Abstract
Background
Mutations in podocyte and basement membrane genes are associated with a growing spectrum of glomerular disease affecting adults and children. Investigation of familial cases has helped to build understanding of both normal physiology and disease.
Methods
We investigated a consanguineous family with a wide clinical phenotype of glomerular disease using clinical, histological, and new genetic studies.
Results
We report striking variability in severity of nephropathy within an X-linked Alport syndrome (XLAS) family. Four siblings each carried a mutant COL4A5 allele, p.(Gly953Val) and p.(Gly1033Arg). Two boys had signs limited to hematuria and mild/moderate proteinuria. In striking contrast, a sister presented with end-stage renal disease (ESRD) at 8 years of age and an infant brother presented with nephrotic syndrome, progressing to ESRD by 3 years of age. Both were subsequently found to have homozygous variants in MYO1E, p.(Lys118Glu) and p.(Thr876Arg). MYO1E is a gene implicated in focal segmental glomerulosclerosis and it encodes a podocyte-expressed non-muscle myosin. Bioinformatic modeling demonstrated that the collagen IV-alpha3,4,5 extracellular network connected via known protein–protein interactions to intracellular myosin 1E.
Conclusions
COL4A5 and MYO1E mutations may summate to perturb common signaling pathways, resulting in more severe disease than anticipated independently. We suggest screening for MYO1E and other non-COL4 ‘podocyte gene’ mutations in XLAS when clinical nephropathy is more severe than expected for an individual’s age and sex.
Keywords: Alport syndrome, Genetics, COL4A5, MYO1E, Nephrotic syndrome, Whole exome sequencing
Introduction
In 1927, Cecil Alport described an inherited nephropathy [1] with the teenage male proband having “an attack of influenza followed by a large increase of blood and albumin in the urine”. Alport noted that the nephropathy could progress to uremia and it was inherited through females. Subsequently, the disease was called X-linked Alport syndrome (XLAS). Affected individuals have glomerular basement membrane (GBM) ultrastructural defects. Transmission electron microscopy (TEM) generally shows “diffuse thickening and splitting with strikingly irregular outer and inner contours”, although, especially in women and children, “irregular alternation of thick and abnormally thin GBM” or “diffusely thin GBM” can occur [2].
Mutations of COL4A5 encoding the alpha-5 chain of collagen IV, a GBM component, cause XLAS [3]. A minority of AS individuals have mutations of autosomal genes encoding collagen IV alpha-3 or alpha-4 chains [4], which form heterotrimers with alpha-5. The same collagen IV network is found in ear and eye BMs and AS can feature sensorineural deafness and ophthalmic complications, although these are uncommon in children [5, 6]. The generally less aggressive course of XLAS nephropathy in women is explained by the fact that they carry both a normal and mutant COL4A5 allele versus males who have only the mutated allele [5]. In men, more severe nephropathy can correlate with predicted null versus missense mutations [2, 7]. However, as now described, there exists another genetic explanation underlying variation in the severity of XLAS nephropathy.
Methods
Genetic studies
Samples were collected following informed consent. COL4 Sanger sequencing was performed as previously described [8]. Analysis of X-inactivation was also performed as previously reported [9].
Whole exome sequencing
Library preparation, sequencing, and data generation was performed in the Genomics Core Facility of the Biomedical Research Centre at Guy’s and St Thomas’ Hospitals and King’s College London. DNA libraries were prepared from 3 μg dsDNA using SureSelect Human All Exon 50 Mb kit (Agilent Technologies). Samples were multiplexed (four samples on each lane) and 100 base pair paired end sequencing was performed on Illumina HiSeq system. The mean coverage was 136× (97 % of target sequenced with depth of ≥5×, 95 % ≥10×, and 92 % ≥20×). Sequence data were aligned to the GRCh37/hg19 human reference genome using Novoalign, variants were called with SAMtools and annotated via multiple passes through Annovar. Exome sequencing data analysis was performed at the University of Bristol (Academic Renal Unit). Variants of interest were confirmed using Sanger sequencing (Eurofins MWG Operon, Germany). Primers used for MYO1E were published by Mele et al. [10] and for COL4A5 by Martin et al. 1998 [11]; the KAPA HiFi PCR Kit (Kapa Biosystems) was used for the amplification.
Transmission electron microscopy
Samples were fixed for at least 1 h in Neutral Buffered Formalin 10 % V/V and fixative was replaced with 2.5 % glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for at least 3 h. Post-fixation samples were incubated in 1 % proprietary (TAAB) osmium tetroxide solution and rinsed in 0.1 M sodium cacodylate buffer followed by dehydration in alcohols graded up to 100 % ethanol. Samples were incubated in propylene oxide (TAAB) to remove the alcohol and heated to 40 °C in 50/50 mix propylene oxide/resin (TAAB Araldite CYC212) followed by 100 % resin for 2 h before embedding in resin blocks for 12 h at 60 °C. Ultrathin 120-nm sections were cut with a Leica UC7 ultramicrotome and placed on copper grids. Grids were stained in 2 % uranyl acetate 10 min before the addition of Reynolds’ lead citrate for 10 min. The grids were observed in a JEOL 100CX transmission electron microscope.
Protein interaction network
Protein interaction network analysis was performed using Cytoscape (version 2.8.1) [12]. Collagen IV alpha-3,4,5 and myosin 1E were mapped onto a merged human interactome built from the Protein Interaction Network Analysis platform Homo sapiens network (release date, June 28, 2011) and Mus musculus network (release date, June 28, 2011) [13], the extracellular matrix (ECM) interactions database MatrixDB (release date, August 26, 2010) [14], and a literature-curated database of integrin-based adhesion-associated proteins [15]. Topological parameters were computed using the NetworkAnalyzer plug-in [16].
Results
Wide phenotypic variation in a family with inherited glomerular disease
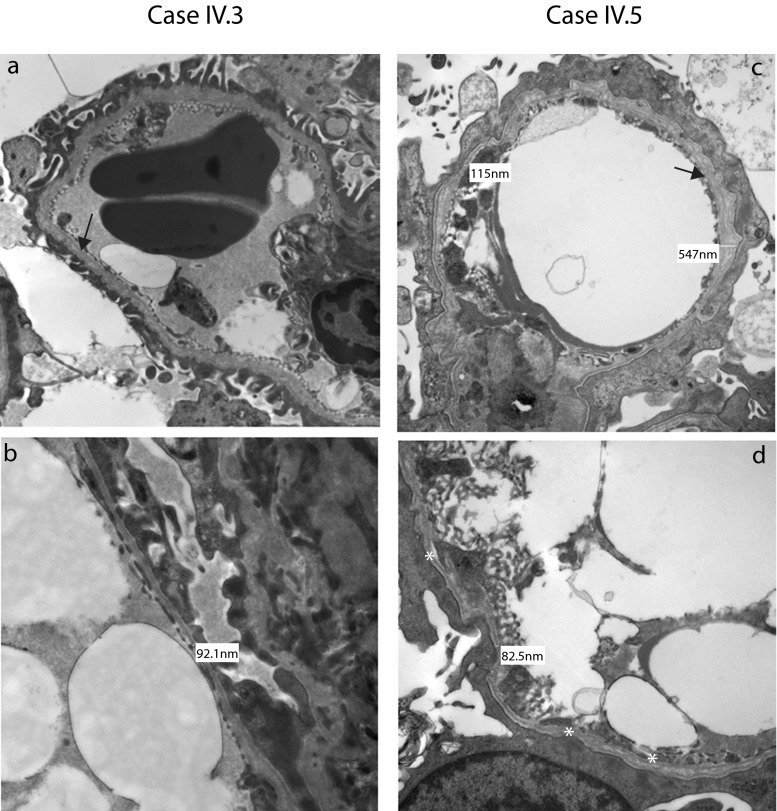
Five siblings from a consanguineous family of Pakistani origin (Fig. 1) were investigated in the Royal Manchester Children’s Hospital’s Renal Genetic Clinic. The proband (IV.3) presented at the age of 2 years with pyrexia and hematuria. Following resolution of the febrile illness, he had persistent microscopic hematuria and mild proteinuria (protein/creatinine ratio 24–50 mg/mmol; normal <20). Plasma creatinine, complement levels, and blood pressure were normal. A renal biopsy at 3 years was normal by light microscopy, with no immune deposits. On TEM, the GBM showed “variable thickness and some lamination” (Fig. 2a, b). Given these changes, after parental consent, COL4A5 Sanger sequencing was undertaken [8]. IV.3 carried two hemizygous variants, confirmed using bidirectional sequencing. The first, c.2858G > T; p.(Gly953Val) in exon 33, was already associated with XLAS [17], with the mutated amino acid highly conserved in evolution as assessed with bioinformatics software Alamut v2.0. The second was c.3097G > C; p.(Gly1033 Arg) in exon 35. This was previously unreported but is predicted to interrupt the GLY-X-Y repeat structure of the collagenous domain in the alpha-5 chain. Other missense changes in this region have been reported in AS [18]. Ophthalmology and audiometry examinations were normal in IV.3 and in his other siblings with renal disease, as presented below.
Fig. 1.
Family pedigree. Black filled symbols represent individuals affected by end-stage renal disease (ESRD) and black filled symbols with a line through are those individuals who had ESRD as a cause of death. Hatched symbols represent individuals with hematuria and or proteinuria. Genotypes for COL4A5 are indicated above the symbols (blue) and for MYO1E the genotype is indicated below (red). Each filled semicircle represents a mutant allele. † indicates age of death
Fig. 2.
Native renal biopsies (a–d). Transmission electron micrograph images from IV.3 (a, b) and IV.5 (c, d), with higher magnification images in the bottom row (b, d). In IV.3, the GBM was thin, with a diameter as low as 92 nm. There were also focal areas of lamination (black arrow in a). In IV.5, glomerular basement membranes extra cellular matrix (GBMs) were irregular, with thick and thin portions, with lamination (black arrow in c). There was extensive podocyte foot process fusion and a generalized increase in the mesangium
The proband’s older sister (IV.1) had been unwell for several years before emigrating from Pakistan. Aged 8 years, her estimated glomerular filtration rate (eGFR) was 10 ml/min/1.73 m2 and a renal biopsy was not performed due to the associated risks. She commenced dialysis and at 9 years old underwent deceased donor renal transplantation; she has satisfactory graft function 8 years later. IV.1 was found to be heterozygous for the mutant COL4A5 allele. To explain her severe renal presentation, we hypothesized that she had skewed X-inactivation of her normal COL4A5 allele. We found an X-inactivation ratio of 90:10 in her leukocyte DNA, reported in only 4 % of adult females [19].
IV.3’s older brother (IV.2) presented aged 5 years with fever and microscopic hematuria. His eGFR of 54 ml/min/1.73 m2 later improved to 104 ml/min/1.73 m2. Over the next year, he had persistent proteinuria (269–423 mg/mmol) and, although normotensive, he was treated with the angiotensin converting enzyme inhibitor (ACEI), enalapril. This follows evidence and guidelines [20–23] that renin-angiotensin system blockade may slow renal disease progression in XLAS males. At age 9 years, his proteinuria (162 mg/mmol) and renal function were stable. He was found to be hemizygous for the same mutant COL4A5 allele. A renal biopsy was not performed. The youngest female sibling (IV.4) is well and has normal urinalysis and COL4A5 alleles.
The youngest male sibling (IV.5) presented at 6 months old with macroscopic hematuria and nephrotic syndrome (urinary protein/creatinine 6220 mg/mmol). He was treated with intravenous furosemide and 20 % human albumin replacement therapy. A renal biopsy was normal by light microscopy but TEM revealed GBM thickening and lamination with foot process fusion (Fig. 2c, d). A trial of glucocorticoids was discontinued at 28 days, having no effect on proteinuria; he was subsequently maintained on oral ACEI and diuretics, which prevented edema despite hypoalbuminemia (10–11 g/l). Over 3 years, his renal function deteriorated and peritoneal dialysis was commenced.
The mother, III.5, was investigated at age 39. She had microscopic hematuria but was normotensive. Her eGFR was 82 ml/min/1.73 m2 and her 24-h urinary protein was 1.70 g. She began treatment with ACEI. She carries the mutant COL4A5 allele. Two of her brothers (III.6 and III.7) died with end-stage renal disease (ESRD) in their twenties, and her mother (II.5) had ESRD aged 58 years. The children’s father has normal urinalysis and borderline hypertension and carries a normal COL4A5 allele. His deceased father (II.3) had a history of nephropathy.
Whole exome sequencing identifies mutations in MYO1E
Nearly a century after Alport’s description of XLAS, we appreciate that mutations in a growing list of genes encoding podocyte proteins can cause glomerular disease [24–29]. Notably, coinheritance of a variant allele of one such gene, podocin-encoding NPHS2, predisposes to proteinuria and ESRD in individuals with hematuria and COL4A4 mutations [30]. In this family, the parents are first cousins so we hypothesized that a second, autosomal recessive, condition modified the severity of nephropathy. Accordingly, we performed next generation sequencing in IV.5, which included analysis of all genes currently associated with podocytopathy (ACTN4, ADCK4, ALG1, ANLN APOL1, ARHGAP24, ARHGDIA, CD151, CD2AP, COL4A3, COL4A4, COL4A5, COQ2, COQ6, CTL4A, CUBN, DGKE, E2F3, EMP2, INF2, ITGA3, ITGB4, LAMB2, LMNA, LMX1B, MYH9, MYO1E, NPHS1, NPHS2, NXF5, PAX2, PDSS2, PLCe1, PMM2, PTPRO, SCARB2, SMARCAL1, SYNPO, TRPC6, TTC21B, WT1 and ZMPSTE24), as described in [29]. This confirmed the COL4A5 mutations, and that COL4A3 and COL4A4 were normal. However, we discovered that IV.5 also carried two homozygous missense variants in MYO1E affecting highly conserved amino acids c.352A > G; p.(Lys118Glu) and c.2627C > G; p.(Thr876Arg). Furthermore, IV.1, the sister with ESRD, was also homozygous for these variants (Fig. 1). IV.2 was wild type and IV.3, IV.4 and the parents were heterozygous.
Collagen IV alpha-5 and myosin 1E share protein interactions
We undertook a protein–protein interaction network analysis [31] based on known protein–protein interactions. As depicted in Fig. 3, myosin 1E and collagen IV-alpha-3,4,5 have shared interactors in a network comprising a number of key matrix and cytoskeletal elements. Thus, COL4A5 and MYO1E mutations may summate to perturb common cell-matrix signaling pathways.
Fig. 3.
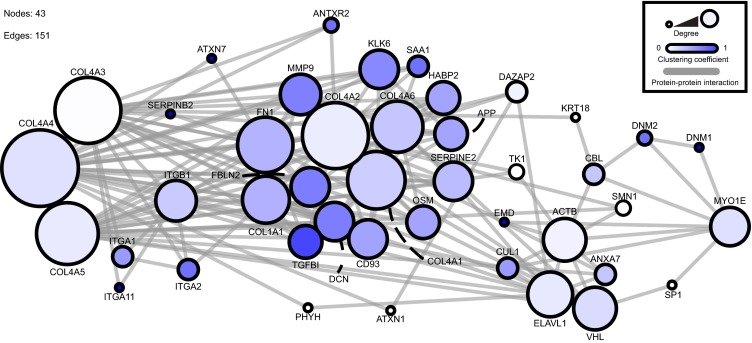
Protein–protein interaction network for collagen IV and myosin 1E. This interaction network was created from a database of known protein–protein interactions. The direct (1-hop) interactors for collagen IV-α3,4,5 and myosin 1E were identified and arranged within the network. The circles (nodes) represent proteins within the interaction network. The lines (edges) represent known interactions. The size of the node represents the degree of connections and the color of the node represents the number of interactions. The network comprises 43 nodes and 151 edges. Network analysis was performed using Cytoscape (version 2.8.1) [12]. Collagen IV alpha-3,4,5 and myosin 1E were mapped onto a merged human interactome built from the Protein Interaction Network Analysis platform Homo sapiens network (release date, 28 June 2011) and Mus musculus network (release date, 28 June 2011) [13], the ECM interactions database MatrixDB (release date, 26 August 2010) [14] and a literature-curated database of integrin-based adhesion-associated proteins [15]. Topological parameters were computed using the NetworkAnalyzer plug-in [16]
Discussion
In this kindred, the coinheritance of COL4A5 mutations and homozygous MYO1E variants were associated with more severe kidney disease within a family initially recognized to have XLAS. Skewed X inactivation may be a second contributing factor in IV.1 and possibly protected against the more rapid progression to renal failure seen in IV.5.
MYO1E encodes a podocyte-expressed non-muscle myosin [10, 32], with TH1 and TH2 domains, functioning in invadosomes; actin-rich adhesion structures modulating ECM degradation and invasion [33]. In vitro, myosin 1E overexpression protects podocytes from puromycin nephrotoxicity [32], while in vivo targeted Myo1e deletion in podocytes causes proteinuria, foot process effacement, and GBM thickening [34]. MYO1E biallelic, generally missense, mutations have been reported in children with focal segmental glomerulosclerosis and glucocorticoid-resistant proteinuria [10, 35, 36]. The variant p.(Lys118Glu) in the myosin head motor domain is a novel variant and is predicted to be deleterious. The p.(Thr876Arg) variant in the myosin tail domain is also predicted to be deleterious and it is recorded in dbSNP (rs147596471) with an allele frequency of 0.003 but only in the heterozygous state. To our knowledge, IV.5 is the youngest individual reported to date who has biallelic MYO1E mutations and who has had a renal biopsy; instead of sclerosis, only severe GBM and foot process anomalies were seen.
This extraordinary family demonstrates that mutations of two podocyte genes can explain a highly variable renal phenotype, inexplicable by conventional pedigree analysis. The mutations summate to generate life-threatening nephropathies, and insights are highly relevant to genetic counseling. We suggest screening for MYO1E and other non-COL4 ‘podocyte gene’ mutations in XLAS when clinical nephropathy is more severe than expected for an individual’s age and sex. Furthermore, the increasing use of global and unbiased genetic analysis with next-generation sequencing is likely to reveal disease-modifying variants across the range of human genetic disease.
Acknowledgments
This work was supported by a Wellcome Trust Intermediate Fellowship award (090006) to R.L. and a Wellcome Trust Clinical Training Fellowship (097208) to H.M.S. K.A.H, B.K., and A.S.W. are supported by a CMFT Strategic Investment Initiative grant. We acknowledge Adam Bryon for generating the protein–protein interaction database used in Fig. 3. The study was facilitated by the Manchester Academic Health Sciences Centre and the Manchester Biomedical Research Centre. In addition, whole exome sequencing was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London.
Author contributions
H.M.S., K.H., B.K., and A.S.W. initiated genetic investigations, H.C., J.C., F.F., A.B..., G.I.W, M.A.S., and A.K. preformed genetic analyses, G.B. reported histopathology findings, M.J.R. and R.L. performed and reviewed the network analysis. R.L., N.J.W., and A.S.W. prepared the manuscript and this was revised and reviewed by all co-authors.
Conflict of interest
The authors declare no conflicts of interests.
References
- 1.Alport AC. Hereditary familial congenital haemorrhagic nephritis. Br Med J. 1927;1:504–506. doi: 10.1136/bmj.1.3454.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidet L, Gubler MC. The renal lesions of Alport syndrome. J Am Soc Nephrol. 2009;20:1210–1215. doi: 10.1681/ASN.2008090984. [DOI] [PubMed] [Google Scholar]
- 3.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- 4.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schröder CH, Smeets HJ, Reeders ST. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet. 1994;8:77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- 5.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European community Alport syndrome concerted action” study. J Am Soc Nephrol. 2003;14:2603–2610. doi: 10.1097/01.ASN.0000090034.71205.74. [DOI] [PubMed] [Google Scholar]
- 6.Hanson H, Storey H, Pagan J, Flinter F. The value of clinical criteria in identifying patients with X-linked Alport syndrome. Clin J Am Soc Nephrol. 2011;6:198–203. doi: 10.2215/CJN.00200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierides A, Voskarides K, Kkolou M, Hadjigavriel M, Deltas C. X-linked, COL4A5 hypomorphic Alport mutations such as G624D and P628L may only exhibit thin basement membrane nephropathy with microhematuria and late onset kidney failure. Hippokratia. 2013;17:207–213. [PMC free article] [PubMed] [Google Scholar]
- 8.Storey H, Savige J, Sivakumar V, Abbs S, Flinter FA. COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol. 2013;24:1945–1954. doi: 10.1681/ASN.2012100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCauley J, Masand N, McGowan R, Rajagopalan S, Hunter A, Michaud JL, Gibson K, Robertson J, Vaz F, Abbs S, Holden ST. X-linked VACTERL with hydrocephalus syndrome: further delineation of the phenotype caused by FANCB mutations. Am J Med Genet A. 2011;155A:2370–2380. doi: 10.1002/ajmg.a.33913. [DOI] [PubMed] [Google Scholar]
- 10.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, Buelli S, Tomasoni S, Piras R, Krendel M, Bettoni S, Morigi M, Delledonne M, Pecoraro C, Abbate I, Capobianchi MR, Hildebrandt F, Otto E, Schaefer F, Macciardi F, Ozaltin F, Emre S, Ibsirlioglu T, Benigni A, Remuzzi G, Noris M, PodoNet Consortium MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med. 2011;365:295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin P, Heiskari N, Zhou J, Leinonen A, Tumelius T, Hertz JM, Barker D, Gregory M, Atkin C, Styrkarsdottir U, Neumann H, Springate J, Shows T, Pettersson E, Tryggvason K. High mutation detection rate in the COL4A5 collagen gene in suspected Alport syndrome using PCR and direct DNA sequencing. J Am Soc Nephrol. 1998;9:2291–2301. doi: 10.1681/ASN.V9122291. [DOI] [PubMed] [Google Scholar]
- 12.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Vallenius T, Ovaska K, Westermarck J, Mäkelä TP, Hautaniemi S. Integrated network analysis platform for protein–protein interactions. Nat Methods. 2009;6:75–77. doi: 10.1038/nmeth.1282. [DOI] [PubMed] [Google Scholar]
- 14.Chautard E, Ballut L, Thierry-Mieg N, Ricard-Blum S. MatrixDB, a database focused on extracellular protein–protein and protein–carbohydrate interactions. Bioinformatics. 2009;25:690–691. doi: 10.1093/bioinformatics/btp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci. 2010;123:1385–1388. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assenov Y, Ramirez F, Schelhorn SE, Lengauer T, Albrecht M. Computing topological parameters of biological networks. Bioinformatics. 2008;24:282–284. doi: 10.1093/bioinformatics/btm554. [DOI] [PubMed] [Google Scholar]
- 17.Tan R, Colville D, Wang YY, Rigby L, Savige J. Alport retinopathy results from “severe” COL4A5 mutations and predicts early renal failure. Clin J Am Soc Nephrol. 2010;5:34–38. doi: 10.2215/CJN.01030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knebelmann B, Breillat C, Forestier L, Arrondel C, Jacassier D, Giatras I, Drouot L, Deschenes G, Grunfeld JP, Broyer M, Gubler MC, Antignac C. Spectrum of mutations in the COL4A5 collagen gene in X-linked Alport syndrome. Am J Hum Genet. 1996;59:1221–1232. [PMC free article] [PubMed] [Google Scholar]
- 19.Amos-Landgraf JM, Cottle A, Plenge RM, Friez M, Schwartz CE, Longshore J, Willard HF. X chromosome-inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet. 2006;79:493–499. doi: 10.1086/507565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol. 2013;24:364–375. doi: 10.1681/ASN.2012020148. [DOI] [PubMed] [Google Scholar]
- 21.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P, Study Group Members of the Gesellschaft für Pädiatrische Nephrologie. Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012;81:494–501. doi: 10.1038/ki.2011.407. [DOI] [PubMed] [Google Scholar]
- 22.Temme J, Peters F, Lange K, Pirson Y, Heidet L, Torra R, Grunfeld JP, Weber M, Licht C, Müller GA, Gross O. Incidence of renal failure and nephroprotection by RAAS inhibition in heterozygous carriers of X-chromosomal and autosomal recessive Alport mutations. Kidney Int. 2012;81:779–783. doi: 10.1038/ki.2011.452. [DOI] [PubMed] [Google Scholar]
- 23.Webb NJ, Lam C, Shahinfar S, Strehlau J, Wells TG, Gleim GW, Le Bailly De Tilleghem C. Efficacy and safety of losartan in children with Alport syndrome–results from a subgroup analysis of a prospective, randomized, placebo- or amlodipine-controlled trial. Nephrol Dial Transplant. 2011;26:2521–2526. doi: 10.1093/ndt/gfq797. [DOI] [PubMed] [Google Scholar]
- 24.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/S1097-2765(00)80057-X. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 26.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 27.Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 28.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy HJ, Bierzynska A, Wherlock M, Ognjanovic M, Kerecuk L, Hegde S, Feather S, Gilbert RD, Krischock L, Jones C, Sinha MD, Webb NJ, Christian M, Williams MM, Marks S, Koziell A, Welsh GI, Saleem MA, RADAR the UK SRNS Study Group Simultaneous sequencing of 24 genes associated with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2013;8:637–648. doi: 10.2215/CJN.07200712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voskarides K, Arsali M, Athanasiou Y, Elia A, Pierides A, Deltas C. Evidence that NPHS2-R229Q predisposes to proteinuria and renal failure in familial hematuria. Pediatr Nephrol. 2012;27:675–679. doi: 10.1007/s00467-011-2084-6. [DOI] [PubMed] [Google Scholar]
- 31.Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, Knight D, Brenchley PE, Zent R, Humphries MJ. Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol. 2014;25:939–951. doi: 10.1681/ASN.2013030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin X, Wang W, Mao J, Shen H, Fu H, Wang X, Gu W, Liu A, Yu H, Shu Q, Du L. Overexpression of Myo1e in mouse podocytes enhances cellular endocytosis, migration, and adhesion. J Cell Biochem. 2014;115:410–419. doi: 10.1002/jcb.24676. [DOI] [PubMed] [Google Scholar]
- 33.Ouderkirk JL, Krendel M. Myosin 1e is a component of the invadosome core that contributes to regulation of invadosome dynamics. Exp Cell Res. 2014;322:265–276. doi: 10.1016/j.yexcr.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chase SE, Encina CV, Stolzenburg LR, Tatum AH, Holzman LB, Krendel M. Podocyte-specific knockout of myosin 1e disrupts glomerular filtration. Am J Physiol Renal Physiol. 2012;303:F1099–F1106. doi: 10.1152/ajprenal.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanna-Cherchi S, Burgess KE, Nees SN, Caridi G, Weng PL, Dagnino M, Bodria M, Carrea A, Allegretta MA, Kim HR, Perry BJ, Gigante M, Clark LN, Kisselev S, Cusi D, Gesualdo L, Allegri L, Scolari F, D'Agati V, Shapiro LS, Pecoraro C, Palomero T, Ghiggeri GM, Gharavi AG. Exome sequencing identified MYO1E and NEIL1 as candidate genes for human autosomal recessive steroid-resistant nephrotic syndrome. Kidney Int. 2011;80:389–396. doi: 10.1038/ki.2011.148. [DOI] [PubMed] [Google Scholar]
- 36.Al-Hamed MH, Al-Sabban E, Al-Mojalli H, Al-Harbi N, Faqeih E, Al Shaya H, Alhasan K, Al-Hissi S, Rajab M, Edwards N, Al-Abbad A, Al-Hassoun I, Sayer JA, Meyer BF. A molecular genetic analysis of childhood nephrotic syndrome in a cohort of Saudi Arabian families. J Hum Genet. 2013;58:480–489. doi: 10.1038/jhg.2013.27. [DOI] [PubMed] [Google Scholar]