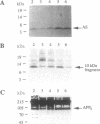
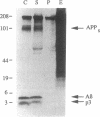
Abstract
The 39- to 43-amino acid amyloid beta-protein (A beta), which is progressively deposited in cerebral plaques and blood vessels in Alzheimer disease (AD), is secreted by cultured human cells during normal metabolism. In studies of cell lines transfected with beta-amyloid precursor protein (beta APP) cDNAs, the beta APP mutation K670N/M671L found in a Swedish familial AD (FAD) pedigree has previously been shown to cause a marked augmentation of A beta secretion. Here, we have conducted blinded analyses of beta APP metabolism in primary skin fibroblasts from affected members of the Swedish FAD pedigree and their unaffected siblings or spouses. These fibroblasts continuously secrete a homogenous population of A beta molecules starting at Asp-1 (D672 of beta APP). We found a consistent and significant approximately 3-fold elevation of A beta release from all biopsied skin fibroblasts bearing the FAD mutation. No significant alterations of other metabolic derivatives of beta APP were detected. The elevated A beta levels were found in cells from both patients with clinical AD and presymptomatic subjects. Thus, A beta overproduction in this FAD pedigree is not a secondary event but is consistent with a causal role in the development of the disease. Increased A beta secretion can begin many years prior to onset of symptoms, even in peripheral tissues, indicating that it does not require preexisting neural abnormalities.
Full text
PDF

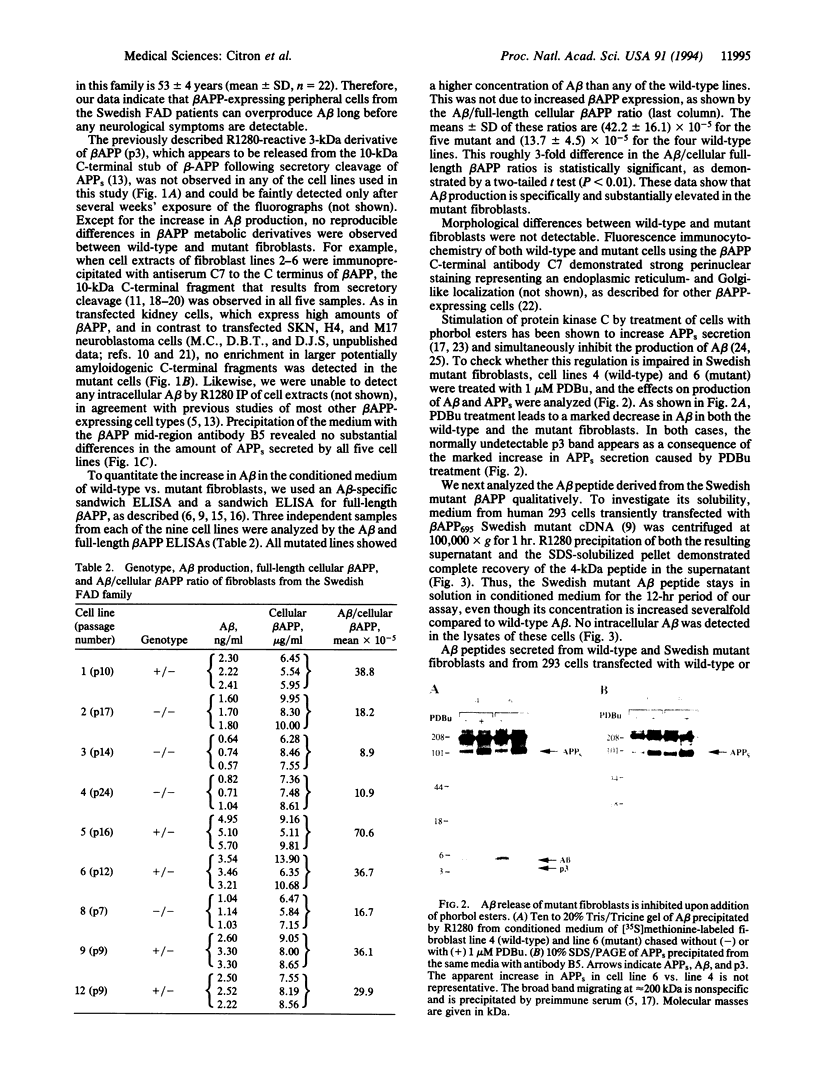
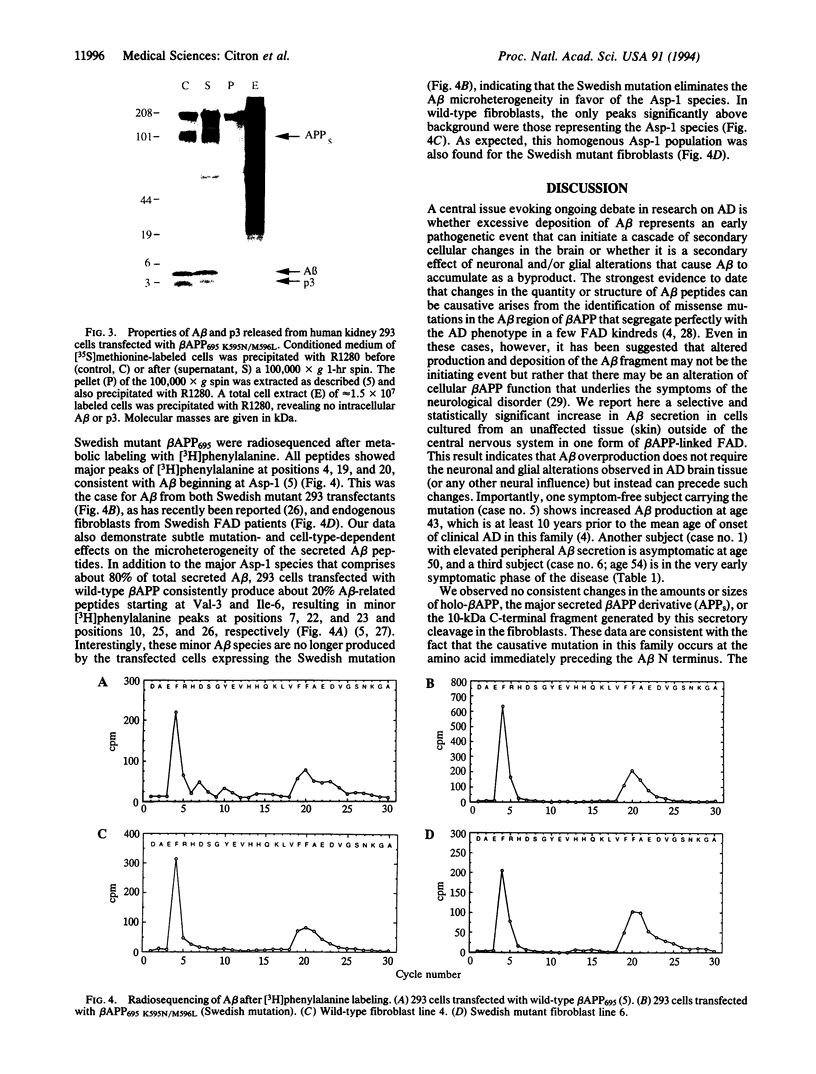

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busciglio J., Gabuzda D. H., Matsudaira P., Yankner B. A. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. D., Koo E. H., Greengard P. Protein phosphorylation inhibits production of Alzheimer amyloid beta/A4 peptide. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9195–9198. doi: 10.1073/pnas.90.19.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Caporaso G. L., Gandy S. E., Buxbaum J. D., Greengard P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2252–2256. doi: 10.1073/pnas.89.6.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Dovey H. F., Suomensaari-Chrysler S., Lieberburg I., Sinha S., Keim P. S. Cells with a familial Alzheimer's disease mutation produce authentic beta-peptide. Neuroreport. 1993 Aug;4(8):1039–1042. doi: 10.1097/00001756-199308000-00011. [DOI] [PubMed] [Google Scholar]
- Dyrks T., Dyrks E., Masters C. L., Beyreuther K. Amyloidogenicity of rodent and human beta A4 sequences. FEBS Lett. 1993 Jun 14;324(2):231–236. doi: 10.1016/0014-5793(93)81399-k. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Felsenstein K. M., Hunihan L. W., Roberts S. B. Altered cleavage and secretion of a recombinant beta-APP bearing the Swedish familial Alzheimer's disease mutation. Nat Genet. 1994 Mar;6(3):251–255. doi: 10.1038/ng0394-251. [DOI] [PubMed] [Google Scholar]
- Gillespie S. L., Golde T. E., Younkin S. G. Secretory processing of the Alzheimer amyloid beta/A4 protein precursor is increased by protein phosphorylation. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1285–1290. doi: 10.1016/0006-291x(92)90442-n. [DOI] [PubMed] [Google Scholar]
- Haass C., Hung A. Y., Schlossmacher M. G., Oltersdorf T., Teplow D. B., Selkoe D. J. Normal cellular processing of the beta-amyloid precursor protein results in the secretion of the amyloid beta peptide and related molecules. Ann N Y Acad Sci. 1993 Sep 24;695:109–116. doi: 10.1111/j.1749-6632.1993.tb23037.x. [DOI] [PubMed] [Google Scholar]
- Haass C., Hung A. Y., Schlossmacher M. G., Teplow D. B., Selkoe D. J. beta-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J Biol Chem. 1993 Feb 15;268(5):3021–3024. [PubMed] [Google Scholar]
- Haass C., Koo E. H., Mellon A., Hung A. Y., Selkoe D. J. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992 Jun 11;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Hardy J. Framing beta-amyloid. Nat Genet. 1992 Jul;1(4):233–234. doi: 10.1038/ng0792-233. [DOI] [PubMed] [Google Scholar]
- Hardy J., Mullan M. Alzheimer's disease. In search of the soluble. Nature. 1992 Sep 24;359(6393):268–269. doi: 10.1038/359268a0. [DOI] [PubMed] [Google Scholar]
- Hung A. Y., Haass C., Nitsch R. M., Qiu W. Q., Citron M., Wurtman R. J., Growdon J. H., Selkoe D. J. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J Biol Chem. 1993 Nov 5;268(31):22959–22962. [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Knops J., Johnson-Wood K., Schenk D. B., Sinha S., Lieberburg I., McConlogue L. Isolation of baculovirus-derived secreted and full-length beta-amyloid precursor protein. J Biol Chem. 1991 Apr 15;266(11):7285–7290. [PubMed] [Google Scholar]
- Mattson M. P. Calcium as sculptor and destroyer of neural circuitry. Exp Gerontol. 1992;27(1):29–49. doi: 10.1016/0531-5565(92)90027-w. [DOI] [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Finch E. A., Dawes L. R. Expression of the Alzheimer amyloid precursor gene transcripts in the human brain. Neuron. 1988 Oct;1(8):669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Podlisny M. B., Tolan D. R., Selkoe D. J. Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer's disease. Am J Pathol. 1991 Jun;138(6):1423–1435. [PMC free article] [PubMed] [Google Scholar]
- Regland B., Gottfries C. G. The role of amyloid beta-protein in Alzheimer's disease. Lancet. 1992 Aug 22;340(8817):467–469. doi: 10.1016/0140-6736(92)91780-c. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Tamaoka A., Kalaria R. N., Lieberburg I., Selkoe D. J. Identification of a stable fragment of the Alzheimer amyloid precursor containing the beta-protein in brain microvessels. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1345–1349. doi: 10.1073/pnas.89.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigo-Pelfrey C., Lee D., Keim P., Lieberburg I., Schenk D. B. Characterization of beta-amyloid peptide from human cerebrospinal fluid. J Neurochem. 1993 Nov;61(5):1965–1968. doi: 10.1111/j.1471-4159.1993.tb09841.x. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wertkin A. M., Turner R. S., Pleasure S. J., Golde T. E., Younkin S. G., Trojanowski J. Q., Lee V. M. Human neurons derived from a teratocarcinoma cell line express solely the 695-amino acid amyloid precursor protein and produce intracellular beta-amyloid or A4 peptides. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9513–9517. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H. M., Wegiel J., Wang K. C., Kujawa M., Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can J Neurol Sci. 1989 Nov;16(4 Suppl):535–542. doi: 10.1017/s0317167100029887. [DOI] [PubMed] [Google Scholar]