Abstract
Trait impulsivity and poor inhibitory control are well-established risk factors for alcohol misuse, yet little is known about the associated neurobiological endophenotypes. Here we examined correlations among brain physiology and self-reported trait impulsive behavior, impaired control over drinking, and a behavioral measure of response inhibition. A sample of healthy drinkers (n=117) completed a pulsed arterial spin labeling (PASL) scan to quantify resting regional cerebral blood flow (rCBF), and measures of self-reported impulsivity (Eysenck I7 Impulsivity scale) and impaired control over drinking. A subset of subjects (n=40) performed a stop signal task during blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging to assess brain regions involved in response inhibition. Eysenck I7 scores were inversely related to blood flow in the right precentral gyrus. Significant BOLD activation during response inhibition occurred in an overlapping right frontal motor/premotor region. Moreover, impaired control over drinking was associated with reduced BOLD response in the same region. These findings suggest that impulsive personality and impaired control over drinking are associated with brain physiology in areas implicated in response inhibition. This is consistent with the idea that difficulty controlling behavior is due in part to impairment in motor restraint systems.
Keywords: Cerebral blood flow, fMRI, Inhibitory control, Stop task, Alcohol
1. Introduction
Impulsivity, which refers broadly to acting without thinking, is a widely accepted risk factor for alcohol abuse (Potenza and de Wit, 2010). Measuring impulsive personality traits encompasses several distinct conceptual and methodological factors. One such factor that has been repeatedly implicated in alcohol abuse is difficulty controlling or inhibiting inappropriate behavior. Such poor behavioral control can be assessed by both self-report personality inventories and behavioral laboratory measures. On personality inventories, greater self-reported difficulty controlling behavior or acting without forethought is associated with increased drug and alcohol use (Petry, 2001; Finn, 2002). Behavioral measures of inhibitory control include stop signal and go/no-go tasks, which measure the ability to inhibit prepotent or instigated motor behavior, such as a finger press. Poor response inhibition on these tasks has also been repeatedly linked with greater alcohol use and problems (Bjork et al., 2004; Nigg et al., 2006; Rubio et al., 2008).
One potential explanation for the increased risk of alcohol-related problems in impulsive individuals is a specific instance of impaired impulse control: impaired control over drinking. Impaired control refers to a decreased ability to limit or abstain from alcohol consumption despite persistent intentions to do so (Heather et al., 1993). Impaired control is a well-established feature of problematic alcohol use, with two DSM-V criteria for alcohol use disorders that reflect impaired control (i.e., drinking greater amounts than intended and inability to quit or control drinking; American Psychiatric Association, 2013). Moreover, impaired control is becoming increasingly recognized as a problem for young adult drinkers, as this is one of the first symptoms endorsed by those transitioning from social- to dependent-drinking (Leeman et al., 2012; Leeman et al., 2014).
Impaired control and impulsivity/behavioral under-control are conceptually linked, in that impaired control refers to difficulty controlling the specific behavior of alcohol consumption. As such, it is reasonable to assume that individuals who have a general difficulty controlling behavior or inhibiting inappropriate responses might also display impaired control over drinking. Indeed, initial studies show correlations between impaired control and both self-report and behavioral measures of inhibitory control (for review, see Leeman et al., 2012). However, little is known about the neurobiological endophenotypes of trait impulsivity in general, and impaired control specifically. Understanding the neural correlates of impulsive traits and impaired control could have important implications for identifying individuals at risk for alcohol use disorders, as well as in developing treatments.
In a reasonably large sample of 117 healthy subjects who spanned a range of drinking, the current study examined anatomic regions in which measures of brain physiology were correlated with self-reported trait impulsive behavior in general, impaired control over drinking specifically, and a behavioral measure of motor response inhibition. Specifically, we identified regions where impulsive personality and impaired control correlated with resting cerebral blood flow. Additionally, we examined fMRI blood oxygenation level-dependent (BOLD) activation during a response inhibition (stop signal) task as a function of impaired control. Given previous evidence about brain areas involved in response inhibition (Congdon et al., 2010; Bari and Robbins, 2013; Rae et al., 2014), we expected trait impulsivity and impaired control to be associated with less activity in right frontal regions.
2. Methods
2.1. Subjects
The sample of 117 right-handed regular drinkers (98 men and 19 women), ranging from moderate to heavy, participated in one of three previous studies (functional magnetic resonance imaging or positron emission tomography) conducted at the Indiana University School of Medicine and the Indiana Alcohol Research Center. Subjects were recruited by community advertisements and provided informed consent as approved by the Indiana University Institutional Review Board. The study was carried out in accordance with the Declaration of Helsinki. Interested volunteers were first screened by phone and then completed an in-person interview to determine medical history and current and past drug and alcohol use. Exclusion criteria included self-reported neurological disorders (injury, disease) of cerebral origin, and any major DSM-IV Axis I psychiatric disorder (aside from alcohol abuse/dependence), including drug dependence. All participants had a zero breath alcohol content at the time of study. Six participants had a positive drug screen (marijuana/THC, n=4; opiates, n=1; TCA, n=1); consequently, the data were analyzed with and without these participants. No differences were observed, and results reported here are based on the entire sample.
2.2. Measures
2.2.1. Alcohol use measures
During the in-person interview, subjects were given the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994), a semi-structured interview that assesses the symptoms of an alcohol use disorder (AUD). The SSAGA was used to create a total AUD symptom count as outlined in the current DSM-V. Subjects also completed the Timeline Follow-back (TLFB; Sobell and Sobell, 1992), a self-reported retrospective timeline calendar of alcohol consumption, estimating the number of standard drinks consumed each day over the past 90 days. From this we calculated participants’ average number of drinks per week and average number of drinks per drinking day. In addition, subjects completed the Alcohol Use Disorder Identification Test (AUDIT; Babor et al., 1989), a 10-item self-report measure that assesses patterns of drinking, dependence, and alcohol-related problems. Scores on the AUDIT range from 0 (no alcohol-related problems) to 40 (most severe alcohol-related problems), with ≥ 8 being a commonly used threshold for hazardous drinking (Babor et al., 2001).
2.2.2. Impulsive personality
Impulsive personality was assessed using the Eysenck I7 Impulsiveness Questionnaire (Eysenck et al., 1985). The impulsivity subscale consists of 19 yes/no questions related to acting on impulse (e.g., ‘Do you often do and say things without stopping to think; Do you need to use a lot of self-control to keep out of trouble?’), with a total possible score range of 0–19. As originally reported by Eysenck et al. (1985) for adults aged 20–29, normative values are 7.9 (SD=4.1) for males and 9.0 (SD=4.2) for females.
2.2.3. Impaired control over drinking (IC)
Subjects were classified as having impaired (IC) or no impaired control (no-IC) over their drinking based on the following two DSM-V symptoms of alcohol dependence as derived from the SSAGA: 1) drinking in larger amounts, or for over a longer period than intended, and 2) persistent desire, or one or more unsuccessful efforts, to cut down or control drinking. Subjects endorsing one or both of these symptoms were grouped in the IC group, and subjects endorsing neither were grouped in the no-IC group.
2.2.4. Response inhibition
A subset of subjects (n=40, 39 of whom were included in Kareken et al. (2013))1 performed a stop signal task during blood oxygenation level-dependent (BOLD) fMRI to assess brain activation during response inhibition. Task procedures are described in detail by Kareken et al. (2013). Briefly, participants responded as quickly as possible to Go signals (horizontal green arrows), but attempted to withhold their responses on trials in which a Stop signal (vertical red arrow) appeared subsequent to the Go signal. An adaptive staircase algorithm adjusted the delay between the Go and Stop signals to target a stop failure rate of 50%. Stop signal reaction time (SSRT), an estimate of the time needed to stop (withdraw) the Go response, was calculated by subtracting a subject’s average stop-signal delay from that subject’s xth percentile Go RT (correct trials only), where x corresponds to the stop failure rate (Band et al., 2003).
2.3. Procedure
Procedural details specific to the individual studies are reported elsewhere (Kareken et al., 2012; Kareken et al., 2013; Oberlin et al., 2013). All subjects completed the self-report measures of alcohol consumption and impulsivity during their initial study sessions at the Indiana Clinical Research Center. Subjects then returned for either one (Kareken et al., 2012; Oberlin et al., 2013) or two (Kareken et al., 2013) magnetic resonance imaging (MRI) sessions. Detailed timelines of the specific MRI protocols for each of these studies are provided in Supplementary Fig. 1. All subjects completed a Pulsed Arterial Spin Labeling (PASL) scan to measure resting regional cerebral blood flow (rCBF) as part of the MRI protocol.
Those subjects participating in the stop signal functional MRI (fMRI) study were imaged under both intravenous alcohol and saline infusions in counter-balanced order (Kareken et al., 2013). Here we re-examined the BOLD fMRI data from these participants as a function of impaired control over drinking, but using data from the saline condition only. This permitted an examination of the overlap between regions where resting rCBF was associated with Eysenck I7 and areas where impaired control over drinking affected BOLD activation during response inhibition. PASL scans from this stop signal study were obtained at baseline (i.e., before saline infusion).
2.4. Imaging
Subjects were imaged on a Siemens (Erlangen, Germany) 3 T Magnetom Trio-Tim scanner equipped with a 12-channel head coil array. A T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence was used to acquire high-resolution anatomical images (1.0×1.0×1.2 mm3 voxels) for co-registration and normalization to the Montreal Neurological Institute (MNI) coordinate system.
PASL scans (5:45 min duration) measured rCBF (ml/100 g/min) using a one-compartment model (Wang et al., 2003), and were acquired using Q2TIPS pulse sequence (Luh et al., 1999) labeling scheme, as detailed in Wang et al. (2011) with a 64 label-control pair readout (single-shot gradient-echo echo planar imaging (EPI)); 18 ascending axial slices; matrix, 64×64; 3.75×3.75×6 mm3 voxels; GRAPPA acceleration factor 2; 3D prospective acquisition correction algorithm). During the PASL acquisition, subjects were instructed to relax with their eyes closed. To ensure that subjects remained awake and in a lightly attentive state throughout the scan, subjects were directed to press a button on a response box (Current Designs, Inc. Philadelphia, PA) when they heard a distinct tone (750 Hz, 750 ms long). This tone was played five times at a random time during 1-min intervals. As a criterion for inclusion, we required that participants respond to at least four of the five tones. Reaction time was not emphasized, and subjects were told that the tone and their response were solely to ensure that they were awake.
In the stop signal task subset, three BOLD contrast sensitive scans measured stop task responses (gradient echoplanar imaging, 193 volumes; repetition time, 2,000 ms; echo time, 29 ms; flip angle, 76°; 35 interleaved 3-mm-thick axial slices; matrix, 88×88; 2.5×2.5×3.0 mm3 voxels; GRAPPA acceleration factor 2; and 3D prospective acquisition correction algorithm).
2.5. Image processing
Images were preprocessed in SPM8 (Wellcome Trust Centre for Neuroimaging). For PASL data, SPM segmentation of participants’ MPRAGE image was used to identify voxels containing at least 75% gray matter, and rCBF analyses were restricted to these voxels (Jahng et al., 2005). The segmentation was also used to convert rCBF volumes to the Montreal Neurological Institute (MNI) stereotactic space, where the resulting volumes were interpolated to 2-mm/side isotropic voxels and smoothed by a 6×6×8 mm full-width at half-maximum Gaussian kernel. To reduce inter-subject variability, each subject’s rCBF values were divided by that subject’s own gray matter rCBF averaged across the whole brain (see Pfefferbaum et al., 2010).
2.6. Data analyses
2.6.1. rCBF correlations
We first tested for differences in resting blood flow between participants with IV cannulae inserted (and who could have thus had some potential alcohol expectancy) and those without (see Supplementary Fig. 1). As we observed no differences, all participants were analyzed together. To estimate the relationship between Eysenck I7 scores and rCBF, we used an SPM8 regression analysis. Gender, recent drinking (drinks/week as reported on the TLFB), and smoking were included as covariates to control for previously reported gender differences in rCBF values (Gur et al., 1982; Gur et al., 1995; Wang et al., 2011) and any influence of alcohol consumption or smoking on rCBF. IC status was also included as a covariate, which controlled for effects related to impaired control over drinking. Given the size of the sample, however, we did not model interactions between these covariates. As our hypotheses about impulsivity and brain physiology were specific to the frontal lobes, statistical inferences were made based on peak voxel significance corrected for family wise error (FWE, p < 0.05) within a frontal-insular-subcortical (FIS) mask. This 382,584 mm3 (47,823 voxels) mask included the following structural regions from AAL library (Tzourio-Mazoyer et al., 2002) available in MarsBar: medial and lateral frontal and orbital regions, bilateral precentral gyri, anterior and middle cingulate cortex, anterior insula, as well as subcortical motor regions consisting of bilateral putamen, pallidum and caudate.
2.6.2. fMRI and stop signal
For the fMRI data, we conducted a voxel-wise analysis using an SPM factorial model to contrast the IC and no-IC groups during stop task performance. The primary contrast of interest, [StopInh>Go], compared stop trials where subjects successfully inhibited their responses (StopInh) and correct “Go” trials (see Kareken et al., 2013). Statistical inferences were made based on peak voxel significance (corrected for family-wise error (pFWE < 0.05) within the FIS mask described above. Then, to test for spatial overlap in regions where rCBF and I7 correlated, and where IC groups differed in stop task activation, a search region was generated from the peak of the negative correlation between rCBF and I7 (6-mm radius sphere centered at [56, 4, 26]; see Section 3); IC group differences were then tested within this small volume, and also corrected for the FIS mask volume’s family-wise error (pFWE < 0.05).
3. Results
3.1. Sample characteristics
Subjects were predominately male, young adult heavy drinkers whose mean and SD on the I7 (Table 1) closely approximated the normative average and variance of impulsivity scores in Eysenck et al. (1985). The subsample of participants who took part in the stop signal fMRI study were generally comparable to the overall sample, with a more balanced gender distribution and slightly lower AUDIT scores and DSM-V symptoms in the subsample.
Table 1.
Sample characteristics for all subjects and by IC group (top panel) and sample characteristics for the stop task subsample as a whole and by IC group (bottom panel)
| Total Sample (n=117) | IC (n=70) | No-IC (n=47) | Contrasts | |
| M (SD) | M (SD) | M (SD) | ||
| Gender M:F | 98:19 | 62:8 | 36:11 | |
| % Male | 83.8% | 88.6% | 76.6% | |
| % Smokers | 13.7% | 18.6% | 6.4% | p = 0.06 |
| Age | 24.1 (3.1) | 24.3 (3.5) | 23.7 (2.3) | ns |
| Education | 15.2 (1.6) | 14.9 (1.7) | 15.5 (1.3) | ns |
| Drinks/week | 17.4 (11.7) | 20.5 (12.5) | 12.8 (8.7) | 0.001 |
| Drinks/drinking day | 5.8 (3.4) | 6.5 (3.6) | 4.8 (2.8) | 0.006 |
| AUDIT | 11.0 (5.6) | 12.9 (6.0) | 8.1 (3.3) | <0.001 |
| DSM-V count | 2.4 (2.1) | 3.4 (2.0) | 0.8 (0.9) | <0.001 |
| Eysenck I7 | 7.8 (4.1) | 8.2 (4.0) | 7.1 (4.3) | ns |
| Stop task subsample (n=40) | IC (n=18) | No-IC (n=22) | ||
| M (SD) | M (SD) | M (SD) | Contrasts | |
| Gender M:F | 23:17 | 11:7 | 12:10 | |
| % Male | 57.5% | 61.1% | 54.5% | |
| % Smokers | 5.0% | 2.5% | 2.5% | ns |
| Age | 23.3 (2.0) | 23.3 (2.2) | 23.3 (1.9) | ns |
| Education | 15.5 (1.5) | 15.2 (1.8) | 15.7 (1.1) | ns |
| Drinks/week | 14.9 (10.4) | 18.2 (13.7) | 12.1 (5.8) | ns |
| Drinks/drinking day | 5.1 (2.7) | 5.1 (2.9) | 5.1 (2.6) | ns |
| AUDIT | 8.7 (3.0) | 9.7 (3.2) | 8.0 (2.7) | ns |
| DSM-V count | 1.5 (1.1) | 2.3 (0.9) | 0.9 (0.9) | <0.001 |
| Eysenck I7 | 7.4 (4.2) | 6.8 (2.9) | 7.9 (5.0) | ns |
| SSRT | 242.7 (24.1) | 235.5 (21.3) | 248.5 (25.0) | ns |
Note. Contrasts were tested by between-groups t-tests (IC compared with no-IC). ns indicates a significance value of p > 0.05.
AUDIT = Alcohol Use Disorder Identification Test; DSM-V = Diagnostic and Statistical Manual of Mental Disorders, 5th ed; SSRT = stop signal reaction time. IC = impaired control over drinking.
3.2. Impaired control over drinking (IC)
Within the entire sample, 70 subjects (62 men) were classified as IC and 47 (36 men) were classified as no-IC. The IC group reported greater alcohol consumption and alcohol problems (ps < 0.05), without group differences in Eysenck I7 scores, age, or education (Table 1). Within the stop signal subsample, 18 subjects (11 men) were classified as IC and 22 (12 men) were classified as no-IC. No significant group differences were observed in alcohol consumption, Eysenck I7 scores, or SSRT.
3.3. Associations between impulsivity/IC and rCBF
All participants responded to the tones during the PASL scan, ensuring that they were awake throughout the imaging. Impulsivity scores were normally distributed, but the alcohol consumption measure (i.e., drinks/week) was positively skewed; the square-root-transformed drinking data were therefore used for analysis.
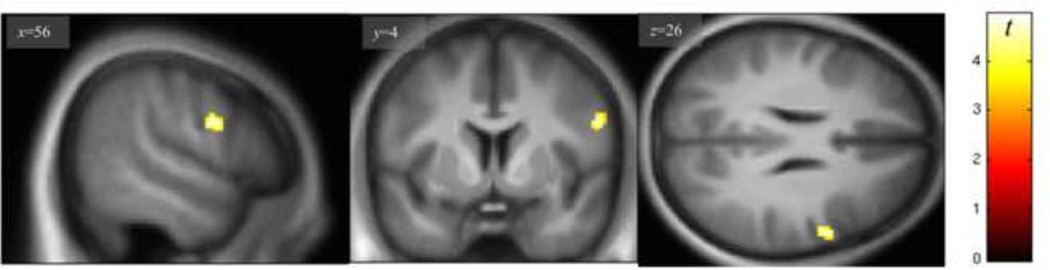
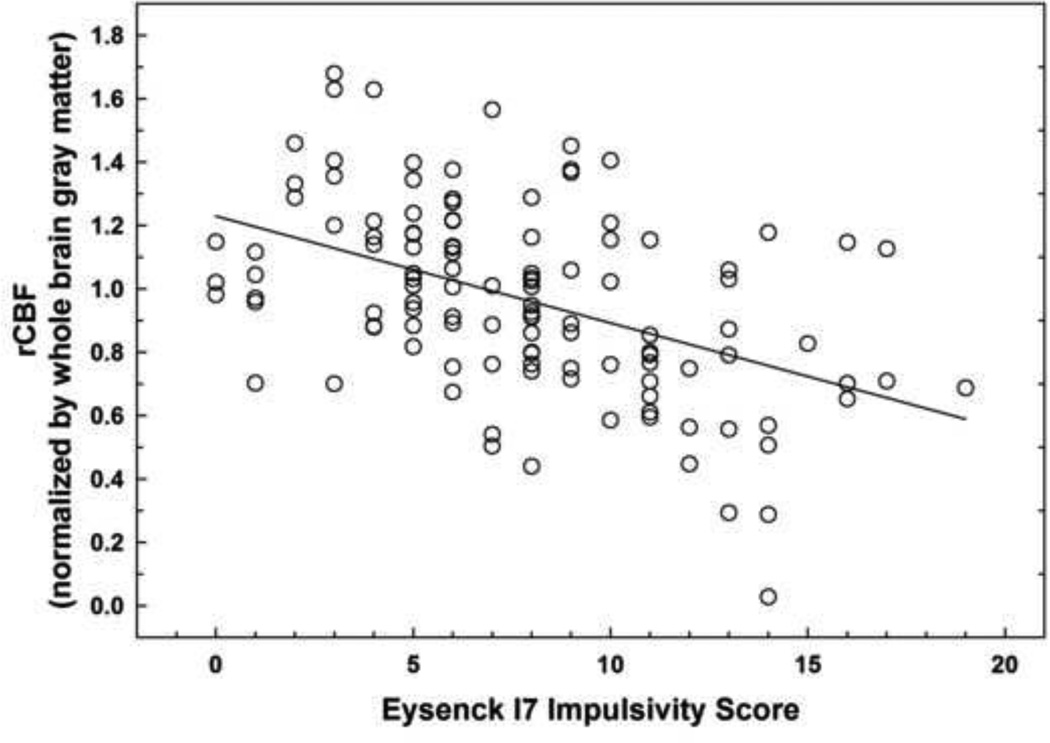
Linear regression analysis (controlling for IC status, smoking, drinks/week, and gender) showed no significant positive associations between rCBF and Eysenck I7 scores. By contrast, there was a negative correlation between rCBF and I7 scores in the anterior right precentral gyrus (Fig. 1; Table 2), with a significant peak voxel (pFWE = 0.029) effect at the [56, 4, 26] MNI coordinate. Fig. 2 presents an illustrative plot of the distribution of the negative association between Eysenck I7 scores and average rCBF in the right precentral gyrus cluster formed at p< 0.001, uncorrected.
Fig. 1.
Negative relationship between regional cerebral blood flow and Eysenck I7 scores in the right precentral gyrus (display height, p<0.001). Peak effect at [56 4 26] is significant after correcting for family wise error (pFWE<0.05) within a frontal-insular-subcortical (FIS) mask.
Table 2.
Brain regions where regional cerebral blood flow (using PASL, n=117) is related to Eysenck I7 or Impaired Control (IC) group effects.
| Comparison | Region | Cluster Size |
MNI coordinates | Peak | Peak significance |
||
|---|---|---|---|---|---|---|---|
| k | x | y | z | Z | p (FWE) | ||
| Negative correlation with Eysenck I7 | R Precentral gyrus | 59 | 56 | 4 | 26 | 4.69 | 0.029* |
| No-IC > IC | Medial superior frontal gyrus | 80 | −2 | 56 | 20 | 4.26 | ns (0.15) |
Note.
Indicates significant (pFWE) peak within frontal-insular-subcortical (FIS) mask. Voxel-wise height threshold, p < 0.001 (uncorrected). Whole brain gray matter rCBF = 58.0 ml/100 g/min (SD = 6.8).
Fig. 2.
Scatter plot illustrating negative relationship between mean rCBF values extracted from the right precentral gyrus cluster (shown in Figure 1) and Eysenck I7 scores in 117 subjects.
The same regression model was also examined for IC group differences (controlling for Eysenck I7 scores, smoking, drinks/week, and gender). While an IC group difference in blood flow (IC < no-IC) in the medial superior frontal gyrus area (Table 2) was present, this effect was not significant after correcting for family-wise error within the FIS mask. There were no group differences in the opposite direction (IC > no-IC).
There were no significant correlations between rCBF and drinks/week or smoking. See Supplementary Table and Supplementary Fig. 2 for significant gender differences in rCBF.
3.4. Overlap of correlation between Eysenck I7 and rCBF and stop task activation
Summary statistics of stop task performance for the sample were as follows: mean SSRT=242.8 (SD=23.8) ms, mean Go RT = 438.5 (SD= 104.0) ms, mean accuracy rate for Go trials = 97.5% (SD= 4.9), and mean stop failure rate = 51% (SD= 2.8). There was no significant correlation between Eysenck I7 scores and SSRT (p = 0.76), and a regression analysis within SPM did not show any significant associations between stop task activation and Eysenck I7 scores in the subsample.
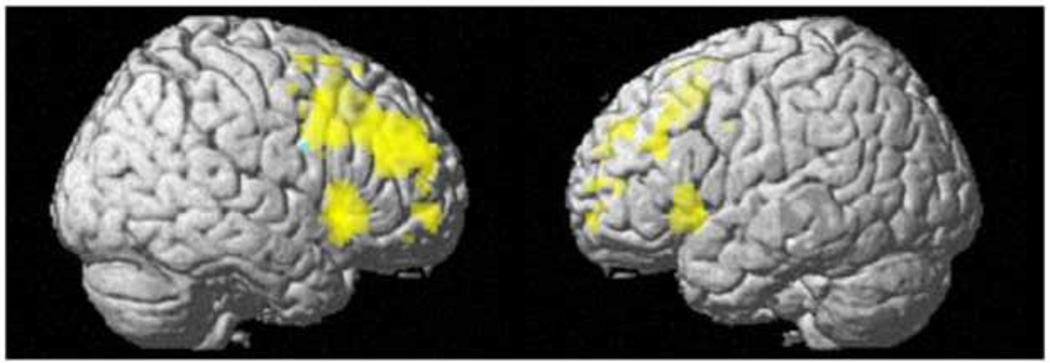
Fig. 3 presents a 3D rendering of the region in which rCBF was negatively related with Eysenck I7 scores in the total sample (cyan) and regions of significant [StopInh>Go] BOLD activation in the subsample (yellow; both effects within the FIS mask). The figure illustrates the right lateralization of both effects, and suggests that some right frontal motor/premotor areas associated with impulsive personality and response inhibition overlap (green).
Fig. 3.
The overlap (green) of negative relationship between rCBF and Eysenck Impulsivity I7 in n=117 (cyan) and the stop signal task [StopInh>Go] BOLD activation in a n=40 subsample (yellow). Displayed at a voxel-wise threshold, pFWE<0.05, family wise error corrected within the FIS mask. Cluster size, k=0.
3.5. IC and stop task performance during fMRI
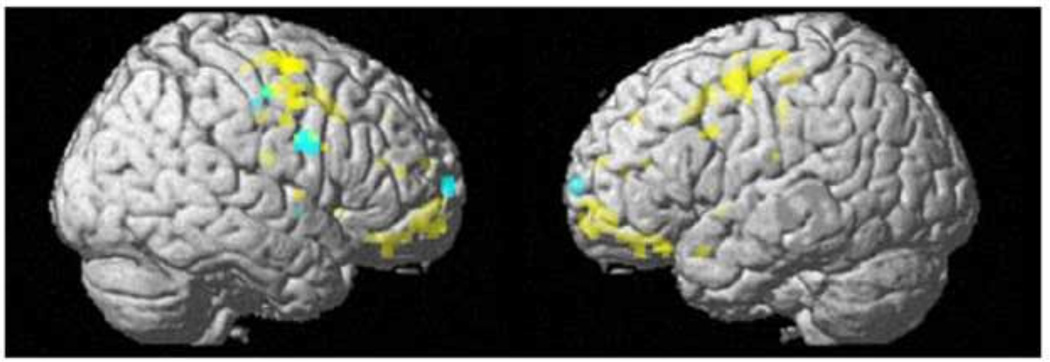
Group differences in [StopInh>Go] BOLD activation during response inhibition were evident in bilateral frontal regions, with IC showing reduced [StopInh>Go] response as compared to no-IC (Fig. 4 (yellow), with a significant peak voxel (pFWE = 0.047) effect at the [− 32, − 4, 50] MNI coordinate). In a spherical (6-mm radius) search volume centered on the peak of the frontal cluster (negative correlation between rCBF and Eysenck I7 scores), IC were significantly lower than no-IC (pFWE=0.019) in the right precentral gyrus. As illustrated by Fig. 4, the cluster of IC-group differences in the [StopInh>Go] BOLD response (yellow) is proximate to, and overlaps with (green), regions where resting rCBF is negatively associated with Eysenck I7 scores (cyan). There were no significant group differences in the opposite direction (IC>no-IC).
Fig. 4.
IC<no-IC group difference in [StopInh>Go] BOLD response (yellow) and negative relationship between regional cerebral blood flow and Eysenck I7 scores (cyan) with green color indicating the right prefrontal area overlap. Both effects are shown within the FIS mask at a display height, p<0.001, uncorrected, and cluster size, k=0.
4. Discussion
This study examined correlations between brain physiology and measures of impulsivity. Specifically, we identified brain areas where blood flow and BOLD activation related to impulsive personality, impaired control over drinking, and a behavioral measure of response inhibition. The results showed that impulsive personality was inversely associated with blood flow in the right precentral/prefrontal region. Importantly, this same region was also activated when restraining a motor behavior (i.e., inhibiting a finger press during the stop signal task). Moreover, IC group differences in BOLD activation while performing the motor inhibition task were present in an area that overlapped this same precentral region. Taken together, these findings show that both impulsive personality and self-reported impaired control over drinking are associated with decreased activity in brain regions associated with motor restraint.
Other studies have similarly implicated this precentral/prefrontal region in response inhibition. For example, Li et al. (2006) isolated processes of response inhibition (as distinct from other cognitive processes involved in stop task performance, including attention, error monitoring, and salience processing), and found that response inhibition was associated with activation of medial and precentral frontal cortices. Additionally, using disruptive transcranial magnetic stimulation (TMS) over dorsal premotor cortex decreased inhibition associated with motor control, suggesting a specific role for premotor cortex in motor response inhibition (Duque et al., 2012). Further, a meta-analysis of 16 fMRI studies (Rae et al., 2014) found that right pre- SMA and right pre-motor cortex were involved in the specific act of response stopping. Finally, in a comprehensive review of studies examining cortical influences of response inhibition, Bari and Robbins (2013) conclude that ‘it is probably the interaction between inferior frontal cortex and pre-SMA…that allows the successful inhibition of a pre-potent motor response.’ Taken together, these studies provide support for the conclusion that the area in which trait impulsivity is correlated with blood flow is also implicated in motor response inhibition.
Associations between simple response inhibition and self-report impulsive personality measures have been tested in several behavioral studies, with conflicting results. Some studies have shown correlations between self-reported impulsive personality and poor inhibitory control on laboratory motor tasks, including the stop signal and go/no-go tasks (Logan et al., 1997; Castellanos-Ryan et al., 2011;), while others have shown no such association (Enticott et al., 2006; Reynolds et al., 2006). A recent meta-analysis of 27 studies showed a small, but significant, association between multi-dimensional self-report and behavioral measures of impulsivity (Cyders and Coskunpinar, 2011). Further, self-reported impulsivity on the Barratt Impulsiveness Scale was associated with BOLD activation during salience processing of stop stimuli on the stop signal task, but not with activation during response inhibition (Farr et al., 2012). Despite our own observation of a significant inverse association between impulsive personality and blood flow in regions involved in motor restraint, we also did not observe direct associations between impulsive personality and either motor restraint (stop signal reaction time) or the BOLD response to inhibitory stimuli (although the number of subjects tested for stop signal responses was comparatively small and perhaps insufficient in power). Similarly, we did not observe differences in self-reported impulsivity or SSRT in the two IC groups. Thus, rather than direct relationships between these three different manifestations of impulsivity, perhaps motor inhibition, the propensity to engage in impulsive acts, and impaired control over drinking specifically may have common origins in the functional efficiency and integrity of non-dominant hemisphere motor and premotor cortex. At least at the level of more elemental motor behavior, it is clear that activation of ipsilateral motor cortex inhibits the contralateral (right) dominant hand, likely through transcallosal mechanisms (Ferbert et al., 1992).
Reduced activity in motor regions during the stop task was also implicated in loss of control over drinking, a finding suggesting that difficulty controlling the impulse to consume alcohol could also be governed in part by motor regions. This is consistent with findings reported in two recent reviews of fMRI studies of response inhibition in alcohol and substance dependent individuals that showed impaired activation in prefrontal areas, including dorsolateral prefrontal cortex and pre-SMA (Feil et al., 2010; Luijten et al., 2014). Such dysregulation of brain regions involved in response inhibition could lead to particular difficulty inhibiting behaviors induced by the urge to consume alcohol, despite the individual’s intent to limit drinking.
Deficits in brain activation in alcohol-dependent individuals during response inhibition suggest that improving inhibitory control could be a viable prevention and treatment strategy for alcohol use disorders. Indeed, researchers are beginning to investigate cognitive enhancers as potential pharmacotherapies for alcohol dependence. For example, Schmaal et al. (2013) recently showed that modafanil enhances response inhibition in alcohol dependent individuals by increasing activation in the SMA and motor (right ventrolateral) thalamus. Moreover, in a 10-week trial of modafinil treatment in alcohol dependent patients, the degree to which modafinil improved inhibition was directly associated with decreases in alcohol consumption (Joos et al., 2013). The current study shows that this decreased prefrontal activation during inhibition and while at rest may exist in young adult drinkers before clinical levels of chronic alcohol dependence (and without any differences in behavioral performance).
There are some limitations to this study. The sample was predominately male, precluding analyses of gender differences and limiting the generalizability of the findings to women. This limitation was due to logistical issues regarding recruitment for the individual studies, and it will be important to replicate these results in a future study with a more gender-balanced sample. It is important to note, however, that within the stop task subsample, comparable numbers of men and women were represented. Moreover, we did not have the power to model interactions between some covariates, such as gender, smoking, and drinking. The drinking habits of the stop signal sample were also slightly different than those of the entire PASL sample. Overall rates of alcohol consumption were lower in the subsample, and a smaller percentage of participants reported impaired control than in the larger sample. Similarly, all participants were regular drinkers. Future studies will be needed to examine the associations observed here in light or non-drinkers. An additional consideration is that the PASL scan is not a true “resting” scan, as participants were asked to maintain a light level of vigilant attention (key press to an approximate once per minute tone) to ensure wakefulness and better standardize mental states across subjects. It is therefore possible that the simple task could affect the nature of blood flow results and its association with impulsive personality traits and motor response inhibition. Additionally, the stop signal task was completed following IV saline infusion, in a protocol in which participants were told that they could receive alcohol or saline. Future studies should replicate these findings with no alcohol expectancy to confirm that such an expectancy did not significantly influence findings. Finally, it is important to note that other factors that were not measured here, including genetic factors and diet and exercise, could contribute to the reported findings.
In sum, and insofar as we are aware, the current study provides the first demonstration that self-reported impulsive personality is associated with resting cerebral blood flow in frontal regions implicated in response inhibition. It is also the first to demonstrate that self-reported loss of control in drinking is related to lower stop-signal activation in this and proximate regions. These findings are consistent with the idea that difficulty controlling behavior is due in part to impairment in motor restraint systems, contributing to a general difficulty in withholding urges to engage in maladaptive behaviors. Targeting brain systems involved in response inhibition could have significant utility in prevention and treatment of alcohol use disorders.
Supplementary Material
Highlights.
We examined correlations between brain physiology and measures of impulsivity.
Impulsivity was inversely correlated with blood flow in the right precentral gyrus.
Significant BOLD activation during response inhibition was observed in an overlapping region.
Reduced activation was observed in individuals with impaired control over drinking
Acknowledgements
This research was supported by National Institute on Alcohol Abuse and Alcoholism, The Indiana University Alcohol Research Center, P60 AA007611 (Crabb); R21 AA018020 (DAK); R01 AA017661 (DAK); and National Institute on Drug Abuse Grant F32 DA033756 (JW). NIAAA and NIDA had no involvement other than financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
One subject was excluded from the Kareken et al. (2013) sample but included here given the subject’s indeterminate status with regard to a family history of alcoholism; one subject included in the Kareken et al. (2013) article was excluded here given technical problems with the subject’s PASL image data.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for Sse in Primary Health Care. Geneva: World Health Organization; 2001. [Google Scholar]
- Babor TF, Kranzler HR, Lauerman RJ. Early detection of harmful alcohol consumption: comparison of clinical, laboratory, and self-report screening procedures. Addictive Behaviors. 1989;14:139–157. doi: 10.1016/0306-4603(89)90043-9. [DOI] [PubMed] [Google Scholar]
- Band GPH, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Rubia K, Conrod PJ. Response inhibition and reward response bias mediate the predictive relationships between impulsivity and sensation seeking and common and unique variance in conduct disorder and substance misuse. Alcoholism Clinical and Experimental Research. 2011;35:140–515. doi: 10.1111/j.1530-0277.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: is there overlap in nomothetic span and construct representation for impulsivity? Clinical Psychology Review. 2011;31:965–982. doi: 10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. Journal of Neuroscience. 2012;32:806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott PG, Ogloff JRP, Bradshaw JL. Associations between laboratory measures of executive inhibitory control and self-reported impulsivity. Personality and Individual Differences. 2006;41:285–294. [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsop J. Age norms for impulsiveness, venturesomeness, and empathy in adults. Personality and Individual Differences. 1985;6:613–619. [Google Scholar]
- Farr OM, Hu S, Zhang S, Li CS. Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. Neuroimage. 2012;63:1070–1077. doi: 10.1016/j.neuroimage.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neuroscience and Biobehavioral Reviews. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. Journal of Physiology. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR. Motivation, working memory, and decision making: a cognitive-motivational theory of personality vulnerability to alcoholism. Behavioral and Cognitive Neuroscience Reviews. 2002;1:183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217:659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, Gur RE. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267:528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- Heather N, Tebbutt JS, Mattick RP, Zamir R. Development of a scale for measuring impaired control over alcohol consumption: a preliminary report. Journal of Studies on Alcohol. 1993;54:700–709. doi: 10.15288/jsa.1993.54.700. [DOI] [PubMed] [Google Scholar]
- Jahng GH, Song E, Zhu XP, Matson GB, Weiner MW, Schuff N. Human brain: reliability and reproducibility of pulsed arterial spin-labeling perfusion MR imaging. Radiology. 2005;234:909–916. doi: 10.1148/radiol.2343031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos L, Goudriaan AE, Schmaal L, Fransen E, van den Brink W, Sabbe BG, Dom G. Effect of modafinil on impulsivity and relapse in alcohol dependent patients: a randomized, placebo-controlled trial. European Neuropsychopharmacology. 2013;23:948–955. doi: 10.1016/j.euroneuro.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Dzemidzic M, Wetherill L, Eiler W, 2nd, Oberlin BG, Harezlak J, Wang Y, O'Connor SJ. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology. 2013;228:335–345. doi: 10.1007/s00213-013-3038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Grahame N, Dzemidzic M, Walker MJ, Lehigh CA, O'Connor SJ. fMRI of the brain's response to stimuli experimentally paired with alcohol intoxication. Psychopharmacology. 2012;220:787–797. doi: 10.1007/s00213-011-2526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Beseler CL, Helms CM, Patock-Peckham JA, Wakeling VA, Kahler CW. A brief, critical review of research on impaired control over alcohol use and suggestions for future studies. Alcoholism: Clinical and Experimental Research. 2014;38:301–308. doi: 10.1111/acer.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Patock-Peckham JA, Potenza MN. Impaired control over alcohol use: An under-addressed risk factor for problem drinking in young adults? Experimental and Clinical Psychopharmacology. 2012;20:92–106. doi: 10.1037/a0026463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stopsignal task: neural correlates independent of signal monitoring and post-response processing. Journal of Neuroscience. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magnetic Resononce in Medicine. 1999;41:1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, Franken IH. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. Journal of Psychiatry and Neuroscience. 2014;39:149–169. doi: 10.1503/jpn.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug and Alcohol Dependence. 2001;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel A, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Volumetric cerebral perfusion imaging in healthy adults: Regional distribution, laterality, and repeatability of pulsed continuous arterial spin labeling (PCASL) Psychiatry Research: Neuroimaging. 2010;182:266–273. doi: 10.1016/j.pscychresns.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, de Wit H. Control yourself: alcohol and impulsivity. Alcoholism: Clinical and Experimental Research. 2010;34:1303–1305. doi: 10.1111/j.1530-0277.2010.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Weaver C, Anderson MC, Rowe JB. Selection and stopping in voluntary action: a meta-analysis and combined fMRI study. Neuroimage. 2014;86:381–391. doi: 10.1016/j.neuroimage.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40:305–315. [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Avila C, Ferre F, Jimenez-Arriero MA, Ponce G, Palomo T. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcoholism: Clinical and Experimental Research. 2008;32:1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE. Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biological Psychiatry. 2013;73:211–218. doi: 10.1016/j.biopsych.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa: 1992. pp. 41–72. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, Zimmerman RA, Detre JA. Pediatric perfusion imaging using pulsed arterial spin labeling. Journal of Magnetic Resonance Imaging. 2003;18:404–413. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- Wang Y, Saykin AJ, Pfeuffer J, Lin C, Mosier KM, Shen L, Kim S, Hutchins GD. Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. Neuroimage. 2011;54:1188–1195. doi: 10.1016/j.neuroimage.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.