functional redundancy [35]. In humans, neuroimaging techniques have also shown both CA and PA are associated with structural brain changes, such as larger hippocampal volume, gray matter increases, and improved white matter microstructure [36], as well functional brain changes evidenced by greater activity patterns and connectivity [37]. There are data suggesting that CA [38], especially in early and mid-life, and PA [39] may affect AD-related pathology, although this is not supported by all studies [40].
We can only speculate why the effect of PA was stronger than CA for reducing risk for MCI. PA increases circulating levels of brain-derived neurotrophic factor, which supports neuronal growth, survival, and synaptic plasticity [35], and may also attenuate vascular risk factors (e.g. glucose intolerance, diabetes mellitus, hypertension, hypercholesterolemia, and obesity) known to contribute to vascular dementia and neurodegenerative dementia [37]. Although physically active older adults in MYHAT did not report less vascular morbidity (data not reported), it is possible that these participants have less subclinical vascular disease. Another possible explanation is that PA provides additional benefits to neurocognitive health by affecting areas of the brain controlling motor coordination [41].
The participants in this study are largely representative of older adults in Southwestern PA. The observed differences in cognition between those included versus those excluded may have led to an underestimation of the observed effects [42]. We based our measures of CA and PA on self-report, which could be influenced by recall or response bias [43]. However, our participants were all cognitively normal when their level of engagement in activities was measured reducing the likelihood that participants misreported. The FCAS, our measure of CA, is a scale that captures a variety of activities engaged in in real life and thus has greater ecological validity compared to prescribed activities. Dichotomizing CA based on the median score may have reduced the variability in this measure and our ability to detect an independent association with risk of cognitive impairment. We relied on a basic yes/no response to whether the participant engaged in everyday activities that provided at least moderate intensity physical activity. The intensity level of activities is subjective and could be subject to response bias. Further, we did not consider the overall frequency of engaging in these activities, however, many of the activities included in the question would likely be engaged in routinely. Despite this limitation, this measure better captures overall physical activity level better than a question focused specifically on “exercise,” that would missed physically active people who do not engage in intentional exercises. Future work needs to use a validated PA scale.
We selected the CDR as our outcome measure, which is specifically based on everyday functioning; this outcome has greater salience for the geriatric population for whom the critical goal is to preserve daily living. The CDR is a categorical entity weighted towards memory but cognitive decline occurs on a continuum across one or more of multiple cognitive domains. Our future analyses will examine whether cognitive and physical activities have general or specific effects across cognitive domains. Finally, even though we adjusted for potentially confounding factors, we acknowledge that the level of engagement in cognitive and physical activities is associated with other health behaviors that may also influence the risk of dementia. We also cannot rule out unmeasured confounding.
In summary, our data suggest that a combination of PA and CA in late life may delay the onset of cognitive impairment and that this effect may be influenced to a greater extent by PA relative to CA. Additional studies are needed to better understand the parameters (i.e. type, duration, intensity, timing) of these activities, separately and combined, that are most effective, and, perhaps most important, who benefits. This will require longitudinal studies with longer followup, perhaps from a life-course perspective or beginning in mid-life. Detailed assessment of CA and PA and careful interpretation of these data are critical when designing nonpharmacological intervention trials. Investigators should also consider embedding intervention trials within well-characterized representative cohorts, which could facilitate a priori selection of a minimally biased sample and/or provide information about differences between participants and non-participants that could be considered when interpreting the study results, and potentially adjusted for, in the analyses [44].
Supplementary Material
Research in Context.
Systematic Review: We reviewed observational studies and intervention trials that examined cognitive activity (CA) and physical activity (PA) in relation to mild cognitive impairment (MCI) and dementia. Few observational studies have examined the independent and combined effects of CA and PA, despite a recent push for combined intervention trials. Interpretation: The findings suggest that both CA and PA are protective against incident MCI, but when examined simultaneously, only the effect of PA remains. This suggests that PA is more important than CA as a modifiable factor to potentially remediate cognitive impairment. Our findings also suggest that the protective effect of PA is most efficacious if combined with higher levels of CA. Future Directions: A validated PA scale should be examined. Longer follow-up of participants is needed to rule out reverse causality. These lessons will have important implications for designing trials combining CA and PA to prevent or delay cognitive impairment.
Acknowledgements
This research was supported in part by funds from the National Institute on Aging to M.G. (R01 AG023651). Additional support came from the Alzheimer’s Disease Research Center at the University of Pittsburgh (AG05133). The authors are grateful for the efforts of Kathryn McMichael in project coordination, Jennifer Jakubcak in database administration, Jack Doman in academic computing support, and all of the MYHAT staff for recruitment, data collection, and data management. They also thank the 2036 senior citizens who participated in the study and made this work possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: None of the authors have any conflicts of interest to disclose.
Authors Role in the Research:
Tiffany F. Hughes, Ph.D. - Study concept, Analysis or interpretation of data, Drafting/revising the manuscript for content
James T. Becker, Ph.D. - Analysis or interpretation of data, Drafting/revising the manuscript for content
Ching-Wen Lee, Ph.D. - Analysis or interpretation of data
Chung-Chou H. Chang, Ph.D. - Analysis or interpretation of data
Mary Ganguli, M.D., M.P.H. - Study concept, Analysis or interpretation of data, Drafting/revising the manuscript for content
REFERENCES
- 1.Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment. Ten years later. Arch Neurol. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 3.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verghese J, LeValley A, Derby C, Kuslansky G, Katz M, Hall C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geda YE, Topazian HM, Roberts LA, Roberts RO, Knopman DS, Pankratz VS, et al. Engaging in cognitive activities, aging, and mild cognitive impairment: a population-based study. J Neuropsychiatry Clin Neurosci. 2011;23:149–154. doi: 10.1176/appi.neuropsych.23.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes TF, Chang C-CH, Vander Bilt J, Ganguli M. Cognitive activity and incident dementia in the community: The MoVIES Project. Am J Alzheimers Dis Other Demen. 2010;25:432–438. doi: 10.1177/1533317510368399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer’s disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- 9.Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 11.Bowen ME. A prospective examination of the relationship between physical activity and dementia risk in later life. Am J Health Promot. 2012;26:333–340. doi: 10.4278/ajhp.110311-QUAN-115. [DOI] [PubMed] [Google Scholar]
- 12.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer’s disease. JAMA. 2010;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etgen T, Sander D, Huntgeburth U, Poppert H, Förstl K, Bickel H. Physical activity and incident cognitive impairment in elderly persons. Arch Intern Med. 2010;170:186–193. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- 14.Martin M, Clare L, Altgassen AM, Cameroon MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011;1 doi: 10.1002/14651858.CD006220.pub2. CD006220. [DOI] [PubMed] [Google Scholar]
- 15.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thom JM, Clare L. Rationale for combined exercise and cognition –focused interventions to improve functional independence in people with dementia. Gerontology. 2011;57:265–275. doi: 10.1159/000322198. [DOI] [PubMed] [Google Scholar]
- 17.Jak AJ. The impact of physical and mental active on cognitive aging. Curr Topics Behav Neurosci. 2012;10:273–291. doi: 10.1007/7854_2011_141. [DOI] [PubMed] [Google Scholar]
- 18.Ganguli M, Snitz BE, Vander Bilt J, Chang C-CH. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry. 2009;24:1277–1284. doi: 10.1002/gps.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. 1996;46:700–706. doi: 10.1212/wnl.46.3.700. l. [DOI] [PubMed] [Google Scholar]
- 20.Hughes CP, Berg L, Danziger WL, Cohen LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 22.Schinka JA, McBride VA, Vanderploeg RD, Tennyson K, Borenstein AR, Mortimer JA. Florida Cognitive Activities Scale: initial development and validation. J Int Neuropsychol Soc. 2005;11:108–116. doi: 10.1017/S1355617705050125. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 26.SAS System for Microsoft Windows [computer software]. Version 9.3. Cary, NC: SAS Institute Inc; 2002–2010. [Google Scholar]
- 27.Geda YE, Silber TC, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, et al. Computer activities, physical exercise, aging, and mild cognitive impairment: A population-based study. Mayo Clin Proc. 2012;87:437–442. doi: 10.1016/j.mayocp.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front Aging Neurosci. 2013;5:8. doi: 10.3389/fnagi.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, et al. The Mental Activity and eXercise (MAX) Trial. A randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173:797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry. 2009;17:179–187. doi: 10.1097/JGP.0b013e3181953b57. [DOI] [PubMed] [Google Scholar]
- 31.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013 doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory MA, Gill DP, Petrella RJ. Brain health and exercise in older adults. Curr Sports Med Rep. 2013;12:256–271. doi: 10.1249/JSR.0b013e31829a74fd. [DOI] [PubMed] [Google Scholar]
- 33.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W, Yu J-T, Tan M-S, Tan L. Cognitive reserve and Alzheimer’s disease. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8720-y. [DOI] [PubMed] [Google Scholar]
- 35.Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: Mental and physical activity as modulators of brain disorders. Prog Neurobiol. 2009;89:369–382. doi: 10.1016/j.pneurobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Kirk-Sanchez NJ, McGough EL. Physical activity and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knopman DS, Roberts R. Vascular risk factors: imaging and neuropathologic correlates. J Alzheimers Dis. 2010;20:699–709. doi: 10.3233/JAD-2010-091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landau SM, Marks SM, Morimino EC, Rabinovici GD, Oh H, O’Neil JP, et al. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch Neurol. 2012;69:623–629. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang KY, Mintun MA, Fagan Am, Goate AM, Bugg JM, Holtzman DM, et al. Exercise and Alzheimer’s Disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, et al. Effects of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neural. 2012;72:730–738. doi: 10.1002/ana.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattler C, Erickson KI, Toro P, Schroder J. Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. J Alzheimers Dis. 2011;26:709–718. doi: 10.3233/JAD-2011-110548. [DOI] [PubMed] [Google Scholar]
- 42.Matthews FE, Chatfield M, Freeman C, McCracken C, Brayne C. MRC CFAS. Attrition and bias in the MRC cognitive functioning and ageing study: an epidemiologic investigation. BMC Public Health. 2004;4:12. doi: 10.1186/1471-2458-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. Gaithrsburg, MD: Aspen Publishers, Inc; 2000. [Google Scholar]
- 44.Ganguli M, Lee C-W, Hughes TF, Snitz BE, Jakubcak J, Duara R, Chang C-CH. Who wants a free brain scan? Assessing and correcting for recruitment biases in a population-based sMRI pilot study. Brain Imaging Behav. 2014 doi: 10.1007/s11682-014-9297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.