Abstract
Background
Despite high efficacy, only 7.7% of women in the United States currently using contraception use an IUD. There is little published contemporary data about fertility rates after IUD use, especially in nulliparous women and women using the hormonal IUD.
Study Design
We recruited sexually active women 18–35 years of age enrolled in the Contraceptive CHOICE Project who had discontinued a contraceptive method and desired pregnancy.
Results
In this pilot project, we enrolled 69 former IUD users (50 copper and 19 levonorgestrel) and 42 former non-IUD users. Pregnancy rates at 12-months were similar between the two groups; 81% of IUD users became pregnant compared to 70% of non-IUD users (p=0.18). In the Cox model, there was no difference in the time to pregnancy in IUD users compared to non-IUD users (HRadj 1.19, 95% CI 0.74–1.92). African American race was the only variable associated with reduced fertility (HRadj 0.40, 95% CI 0.24–0.67).
Conclusions
We found no difference in 12-month pregnancy rates or time to pregnancy between former IUD users and users of other contraceptive methods. However, there was a clinically and statistically significant reduction in fertility in African American women.
Introduction
Intrauterine devices (IUDs) are among the most effective forms of contraception available to women in the United States today. Despite high efficacy, only 7.7% of women in the United States currently using contraception use an IUD.[1] IUD use is lower in the U.S. than in many other developed nations.[2] Health care providers and patients’ concerns about sexually transmitted infections (STIs), pelvic inflammatory disease (PID) and risk of infertility are, in part, responsible for this low uptake.[3] In particular, reluctance to use IUDs can be attributed to complications surrounding the Dalkon Shield in the 1970s. Research over the two decades after the introduction of the Dalkon Shield supported an association between IUD use, PID, and tubal infertility. In 1985, Daling et al. published the results of a case control study that showed a 2.6-fold increased risk for tubal infertility among previous IUD users.[4] Another case-control study from the same year found a 2-fold increased risk for tubal infertility among previous users of an IUD.[5] While the significant associations between IUD use and tubal infertility were seen primarily in patients who had used the Dalkon Shield, both of these reports suggested that the IUD should be avoided nulliparous women, and considered with caution for those who desire future fertility.[4, 5] Concerns about the health risks of the Dalkon Shield led to a large reduction in all IUD use in the U.S.[6]
Many previous studies investigating the relationship between the IUD and fertility failed to control for important confounding variables such as history of infection or PID.[7–9] Many were also limited to parous women and women using copper or inert IUDs. [10–12] More recent studies have attempted to address some of these limitations, and the results are mixed regarding a link between IUD use and infertility. In 2001, Hubacher et al. conducted a case-control study of the association between the copper T380A and tubal infertility in nulliparous women. They found that the presence of serum antibodies to Chlamydia trachomatis was associated with an increased risk of tubal infertility; whereas there was no association between copper IUD use and infertility observed.[13] In contrast, Doll et al. conducted a prospective cohort study of 558 married nulliparous women and found that increasing duration of copper IUD use was significantly associated with lower fertility even after adjusting for age (HRadj of term birth: IUD duration 48–72 months 0.69 (95% CI 0.50–0.97) and IUD duration 78+ months 0.50 (95% CI 0.34–0.73)).[14] It is important to note that neither of these studies included users of levonorgestrel-containing IUDs. A 2013 study from China found no relationship between IUD use and reduced fertility. This study was limited to parous women who were using mostly copper IUDs.[15]
The lack of consistent results about the presence or absence of an association between IUD use and fertility is confusing to healthcare providers and women. The objective of this pilot study was to compare pregnancy rates at 12 months in women who discontinued the copper T-380A or the levonorgestrel intrauterine system (LNG-IUS) to women discontinuing other contraceptive methods. We hypothesized that there would be no difference in 12-month pregnancy rates between the two groups. Our goal was to use these results to plan a larger prospective cohort study to assess the relationship between IUDs, sexually transmitted infections (STIs), and fertility.
Materials and Methods
We conducted a sub-study of the Contraceptive CHOICE Project (CHOICE). CHOICE is a prospective cohort study developed to promote the use of long-acting reversible contraceptive methods (LARC: IUD and implant) in the St. Louis region. The methodologic details of CHOICE have previously been described.[16] Eligibility for this sub-study included age 18–35 years; sexually active with a male partner; discontinuing oral contraceptive pills (OCPs), transdermal contraceptive patch, contraceptive vaginal ring, subdermal etonogestrel implant, copper T-380A or LNG-IUS in order to attempt pregnancy; English speaking; and willing to comply with study protocol. We excluded women who used depot medroxyprogesterone acetate (DMPA) in the 5 months prior to trying to conceive from this sub-study because DMPA can be associated with a longer return to fertility.[17] We identified potentially eligible participants from the CHOICE database and screened them for eligibility by telephone. Eligible participants who agreed to participate were then verbally consented and enrolled into the study. A copy of the informed consent form was subsequently mailed to the participants. Following enrollment, a research assistant administered a survey by telephone. This survey included questions regarding date of contraceptive method discontinuation, date they started trying to conceive, prior history of infertility, partner’s reproductive history, frequency and timing of intercourse, pregnancy since stopping their method of contraception, and the outcome of any pregnancies. We obtained institutional review board approval prior to participant recruitment.
Participants who were pregnant at the time of the initial survey did not require any additional follow-up. Additionally, participants who had been trying to conceive for 12 months or greater at the time of the initial contact were not re-contacted. Participants who were not yet pregnant at the time of enrollment, and had been trying to conceive for less than 12 months, were followed up with telephone surveys at 6 months and 12 months after method discontinuation to assess for pregnancy. Medical record request authorization forms were sent to all participants so that we could perform medical chart reviews to validate pregnancy outcomes.
We compared demographic, socioeconomic and other fertility-related characteristics of women in the IUD and non-IUD groups using chi-square for categorical variables and student’s t-test for continuous variables. Pregnancy at 12 months was compared as a dichotomous variable with the chi-square test. Time to pregnancy was estimated using Kaplan-Meier estimator of survival function. The log-rank test was used to test for difference in time to pregnancy. Participants who were lost to follow-up were censored at the time of the last completed survey. Hazard rate ratios (HRs) and corresponding 95% confidence intervals (CI) were calculated using Cox proportional hazard models. We performed univariate and multivariable Cox proportional hazards regression to calculate the hazard ratio for factors associated with fertility. All covariates that were significantly associated with pregnancy in the univariate model (at the 0.05 level) or altered the hazard ratio by 10% or greater were included in the multivariable model. We planned to include age and parity a priori since these characteristics are associated with fertility.[18] We performed all statistical analyses using STATA 11 (StataCorp, College Station, TX).
Results
We identified 176 potentially eligible participants from the CHOICE database. Between June 2011 and January 2012, 132 of these women underwent telephone screening for eligibility and 111 women (69 former IUD users and 42 former non-IUD users) were enrolled. Of the 69 former IUD users, 50 (72.5%) used the levonorgestrel intrauterine system, and 19 (27.5%) used the copper T380A IUD. Baseline demographic characteristics of IUD users and non-IUD contraceptive users are compared in Table 1. IUD users were older and were more likely to be parous, compared to non-IUD users. Otherwise, the two groups were similar in terms of race, socioeconomic status, smoking status, body mass index (BMI), and history of prior gonorrhea or chlamydia infection.
Table 1.
Demographic and Reproductive Characteristics of Study Participants
| Non-IUD (n=42) | IUD (n=69) | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| Mean age, years (SD) | 27.6 | (3.9) | 29.5 | (3.5) | 0.03 |
|
| |||||
| Race | n | % | n | % | |
| Black | 16 | 38.1 | 22 | 31.9 | 0.77** |
| White | 25 | 59.5 | 44 | 63.8 | |
| Other | 1 | 2.4 | 3 | 4.3 | |
|
| |||||
| Body mass index (kg/m2) | 0.46** | ||||
| <25 | 22 | 52.4 | 33 | 49.3 | |
| 25 to <30 | 6 | 14.3 | 16 | 23.9 | |
| ≥30 | 14 | 33.3 | 18 | 26.9 | |
|
| |||||
| Marital status | 0.06** | ||||
| Single | 22 | 52.4 | 24 | 34.8 | |
| Married/living with a partner | 20 | 47.6 | 40 | 58.0 | |
| Separated/divorced/widowed | 0 | 0 | 5 | 7.2 | |
|
| |||||
| Education | 0.46 | ||||
| <= High school | 10 | 23.8 | 11 | 15.9 | |
| Some college | 18 | 42.9 | 28 | 40.6 | |
| College/Graduate school | 14 | 33.3 | 30 | 43.5 | |
|
| |||||
| Low SES* | 0.65 | ||||
| Yes | 17 | 40.5 | 25 | 36.2 | |
| No | 25 | 59.5 | 44 | 63.8 | |
|
| |||||
| Nulliparous | 0.001 | ||||
| Yes | 28 | 66.7 | 24 | 34.8 | |
| No | 14 | 33.3 | 45 | 65.2 | |
|
| |||||
| Nulligravid | 0.03 | ||||
| Yes | 18 | 42.9 | 16 | 23.2 | |
| No | 24 | 57.1 | 53 | 76.8 | |
|
| |||||
| Current smoker | 0.41 | ||||
| Yes | 7 | 16.7 | 16 | 23.2 | |
| No | 35 | 83.3 | 53 | 76.8 | |
|
| |||||
| History of chlamydia*** | 0.41 | ||||
| Yes | 7 | 16.7 | 16 | 23.2 | |
| No | 35 | 83.3 | 53 | 76.8 | |
|
| |||||
| History of gonorrhea*** | 0.47** | ||||
| Yes | 4 | 9.5 | 4 | 5.8 | |
| No | 38 | 90.5 | 65 | 94.2 | |
SES – socioeconomic status; PID – pelvic inflammatory disease
Low SES defined as receipt of public assistance or reported difficulty paying for transportation, housing, medical expenses or food in past 12 months
Fisher exact test
Patient self-reported
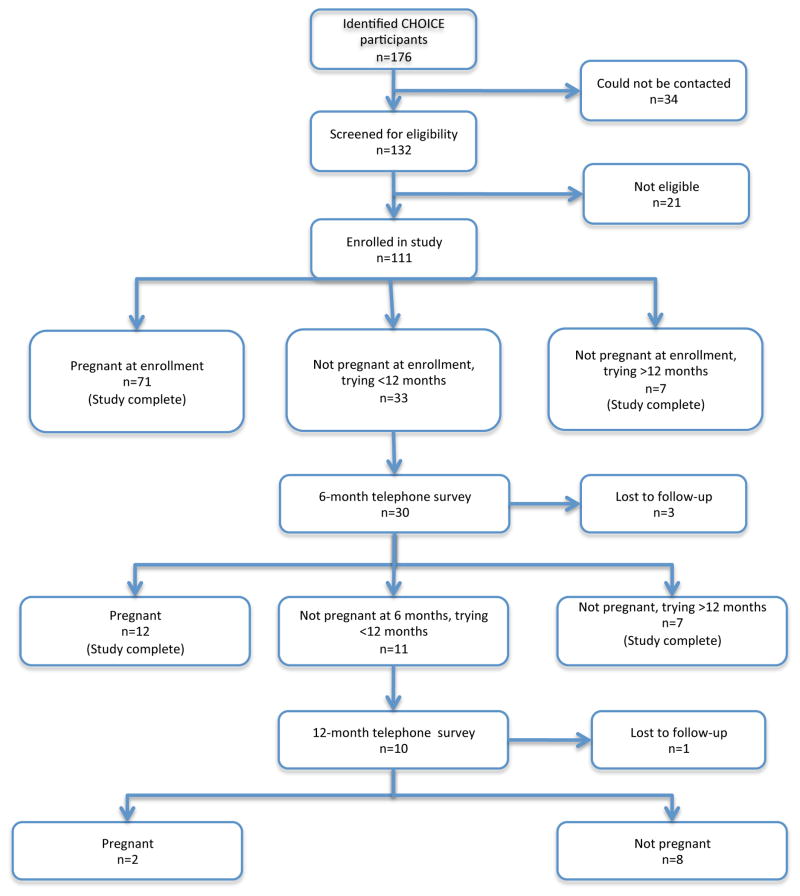
Figure 1 diagrams the study enrollment and follow-up. There were 71 women who were pregnant at the time of their initial telephone contact. Seven women were not pregnant, but had been trying for over 12 months. There were 33 women who had stopped their method of contraception within the past 12 months and who were still trying to conceive; 30 were successfully contacted for follow-up surveys. Of these, 12 were pregnant at follow-up phone interviews, and 7 were not pregnant and had been trying for 12 months or more. The additional 11 participants were eligible for a second follow-up survey at the 12-month endpoint. Ten of the 11 were contacted. Of these 2 were pregnant and 8 were not pregnant. The distribution of IUD and non-IUD users in each group at each time-point can be seen in Figure 1. There were 3 participants who were lost to follow-up at the 6-month time point and 1 participant who was lost to follow-up at the 12-month time point [total loss to follow-up = 4/132 (3%)]. There was no difference in loss to follow up between the two groups.
Figure 1.
Participant flow diagram.
The 12-month pregnancy rates are shown in Table 2 and were similar between the two groups; 81% for former IUD users compared to 70% for non-IUD users achieved pregnancy within 12 months of stopping their method of contraception (p = 0.18). Similarly, when we stratified by age (≤ 28 or >28 years) we found no difference in pregnancy rates between the two groups. For women age 28 years or less (at the time they started trying to conceive), 81% of former IUD users were pregnant at 12 months compared to 64% of former non-IUD users (p=0.15). For women age 29 years or greater at the time of enrollment, the pregnancy rates were 81% for IUD users and 100% for non-IUD users (p=0.49). When we stratified by parity, nulliparous IUD users were more likely to become pregnant than nulliparous non-IUD users (p=0.02)
Table 2.
Twelve month pregnancy rates
| Non-IUD (n=42) | IUD (n=69) | p-value | |
|---|---|---|---|
|
| |||
| Overall | 70% | 81% | 0.18 |
|
| |||
| Stratified by Age: | |||
| Age ≤ 28 (n= 49) | 64% | 81% | 0.15 |
| Age ≥ 29 (n= 62) | 100% | 81% | 0.49 |
|
| |||
| Stratified by Parity: | |||
| Nulliparous (n= 52) | 61% | 92% | 0.02 |
| Parous (n= 59) | 89% | 74% | 0.38 |
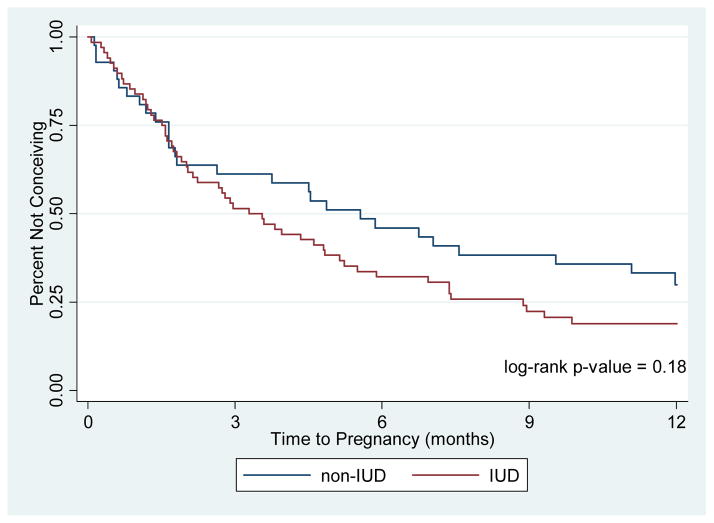
Figure 2 shows a Kaplan-Meier survival analysis of pregnancy rates over time for the IUD users compared to the non-IUD users. There was no statistically significant difference between the two curves (log-rank p=0.18). Table 3 shows the results of the univariate and multivariable Cox proportional hazards model for factors associated with successful pregnancy within 12 months of contraceptive discontinuation. Of the demographic and reproductive characteristics described in Table 1, only black race was significantly associated with pregnancy at 12 months in the univariate model; black women were less likely to become pregnant compared to white women (HR 0.40; 95% CI 0.24–0.67). After adjusting for the race, age, and nulliparity, we still did not observe any association between IUD use and pregnancy at 12 months (HRadj 1.19, 95% CI 0.74–1.92) compared to non-IUD users. In the adjusted model, black race remained significantly associated with decreased fertility.
Figure 2.
Time to Pregnancy by Former Contraceptive Method
Table 3.
Univariate and multivariable Cox proportional hazards model of factors associated with pregnancy at 12-months
| Hazard Ratio | 95% Confidence Interval | ||
|---|---|---|---|
|
| |||
| Univariate | |||
|
| |||
| IUD | 1.36 | 0.87 | 2.14 |
|
| |||
| Age (years) | 1.04 | 0.99 | 1.10 |
|
| |||
| Race | |||
| White | Ref | ||
| Black | 0.45 | 0.27 | 0.76 |
| Other | 0.51 | 0.16 | 1.63 |
|
| |||
| Body mass index (kg/m2) | |||
| <25 | Ref | ||
| 25 to<30 | 1.56 | 0.89 | 2.72 |
| ≥30 | 0.66 | 0.39 | 1.12 |
|
| |||
| Education | |||
| <= High school | Ref | ||
| Some college | 1.01 | 0.52 | 1.96 |
| College/Graduate school | 1.50 | 0.78 | 2.89 |
|
| |||
| Low SES | 0.92 | 0.58 | 1.47 |
|
| |||
| Nulligravid | 1.16 | 0.72 | 1.87 |
|
| |||
| Nulliparous | 0.81 | 0.52 | 1.25 |
| Current smoker | 1.21 | 0.71 | 2.07 |
|
| |||
| History of chlamydia | 1.15 | 0.67 | 1.99 |
|
| |||
| History of gonorrhea | 0.61 | 0.22 | 1.67 |
|
| |||
| Multivariable* | |||
|
| |||
| IUD | 1.19 | 0.74 | 1.92 |
|
| |||
| Age | 1.04 | 0.98 | 1.11 |
|
| |||
| Race | |||
| Black | 0.40 | 0.24 | 0.67 |
| Other | 0.49 | 0.15 | 1.58 |
|
| |||
| Nulliparous | 0.86 | 0.54 | 1.38 |
multivariable model adjusted for age, race, and parity
Discussion
We found no difference in pregnancy rates or time to pregnancy between former IUD users and users of other contraceptive methods including combined oral contraceptives, the contraceptive ring, the contraceptive patch and the etonogestrel implant. The findings of this study are in contrast to the previous studies that found an association between previous IUD use and infertility,[4, 5, 14] but are consistent with older studies that found no association between IUD use and reduced fertility.[new 7–13, 15] Our findings are also consistent with Hubacher et al. who found no association between IUD use and infertility once they controlled for important confounding variables, such as history of Chlamydia trachomatis infection.[13] It is important to note that most of these published studies included IUDs that are different from the ones currently available in the U.S.
Interestingly, we found that nulliparous IUD users were more likely to achieve pregnancy than nulliparous non-IUD users. One possible explanation for this is that users of pills, the patch or the ring were more likely to have irregular menstrual cycles and were using hormonal methods for cycle control. Given the small size of the study, it is also possible that these findings are spurious. Additional research with a larger study population is needed to verify this finding.
Our findings add to the body of scientific literature evaluating fertility after contraceptive use. There are still many women and health care providers who continue to believe that IUDs put women at risk for pelvic inflammatory disease and subsequent infertility. In a survey of women’s knowledge of intrauterine contraception performed in the St. Louis area, approximately 30% of respondents agreed with the statement that IUDs increased the risk of future infertility.[19] Similarly, in a survey of U.S. office-based and Title X clinic providers, approximately 30% of respondents had misconceptions about the safety of IUDs for nulliparous women. [20] IUDs are among the most highly effective forms of contraception available; thus, it is important to dispel the negative associations between IUD use and future fertility. A study published in the New England Journal of Medicine showed the contraceptive failure rate of women who used oral contraceptive pills, the patch or the contraceptive ring to be more than 20 times higher than women who used long-acting reversible contraception such as the IUD or the contraceptive implant. Additionally, this study showed that the risk of unintended pregnancy was twice as high among women under the age of 21, making it essential for providers to consider IUDs a first-line contraceptive option in young women. [21]
We also found an association between black race and decreased fertility. This is consistent with some reports in the medical literature, which show that married, non-Hispanic, black women are more likely to experience infertility compared to white women.[22] Other studies have shown that black women are more likely to present with tubal factor infertility than white women. In a survey of women presenting to an infertility clinic in Boston, 24.0% of African American respondents had tubal factor infertility, compared with 5.3% of white participants.[23] Again, this association of African-American race and reduced fertility should be confirmed in a larger study that is able to control for important confounders such as infection and socioeconomic status. Our study did not find an association between a self-reported history of chlamydia infection and infertility (HR 1.15, 95% CI 0.67–1.99). However, we did not perform serologic testing for chlamydial antibodies, and it is possible that inaccurate recall of a history of chlamydia led to misclassification bias.
One strength of our study is that it addresses the issue of fertility in a cohort of modern IUD users, including users of the LNG-IUS. Since the LNG-IUS is the most commonly used IUD in the US, it is important to include LNG-IUS users in studies of fertility. Our study does have limitations. Because participants were contacted sometimes a year or more after contraceptive discontinuation, it is possible that they may have not accurately recalled the date they stopped their method. Even as a pilot study, our study was underpowered. Our a priori sample size calculations indicated that we needed to enroll 120 IUD users and 60 non-IUD user controls (based on a 20% difference in fertility, alpha of 0.05 and power of 80%). This study was undertaken as we planned a larger, prospective study of contraception and fertility, including serologic analysis for infections; thus, our recruitment period was limited. Our small sample size resulted in decreased power to find a statistically significant difference. Given our observed rates of infertility, we would have needed a sample size of over 850 women to find a significant difference. Finally, serologic testing was not performed in this study. Thus, we were not able to control for previous STI in the relationship between IUD use and fertility. Prospective studies among a cohort of modern IUD users are needed to further assess the relationship between IUD use, prior history of STI and fertility.
We found no difference in pregnancy rates or time to pregnancy between former IUD users and users of other contraceptive methods. We also noted an association between black race and decreased fertility. Both findings should be validated in a prospective study including measures of current and past infection. Recruitment of this cohort is underway, over 340 participants are enrolled, and we will assess current and past STDs, duration and type of IUD, race, and other factors that may impact fertility. This ongoing prospective cohort study will provide additional evidence regarding the relationship of IUD use, infection, and infertility.
Acknowledgments
This research was supported in part by: 1) the Society of Family Planning; 2) Award number K23HD070979 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD); and 3) Clinical and Translational Science Award (UL1RR024992), from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR, NICHD, or NIH. Information on NCRR is available at http://www.ncrr.nih.gov. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp
Footnotes
Disclosures: Dr. Peipert receives research funding from Bayer Healthcare Pharmaceuticals & Teva Pharmaceuticals, and serves on an advisory board for Teva Pharmaceuticals, Bayer Healthcare Pharmaceuticals, and MicroCHIPS. Dr. Madden receives research funding from Merck & Co, Inc. and serves on an advisory board for Bayer Healthcare Pharmaceuticals. Dr. Allsworth receives research funding from Bayer Healthcare Pharmaceuticals.
References
- 1.Finer LB, Jerman J, Kavanaugh ML. Changes in use of long-acting contraceptive methods in the United States, 2007–2009. Fertil Steril. 2012;98(4):893–7. doi: 10.1016/j.fertnstert.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonfield A. Popularity Disparity: Attitudes about the IUD in Europe and the United States. Guttmacher Policy Rev. 2007;10:19–24. [Google Scholar]
- 3.Madden T, et al. Intrauterine contraception in Saint Louis: a survey of obstetrician and gynecologists’ knowledge and attitudes. Contraception. 2010;81(2):112–6. doi: 10.1016/j.contraception.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daling JR, et al. Primary tubal infertility in relation to the use of an intrauterine device. N Engl J Med. 1985;312(15):937–41. doi: 10.1056/NEJM198504113121501. [DOI] [PubMed] [Google Scholar]
- 5.Cramer DW, et al. Tubal infertility and the intrauterine device. N Engl J Med. 1985;312(15):941–7. doi: 10.1056/NEJM198504113121502. [DOI] [PubMed] [Google Scholar]
- 6.Piccinino LJ, Mosher WD. Trends in contraceptive use in the United States: 1982–1995. Fam Plann Perspect. 1998;30(1):4–10. 46. [PubMed] [Google Scholar]
- 7.Vessey MP, Lawless M, McPherson K, Yeates D. Fertility after stopping use of the intrauterine contraceptive device. Br Med J (Clin Res Ed) 1983;286(6359):106. doi: 10.1136/bmj.286.6359.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randic L, Vlasic S, Matrljan I, Waszak CS. Return to fertility after IUD removal for planned pregnancy. Contraception. 1985;32(3):253–9. doi: 10.1016/0010-7824(85)90048-4. [DOI] [PubMed] [Google Scholar]
- 9.Gupta BK, Gupta AN, Lyall S. Return of fertility in various types of IUD users. Int J Fertil. 1989;34(2):123–5. [PubMed] [Google Scholar]
- 10.Andolsek L, Teeter RA, Kozuh-Novak M, Wheeler R, Fortney JA, Rosenberg MJ. Time to conception after IUD removal: importance of duration of use, IUD type, pelvic inflammatory disease and age. Int J Gynaecol Obstet. 1986;24(3):217–23. doi: 10.1016/0020-7292(86)90100-1. [DOI] [PubMed] [Google Scholar]
- 11.Skjeldestad F, Bratt H. Fertility after complicated and non-complicated use of IUDs. A controlled prospective study. Adv Contracept. 1988;4(3):179–84. doi: 10.1007/BF01849435. [DOI] [PubMed] [Google Scholar]
- 12.Delbarge W, Bátár I, Bafort M, Bonnivert J, Colmant C, Dhont M, Fonzé V, Gevers R, Janssens D, Lavalley P, Salmin E, Degueldre M, Vrijens M, Van Kets H, Wildemeersch D. Return to fertility in nulliparous and parous women after removal of the GyneFix intrauterine contraceptive system. Eur J Contracept Reprod Health Care. 2002;7(1):24–30. [PubMed] [Google Scholar]
- 13.Hubacher D, et al. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345(8):561–7. doi: 10.1056/NEJMoa010438. [DOI] [PubMed] [Google Scholar]
- 14.Doll H, Vessey M, Painter R. Return of fertility in nulliparous women after discontinuation of the intrauterine device: comparison with women discontinuing other methods of contraception. BJOG. 2001;108(3):304–14. doi: 10.1111/j.1471-0528.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Lei H, Huang W, Fu J, Wang Q, Shen L, Wang Q, Ruan J, Liu D, Song H, Hu L. Fertility in older women following removal of long-term intrauterine devices in the wake of a natural disaster. Contraception. 2013;87(4):416–420. doi: 10.1016/j.contraception.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Secura GM, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(2):115 e1–7. doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardthaisong T. Return of fertility after use of the injectable contraceptive Depo Provera: up-dated data analysis. J Biosoc Sci. 1984;16(1):23–34. doi: 10.1017/s0021932000014760. [DOI] [PubMed] [Google Scholar]
- 18.Mutsaerts MA, et al. The influence of maternal and paternal factors on time to pregnancy--a Dutch population-based birth-cohort study: the GECKO Drenthe study. Hum Reprod. 2012;27(2):583–93. doi: 10.1093/humrep/der429. [DOI] [PubMed] [Google Scholar]
- 19.Hladky KJ, et al. Women’s knowledge about intrauterine contraception. Obstet Gynecol. 2011;117(1):48–54. doi: 10.1097/AOG.0b013e318202b4c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyler CP, et al. Health care provider attitudes and practices related to intrauterine devices for nulliparous women. Obstet Gynecol. 2012;119(4):762–71. doi: 10.1097/AOG.0b013e31824aca39. [DOI] [PubMed] [Google Scholar]
- 21.Winner B, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 22.Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982–2002. Fertil Steril. 2006;86(3):516–23. doi: 10.1016/j.fertnstert.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 23.Jain T. Socioeconomic and racial disparities among infertility patients seeking care. Fertil Steril. 2006;85(4):876–81. doi: 10.1016/j.fertnstert.2005.07.1338. [DOI] [PubMed] [Google Scholar]