Abstract
Objective
To test the feasibility and preliminary efficacy of self-delivered home-based mirror therapy for phantom pain.
Design
Uncontrolled prospective treatment outcome pilot study.
Participants
Forty community-dwelling adults with unilateral amputation and phantom pain >3 on a 0–10 numeric rating scale enrolled either during a one-time study visit (n = 30) or remotely (n = 10).
Methods
Participants received an explanation of mirror therapy and were asked to self-treat for 25 min daily. Participants completed and posted back sets of outcomes questionnaires at months 1 and 2 post-treatment. Main outcome was mean phantom pain intensity at post-treatment.
Results
A significant reduction in mean phantom pain intensity was found at month 1 (n = 31, p = 0.0002) and at month 2 (n = 26, p = 0.002). The overall median percentage reduction at month 2 was 15.4%. Subjects with high education (>16 years) compared with low education (<16 years) (37.5% vs 4.1%) had greater reduction in pain intensity (p = 0.01).
Conclusion
These findings support the feasibility and efficacy of home-based self-delivered mirror therapy; this low-cost treatment may defray medical costs, therapy visits, and the patient travel burden for people with motivation and a high level of education. More research is needed to determine methods of cost-effective support for people with lower levels of education.
Keywords: phantom pain, mirror therapy, amputee, limb loss, self-treatment
INTRODUCTION
Phantom pain is a common adverse and chronic condition that affects 43–85% of people after limb amputation (1–4). Phantom pain is associated with disability (2), psychological distress (5), and substantial medical costs incurred by various pain management treatment strategies. Given that the prevalence of limb loss is expected to double in the next 4 decades (6), the importance of identifying accessible and cost-effective treatments for phantom pain is increasing.
A review of phantom pain treatments described mirror therapy as being the most promising method of treatment (7). The first report of mirror therapy for phantom pain was described by Ramachandran and colleagues in 1995 (8). Nine patients received guided training during an initial treatment session, and the researchers collected data on specific post-treatment sensory experiments. Findings from this first study suggested that mirror therapy reduced phantom pain. Results from subsequent case studies (9–11), case series (10, 12), and one randomized controlled trial (13) have provided further support for mirror therapy as a treatment for phantom pain. In a randomized controlled study, researchers compared mirror therapy with a control group (covered mirror) and with a mental-visualization treatment (comparison group) in 22 adults with lower extremity amputation and phantom pain. Eighteen subjects (6 in each group) completed the study. For 4 weeks, subjects in the mirror therapy condition performed 15 min of treatment daily under the direct supervision of study staff. The researchers found that 100% of subjects in the mirror therapy group reported a decrease in pain (mean −24 mm on a 100-mm visual analogue scale) compared with one person in the covered mirror group and two in the mental-visualization group. Furthermore, people in the non-mirror therapy groups were more likely to report worsening pain.
Mirror therapy is typically described as being therapist-guided (13–15) and involving a structured protocol of exercises (12, 14, 15). Such specifications have suggested that mirror therapy requires therapist support for treatment initiation at minimum, and broader therapist supervision and personalization of mirror therapy exercises at maximum (16). In contrast, one case study reported success with fully home-based self-delivered mirror therapy for a patient with lower limb phantom pain (9). In this case, only a basic rationale and verbal description of mirror therapy was provided to the patient. and no in-session mirror therapy practice or guidance took place. The patient then self-treated his phantom pain at home in the complete absence of therapist supervision and without following a structured protocol of exercises. The patient performed 25 min of mirror therapy daily in his own home. During his mirror therapy sessions he moved his intact limb in any way he wished. The goal of the leg movement was to create visual interest while he observed the image in the mirror and to create the visual representation that suggested he had two intact and fully functioning legs. Within approximately 6 weeks the patient reported significant reduction in phantom pain, and within 3 months his phantom pain resolved. Mood and function were concomitantly restored and pain medication was stopped.
The success of the case study offered promise that mirror therapy may be a simpler treatment modality than is currently described, and that mirror therapy may be successful with simple education and full self-delivery outside of the clinic and the research laboratory. If patients were able to fully self-administer mirror therapy with minimal instruction, the treatment would have much broader application in areas of the world where physical therapy and pain specialists are scarce (e.g. in rural locations or in countries with poor healthcare resources). As such, global patient access to mirror therapy, a low-cost phantom pain treatment, would expand.
Accordingly, the aims of the current pilot study were: (i) to determine whether the majority of subjects would self-treat phantom pain with mirror therapy without therapist guidance; and (ii) to report outcomes for participants who initiated fully home-based self-delivered mirror treatment for phantom pain (given verbal and visual instruction only and without a single guided practice/treatment session). The main outcome was mean phantom pain intensity ratings at post-treatment months 1 and 2. We aimed to determine the short-term preliminary efficacy of self-delivered mirror therapy and whether demographic variables were related to treatment response.
METHODS
This was an uncontrolled prospective treatment outcome pilot study.
Subjects and setting
Forty community-dwelling adults with unilateral upper or lower extremity amputation who responded to study flyers and online study advertisements between April 2009 and April 2010 participated in this study. The study was approved by the Institutional Review Board of Oregon Health & Science University, Portland, Oregon, USA.
Inclusion criteria consisted of: (i) ages 18–75 years, (ii) phantom pain intensity rated >3 on the numeric rating scale, and (iii) complete amputation surgical healing. Subjects were excluded if they did not speak English, or if they had: (i) bilateral amputation, (ii) diabetic vascular disease aetiology of amputation, or (iii) cognitive impairment. We aimed first to test the intervention in a sample of non-diabetic vascular disease aetiology of amputation with the rationale that diabetic dysvascular disease might be a proxy for health/behavioural compliance. A larger second-phase study including (or focused on) diabetic dysvascular aetiology could then determine any aetiology effects.
Procedures
Subjects were first screened via telephone for eligibility by the study coordinator. Enrolment occurred in one of two ways, either during a one-time study visit for local subjects, or remotely if the person lived out of state. The purpose of the study visit was to obtain informed consent, administer baseline measures, provide a brief demonstration of mirror therapy, and distribute the study materials. Eleven persons were enrolled remotely; informed consent and baseline measurements were completed and returned via standard mail. All subjects were paid $10 USD for completing month 1 questionnaires. The study coordinator called each subject weekly during the first month of treatment to ask if they had any questions about the study procedures. If study diaries and questionnaires were not promptly received following treatment weeks 4 and 8, the study coordinator reminded subjects to return their completed diaries and questionnaire packet by post. The study coordinator was also available by telephone and e-mail to respond to any additional questions study subjects may have had.
Each subject received a study binder that contained an information sheet on mirror therapy; a set of self-addressed, postage paid envelopes; and daily mirror therapy diaries and study questionnaires to complete and post back at the 1- and 2-month time-points. Subjects enrolled in person were also given a mirror (a full-length mirror for lower extremity amputation or a shorter mirror for upper extremity amputation). Subjects enrolled remotely received the same study binder and they also received a 7-min DVD that showed a brief demonstration of home-based self-delivered mirror therapy and reviewed all study instructions; they also received an additional $10 USD to purchase a mirror for the purposes of the study. Whether subjects were enrolled in person or remotely, the mirror therapy instructions were brief and consistent. Subjects were shown how to position a mirror to hide their amputation site behind it and thus be able to view the reflected image of their non-amputated limb in the mirror. As such, when looking down at their body they would see the image of having two intact and functioning limbs. All subjects were also told the following key points: “(1) Set aside 25 min daily to practice your mirror therapy; (2) find a comfortable position with your mirror; (3) keep your eyes positioned such that you see the image of having 2 intact limbs (i.e. look down and see your intact limb and the mirror image of that limb); (4) move your intact limb gently, in any way you wish, for the 25 minutes. The goal of performing mirror therapy is for you to see 2 healthy and functioning limbs; (5) varying your movements may prevent boredom.” Participants were not instructed to either move or not move their phantom limb. The remainder of the DVD content involved orienting subjects to the study binder, the questionnaires to be completed at months 1 and 2, and procedures for posting completed questionnaires back to study staff.
Subjects were instructed to self-deliver mirror therapy daily for 25 min, and to complete and return diaries and questionnaires at months 1 and 2.
Measures
Demographics and medical history
Demographic information collected at enrolment (baseline) included sex, age, race, number of years of education, veteran status, and employment status. Amputation-related information collected at baseline included aetiology of amputation, amputation location, average phantom pain intensity, presence of residual limb pain (yes/no), frequency of phantom sensations (never, monthly, weekly, daily, constant), bothersomeness of phantom sensations (no sensations, not bothered, somewhat bothered, extremely bothered), time since amputation and current prosthesis use (yes/no).
Depressive symptoms
The Centers for Epidemiologic Studies Depression subscale (CES-D) (17) was used at baseline to quantify the level of depressive symptoms. The CES-D score was used to determine whether baseline depressive symptoms predicted either initiation of treatment or response to treatment if treatment was initiated. Subjects respond to the 20 items by indicating how often they experienced each symptom in the past week (0, rarely or none of the time; 1, some of the time; 2, much of the time; 3, most or all of the time), with a possible total of 60 points. The CES-D is a commonly used measure in limb loss outcomes research (5, 18, 19). The widely used cut-score of 16 was used to distinguish persons with a significant level of depressive symptoms (20).
Phantom pain intensity
At each time-point subjects were asked to rate the mean intensity of their phantom pain. At each monthly interval participants were asked to rate their mean pain intensity over the past month using an 11-point numeric rating scale (–10) anchored by 0 = no pain and 10 = worst pain imaginable. A monthly post-treatment mean pain intensity rating was used because it represented a more conservative estimate of pain change.
Daily mirror therapy diary
As a measure of treatment adherence and treatment experience, subjects were asked to track their mirror therapy practice with a daily diary. The diary form included the day’s date, a phantom pain intensity rating (0–10), quantification of mirror therapy (in minutes), and space for a brief description of the practice session.
Statistical analysis
All data analyses were performed using SAS® software release 9.2. Baseline characteristics for the sample were summarized descriptively by median and interquartile range (IQR) for continuous variables, and frequency and percentage for categorical variables. The continuous variables, such as age and years of amputation, were dichotomized into two groups based on the median value, and the baseline level of depressive symptoms (CES-D score) was categorized into two groups based on previous literature (20, 21). Subjects’ baseline pain level served as their own control. The time-effect of mirror therapy on mean phantom pain intensity and changes from baseline were evaluated using the GLIMMIX procedure to accommodate non-normal distribution and repeated measurement. In addition, absolute changes from baseline to both month 1 and month 2 for all subjects who remained in the study were displayed graphically and the magnitude of change was examined using the Wilcoxon signed-rank test. Finally, we compared baseline phantom pain intensity and percentage change from baseline between demographic and clinical characteristic groups using the Wilcoxon two-sample test. All reported p-values were two-sided, and p-values less than 0.05 were considered significant.
RESULTS
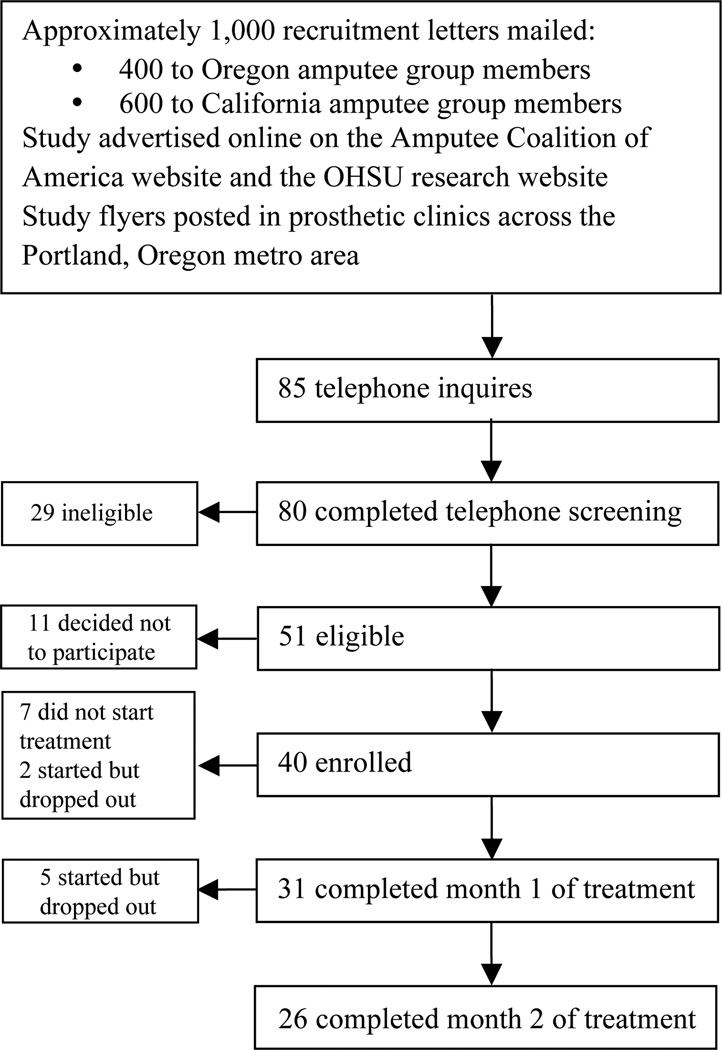
Fig. 1 shows a flowchart for the study population. Of the 40 persons who enrolled in the study, 9 (22.5%) did not initiate treatment; of these, 5 reported that they discontinued study participation due to other life concerns taking precedence (e.g. a move or an acute illness). Contact was lost with the remaining 4 persons who did not initiate treatment, and therefore their reasons for withdrawing from the study are unknown. The 9 subjects who did not initiate treatment were excluded from the analysis.
Fig. 1.
Subject flowchart. OHSV: Oregon Health & Science University.
Final analysis included the 31 subjects who initiated treatment (response rate 77.5%) and completed month 1. Table I presents the baseline characteristics for the study subjects (n = 31). The sample is noted to be predominantly White or Hispanic (90.3%), >60 years of age (54.8%), with an almost even gender split, highly educated (58.1% have >16 years of education), and 51.6% were at least 5 years post-amputation. At baseline, 87.1% of subjects reported “daily” or “constant” phantom sensations, and 90.4% reported being “somewhat bothered” or “extremely bothered” by phantom sensations. This information is omitted from Table I because the majority of subjects reported these symptoms and because the sample size is too small to split the group and test the difference. Of the 31 subjects who initiated treatment, 26 completed month 2 therapy.
Table I.
Sample characteristics at baseline (n =31)
| Factor | Median (IQR) |
n(%) |
|---|---|---|
| Gender | ||
| Female | 13(41.9) | |
| Male | 18(58.1) | |
| Age(range 32–74) | 61(50–64) | |
| <60 years | 14(45.2) | |
| >60 years | 17(54.8) | |
| Race | ||
| White or Hispanic | 28 (90.3) | |
| Non-White/Non-Hispanic | 3 (9.7) | |
| Education, (range 8–21 years) | 16 (13–17) | |
| <16 years | 13 (41.9) | |
| >16 years (college) | 18 (58.1) | |
| Time since amputation, (range 0.2–59 years) | 6 (2–16) | |
| <5 years | 15 (48.4) | |
| >5 years | 16 (51.6) | |
| Mean phantom pain intensity (range 4–10) | 6 (5–8) | |
| Moderate (4–5) | 12 (38.7) | |
| Severe (6–10) | 19 (61.3) | |
| Employment status | ||
| Retired or working (full- or part-time) | 21 (67.7) | |
| Not working due to disability | 10 (32.3) | |
| Veteran status | ||
| Veteran | 6 (19.4) | |
| Non-veteran | 25 (80.6) | |
| Aetiology of amputation | ||
| Trauma | 14 (45.2) | |
| Non-trauma | 17 (54.8) | |
| Amputation location | ||
| Lower extremity | 20 (64.5) | |
| Upper extremity | 11 (35.5) | |
| Amputation side | ||
| Right | 15 (48.4) | |
| Left | 16 (51.6) | |
| Prosthetic user | ||
| No | 11 (35.5) | |
| Yes | 20 (64.5) | |
| Depressive symptoms (CES-D) (range 0–43) | 9 (4–16) | |
| <16 | 20 (64.5) | |
| >16 | 7 (22.6) | |
| Unknown | 4 (12.9) | |
| Residual limb (stump) pain | ||
| No | 12 (38.7) | |
| Yes | 17 (54.8) | |
| Unknown | 2 (6.5) |
IQR: interquartile range; CES-D: Centers for Epidemiologic Studies Depression subscale.
Table II.
Median baseline pain and % change from baseline to month 2
| Baseline |
% change |
|||
|---|---|---|---|---|
| Factor | Median (IQR) | p-value | Median (IQR) | p-value |
| Overall | ||||
| Baseline | 6.0 (5.0–8.0) | |||
| Month 2 | 5.0 (3.0–6.0) | −15.5 (0 to –40.0) | ||
| Age | ||||
| <60 years | 6.0 (5.0–7.0) | 0.46 | −11.8 (0 to 34.3) | 0.39 |
| >60 years | 6.0 (5.0–8.0) | −24.3 (0 to –62.5) | ||
| Race | ||||
| White/Hispanic | 6.0 (4.5–8.0) | 0.76 | −11.1 (–33.3 to –62.5) | 0.29 |
| Non-White/Hispanic | 6.5 (5.0–8.0) | −16.7 (0 to –40.0) | ||
| Gender | ||||
| Male | 7 (5.0–8.0) | 0.34 | −12.5 (0 to –60) | 0.92 |
| Female | 6.0 (5.0–7.0) | −16.7 (0 to –40) | ||
| Education | ||||
| <16 years | 6.0 (5.0–7.0) | 0.64 | 0 (16.7 to –16.7) | 0.01 |
| >16 years | 7.0 (5.0–8.0) | −28.6 (–11.1 to –71.4) | ||
| Time since amputation | ||||
| <5 years | 6.0 (5.0–8.0) | 0.97 | −15.5 (0 to –61.3) | 0.86 |
| >5 years | 6.5 (5.0–8.0) | −20.5 (0 to –38.9) | ||
| Not working due to disability | ||||
| No | 7.0 (5.0–8.0) | 0.39 | −28.6 (–6.3 to –61.3) | 0.06 |
| Yes | 5.5 (5.0–7.0) | 0 (11.1 to –14.3) | ||
| Aetiology of amputation | ||||
| Non–trauma | 6.0 (5.0–7.0) | 0.43 | −13.9 (0 to –33.3) | 0.57 |
| Trauma | 6.5 (5.0–8.0) | −21.4 (0 to –50.0) | ||
| Prosthesis use | ||||
| No | 6.0 (5.0–7.5) | 0.32 | −14.3 (0 to –38.9) | 0.80 |
| Yes | 7.0 (5.0–8.0) | −16.7 (0 to –40.0) | ||
| Depressive symptoms (CES-D) | ||||
| <16 | 6.0 (5.0–8.0) | 0.34 | −18.3 (0 to –38.9) | 0.50 |
| >16 | 7.0 (5.0–8.5) | 0 (0 to –14.3) | ||
| Amputation side | ||||
| Left | 7.0 (5.0–8.0) | 0.33 | −16.7 (0 to –60.0) | 0.87 |
| Right | 5.5 (5.0–7.0) | −12.5 (0 to –38.9) | ||
| Residual limb (stump) pain | ||||
| No | 7.0 (5.0–8.0) | 0.57 | −14.3 (0 to –40.0) | 0.81 |
| Yes | 6.0 (5.0–8.0) | −16.7 (0 to –38.9) | ||
IQR: interquartile range; CES-D: Centers for Epidemiologic Studies Depression subscale.
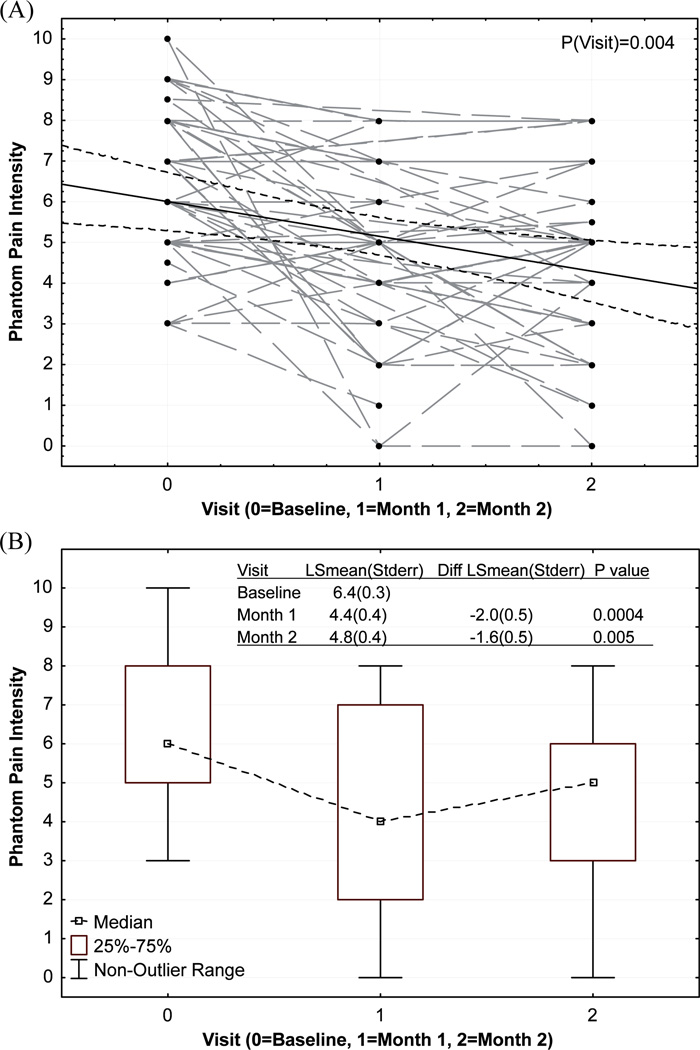
To test the hypothesis that mean phantom pain intensity would be reduced from baseline to post-treatment (months 1 and 2), phantom pain intensity for all subjects at each time-point, we fitted an estimated regression line with 95% confidence bands and these results are displayed in Fig. 2A, and a box-plot of median and interquartile range of pain intensity at each time-point are shown in Fig. 2B. Estimated least square means (LSmean) at each time-point were also shown in Fig. 2B. A trend of reduction was observed from the regression line and a significant time effect was detected. Mean phantom pain intensity at months 1 and 2 were compared with mean phantom pain intensity at baseline. A significant reduction in mean phantom pain intensity was found at both time-points during treatment. While a small rebound in pain intensity was observed at month 2, this rebound difference (between months 1 and 2) was not statistically significant.
Fig. 2.
Phantom pain intensity. (A) Raw points and estimated regression line with 95% confidence bands. (B) Box-plot. LSmean: estimated least square means; Sterr: standard error; diff: difference.
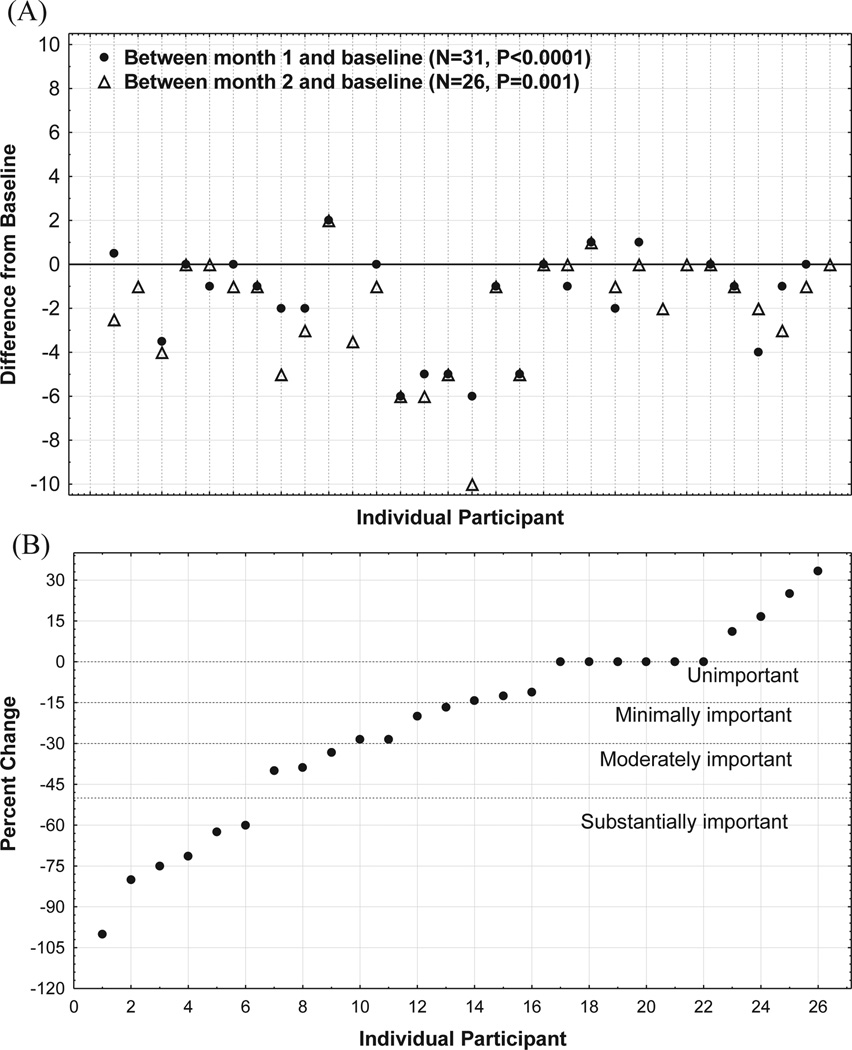
The median percentage reduction in phantom pain intensity for the entire sample from baseline to month 2 was 15.5%. Fig. 3A presents the absolute reduction in mean phantom pain intensity from baseline to months 1 and 2 for each participant. Four subjects reported worse phantom pain at the end of the study (increases ranged from 0.5 to 2 on the 11-point numeric rating scale), 6 subjects reported no change, and 16 subjects reported reductions in phantom pain ranging from 1 to 6 points on the numeric rating scale. Fig. 3B presents the percentage change from baseline to month 2 for each participant. Included in this figure is a description of the level of clinical importance. Thirteen participants reported pain reductions that were at least minimally important in magnitude (>15%), with 9 of these 13 participants achieving moderately (>30%) or substantially important (>50%) reductions in mean phantom pain intensity.
Fig. 3.
(A) Absolute change in phantom pain from baseline to month 2 for each individual participant, and (B) percentage change. 0 for same as baseline, <0 for reduction, >0 for increase.
To determine whether demographic and clinical characteristics were associated with baseline phantom pain and pain reduction after mirror therapy, baseline pain intensity was compared between groups for each factor in Table I. To control for baseline impact, the percentage reduction from baseline was examined between groups (Table II). Baseline phantom pain levels were similar for all listed groups. Although baseline pain levels were similar between “years of education” groups, a significant difference in percentage reduction between the “ <16 and >16 years of education” groups was found (median 0 vs –28.6%, p = 0.01). Among the 7 subjects who had >3 phantom pain intensity reduction at month 2, 6 of them had >16 years of education (4 or more years of university education).
In terms of adverse effects, 2 people cited boredom, 2 reported that the mirror therapy made them more aware of the missing limb, 2 reported increased phantom pain, 2 reported having increased phantom sensations that resolved fairly quickly, and 1 reported feeling depressed at seeing their leg in the mirror. Two people reported having cramping in their existing limb, which was determined to be related to aggressive movements during mirror therapy. These subjects were told to perform gentler movements and, after doing so, their cramping resolved.
DISCUSSION
This pilot study aimed to determine the whether the majority of participants would self-treat phantom pain with mirror therapy without therapist guidance. Of the 31 subjects who initiated self-treatment (77.5%), 31 completed month 1 (100%), 26 completed month 2 (84%), and 5 dropped out of the study (16%). These findings confirmed our hypothesis that the majority of study subjects would engage in the short-term self-treatment. Findings also suggested that subjects who begin self-treatment are likely to complete it. A high level of missing data in the daily diaries precluded our ability to correlate the amount of time practiced with treatment response. Participants were called by the study coordinator (weekly for month 1 and also at month 2) and verbally confirmed their ongoing mirror practice; however, they were not asked to verbally report an average number of minutes practiced each day. Those who reported continuing the treatment remained in the study. The consequence of this limitation in study design is that we were unable to correlate dose-response of mirror therapy (time of practice) to the outcome of phantom pain reduction, and this is highlighted as an objective for a future study. This limitation does not affect the pain level measured at months 1 and 2. The poor completion rate of daily diaries may be inherent to the handwritten diary format (22). Future research may improve data collection by utilizing electronic diaries, as these have been shown to be a superior method of data collection (22). Alternatively, the study coordinator may call participants weekly and ask participants to provide an average for the minutes of daily practice, although this latter method may introduce recall bias.
Clinically, it would be useful to know which patients are likely to initiate self-delivered mirror therapy. We found a trend for those with depression to be less likely to initiate treatment and more likely to drop out, which may reach significance with a larger sample size. The trend observed here is concordant with the relationship between depression and self-management behaviours described in the extant healthcare literature (23). Outcomes may be improved by screening patients for depression and either treating the depression prior to recommending self-delivered mirror therapy, or referring them to a therapist for in-clinic supervision with mirror therapy.
A recently published literature review found that mirror therapy studies have not provided sufficient evidence to determine which patients might benefit most from mirror therapy (24). For the current study, while we observed a trend for depression, only the number of years of education was found potentially to affect the magnitude of the response to self-delivered mirror therapy. Subjects with university-level education reported greater phantom pain reduction. Prior work has similarly shown that amputees with less education have poorer health outcomes. For instance, compared with amputees with university education, amputees with high-school education or less are more likely to be depressed (5) and to have greater levels of non-phantom amputation-related pain (1). Research in other populations has also linked lower levels of education to greater disability (25) and poorer response to medical treatment (25).
Unfortunately, the treatment diary data were not complete enough for us to test whether education level related to treatment compliance (some subjects did not return treatment diaries and others had difficulty writing and only tracked their phantom pain intensity levels over time). It is possible that people with greater education understand mirror therapy better and are more engaged during therapy regardless of whether the treatment is supervised or self-delivered in the home. We found no other studies reporting an association between level of education and response to mirror therapy. Further studies are needed to replicate our finding. Future research may also determine whether an education effect is explained by adherence to treatment or treatment expectations. Perhaps unsupervised, fully self-delivered mirror treatment may be most appropriate for people with university-level education. Future research may determine whether response to self-treatment may be boosted by additional support (e.g. providing additional background information, online support or telesupport), or whether working with a physical therapist is the best course of care for this subpopulation.
Other factors, such as depressive symptoms, employment status (factors known to associate with education level) and age may also have some impact on whether patients are likely to initiate and complete self-treatment.
We found that the attrition rate varied by enrolment type, such that those who enrolled remotely appeared more likely to either not initiate treatment or to drop out (25.6% attrition rate for in-person enrolment compared with 54.5% for remotely enrolled persons). The reasons for attrition were varied. The variance in attrition for in-person and remote enrolment could be due to several factors, but may partially reflect differences in baseline motivation, as the in-person visit required greater commitment and effort to enrol in the study.
A previous report suggested that the majority of patients who initiate mirror therapy experience adverse effects (58%), with the predominant adversities being dizziness, irritation and uneasiness (26). The patients in this study were undergoing acute rehabilitation and therefore these reported effects may be uniquely related to time since amputation. For the current study, we found a lower rate of adverse effects (33%) with greater variation in effect type (boredom, increased awareness of the missing limb, increased phantom pain, increased phantom sensations that resolved fairly quickly, feelings of depression, and cramping in their existing limb. Clinically, it may be useful to provide patient education about potential adverse effects of mirror therapy prior to initiation of treatment, and to provide information about how to deal with these possible experiences. For instance, study subjects found it helpful to learn that cramping in their existing limb was probably due to overly aggressive movements while performing mirror therapy; indeed, their symptoms resolved when they made gentler movements. People should be made aware of the potential for increased symptoms of depression or a grief reaction, and should be given information on accessing appropriate psychological services. Lastly, increased phantom pain may be an indication that mirror therapy is contraindicated.
In terms of preliminary efficacy of fully self-delivered mirror therapy, the clinical meaningfulness of phantom pain reduction should be considered. In accordance with the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials consensus statement on the clinical importance in pain research, a decrease in pain of <15 % is considered an unimportant change, >15% a minimally important change, >30% a moderately important change, and >50% a substantially important change (27). Results from this pilot study generally achieve the threshold for minimally important change overall (median 15.5% reduction). The true “treatment responders” of this study (n = 9) achieved the moderately important change threshold by achieving phantom pain reductions that ranged from 3 to 6 points (range 33.3–100% reduction).
Interestingly, we did not find that level of depressive symptoms correlated with treatment response, but acknowledge that only 7 persons in the study exceeded the cut-score for significant level of depressive symptoms. Thus, we observed a trend toward depression attenuating treatment response, but our ability to test meaningful differences was limited by the small sample size. Indeed, the current study did not detect significant effects for either level of depressive symptoms or employment status (factors that are frequently associated with level of education), it is possible that self-delivered mirror treatment may be less appropriate for people who are severely depressed and are more disabled. Instead, these patients may benefit from working with a physical therapist or psychologist, with the latter potentially bolstering self-efficacy, self-care behaviours, motivation, and adherence to mirror therapy.
Findings from this study appear to support fully self-delivered mirror therapy as a phantom pain treatment for some patients. For those with greater education, simple instructions and a visual (or DVD) demonstration may provide sufficient training to begin and complete self-treatment without any therapist supervision. As is the case with many behavioural programmes (e.g. diet, exercise), motivation is required to initiate and adhere to daily mirror therapy. Fully self-delivered mirror therapy may not be appropriate for all patients, such as those with a lower level of education or motivation.
As with most pilot studies, the main limitations of this work include the small sample and the lack of a control group. A larger, better powered-study, ideally randomized and controlled, is needed to determine true efficacy rates and predictors for success with self-delivered mirror therapy. Furthermore, these findings require replication and extension to a diabetic dysvascular population. The study design includes selection bias, in that participants responded to a study flyer and self-referred, and it is possible that enrolees were more functional and motivated than the general population of amputees. Another limitation is that study subjects may have been unduly influenced by the research milieu. For instance, subjects were called weekly for the first month of the study to ask whether they had any questions about the treatment, and this contact with the study coordinator may have bolstered motivation to self-deliver the treatment.
The strength of this study is that the findings extend our understanding of mirror therapy by suggesting that self-delivered treatment is effective for phantom pain reduction in highly educated amputees. While replication of these findings is needed, this pilot study offers promise that patient access to mirror therapy may broaden for educated patients without therapist guidance. The factors that may moderate treatment response in less-educated amputees remain unknown, but may include levels of motivation, depressive symptoms, and disability. Until further research elucidates the factors that moderate treatment response in less-educated individuals, clinicians should consider recommending additional support for these patients.
ACKNOWLEDGEMENTS
This research was supported by the NIH Office for Women’s Health Research (BIRCWH) 2K12HD043488-06 (BD Darnall) and by the research division of the Department of Anesthesiology and Perioperative Medicine at Oregon Health & Science University.
We thank Kathy Parker, MSN, for assistance with study coordination.
Footnotes
A part of the results were presented in poster format at the American Pain Society annual conference, 6 May 2010, Baltimore, MD, USA, and at the Egyptian Society for the Management of Pain, 29 October 2010, Cairo, Egypt.
REFERENCES
- 1.Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–1919. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 2.Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81:1039–1044. doi: 10.1053/apmr.2000.7583. [DOI] [PubMed] [Google Scholar]
- 3.Nikolajsen L, Jensen TS. Phantom limb pain. Br J Anaesth. 2001;87:107–116. doi: 10.1093/bja/87.1.107. [DOI] [PubMed] [Google Scholar]
- 4.Desmond DM, Maclachlan M. Prevalence and characteristics of phantom limb pain and residual limb pain in the long term after upper limb amputation. Int J Rehabil Res. 2010;33:279–282. doi: 10.1097/MRR.0b013e328336388d. [DOI] [PubMed] [Google Scholar]
- 5.Darnall BD, Ephraim P, Wegener ST, Dillingham T, Pezzin L, Rossbach P, et al. Depressive symptoms and mental health service utilization among persons with limb loss: results of a national survey. Arch Phys Med Rehabil. 2005;86:650–658. doi: 10.1016/j.apmr.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Weeks SR, Anderson-Barnes VC, Tsao JW. Phantom limb pain: theories and therapies. The Neurologist. 2010;16:277–286. doi: 10.1097/NRL.0b013e3181edf128. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran VS, Rogers-Ramachandran D, Cobb S. Touching the phantom limb. Nature. 1995;377:489–490. doi: 10.1038/377489a0. [DOI] [PubMed] [Google Scholar]
- 9.Darnall BD. Self-delivered home-based mirror therapy for lower limb phantom pain. Am J Phys Med Rehabil. 2009;88:78–81. doi: 10.1097/PHM.0b013e318191105b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 11.MacLachlan M, McDonald D, Waloch J. Mirror treatment of lower limb phantom pain: a case study. Disabil Rehabil. 2004;26:901–904. doi: 10.1080/09638280410001708913. [DOI] [PubMed] [Google Scholar]
- 12.Mercier C, Sirigu A. Training with virtual visual feedback to alleviate phantom limb pain. Neurorehabil Neural Repair. 2009;23:587–594. doi: 10.1177/1545968308328717. [DOI] [PubMed] [Google Scholar]
- 13.Chan BL , Witt R, Charrow AP, Magee A, Howard R, Pasquina PF, et al. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357:2206–2207. doi: 10.1056/NEJMc071927. [DOI] [PubMed] [Google Scholar]
- 14.Brodie EE, Whyte A, Niven CA. Analgesia through the looking-glass? A randomized controlled trial investigating the effect of viewing a ‘virtual’ limb upon phantom limb pain, sensation and movement. Eur J Pain. 2007;11:428–436. doi: 10.1016/j.ejpain.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Brodie EE, Whyte A, Waller B. Increased motor control of a phantom leg in humans results from the visual feedback of a virtual leg. Neurosci Lett. 2003;341:167–169. doi: 10.1016/s0304-3940(03)00160-5. [DOI] [PubMed] [Google Scholar]
- 16.Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DM, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353:2035–2036. doi: 10.1016/s0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–396. [Google Scholar]
- 18.Jensen MP, Ehde DM, Hoffman AJ, Patterson DR, Czerniecki JM, Robinson LR. Cognitions, coping and social environment predict adjustment to phantom limb pain. Pain. 2002;95:133–142. doi: 10.1016/s0304-3959(01)00390-6. [DOI] [PubMed] [Google Scholar]
- 19.Wegener ST, Mackenzie EJ, Ephraim P, Ehde D, Williams R. Self-management improves outcomes in persons with limb loss. Arch Phys Med Rehabil. 2009;90:373–380. doi: 10.1016/j.apmr.2008.08.222. [DOI] [PubMed] [Google Scholar]
- 20.Boyd JH, Weissman MM, Thompson WD, Myers JK. Screening for depression in a community sample. Understanding the discrepancies between depression symptom and diagnostic scales. Arch Gen Psychiatry. 1982;39:1195–1200. doi: 10.1001/archpsyc.1982.04290100059010. [DOI] [PubMed] [Google Scholar]
- 21.Phillips LA, Carroll LJ, Cassidy JD, Cote P. Whiplash-associated disorders: who gets depressed? Who stays depressed? Eur Spine J. 2010;19:945–956. doi: 10.1007/s00586-010-1276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Controlled Clinical Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 23.Linn T, Ebener K, Raptis G, Laube H, Federlin K. Natural course of insulin sensitivity and insulin reserve in early insulin-dependent diabetes mellitus. Metabolism. 1995;44:617–623. doi: 10.1016/0026-0495(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 24.Rothgangel AS, Braun SM, Beurskens AJ, Seitz RJ, Wade DT. The clinical aspects of mirror therapy in rehabilitation: a systematic review of the literature. Int J Rehabil Res. 2011;34:1–13. doi: 10.1097/MRR.0b013e3283441e98. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Olivo MA, Landon GC, Siff SJ, et al. Psychosocial determinants of outcomes in knee replacement. Ann Rheum Dis. 2011;70:1775–1781. doi: 10.1136/ard.2010.146423. [DOI] [PubMed] [Google Scholar]
- 26.Casale R, Damiani C, Rosati V. Mirror therapy in the rehabilitation of lower-limb amputation: are there any contraindications? Am J Phys Med Rehabil. 2009;88:837–842. doi: 10.1097/PHM.0b013e3181b74698. [DOI] [PubMed] [Google Scholar]
- 27.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]