Summary
Effective inhibitory synaptic transmission requires efficient stabilization of GABAA receptors (GABAARs) at synapses, which is essential for maintaining the correct excitatory-inhibitory balance in the brain. However, the signaling mechanisms that locally regulate synaptic GABAAR membrane dynamics remain poorly understood. Using a combination of molecular, imaging, and electrophysiological approaches, we delineate a GIT1/βPIX/Rac1/PAK signaling pathway that modulates F-actin and is important for maintaining surface GABAAR levels, inhibitory synapse integrity, and synapse strength. We show that GIT1 and βPIX are required for synaptic GABAAR surface stability through the activity of the GTPase Rac1 and downstream effector PAK. Manipulating this pathway using RNAi, dominant-negative and pharmacological approaches leads to a disruption of GABAAR clustering and decrease in the strength of synaptic inhibition. Thus, the GIT1/βPIX/Rac1/PAK pathway plays a crucial role in regulating GABAAR synaptic stability and hence inhibitory synaptic transmission with important implications for inhibitory plasticity and information processing in the brain.
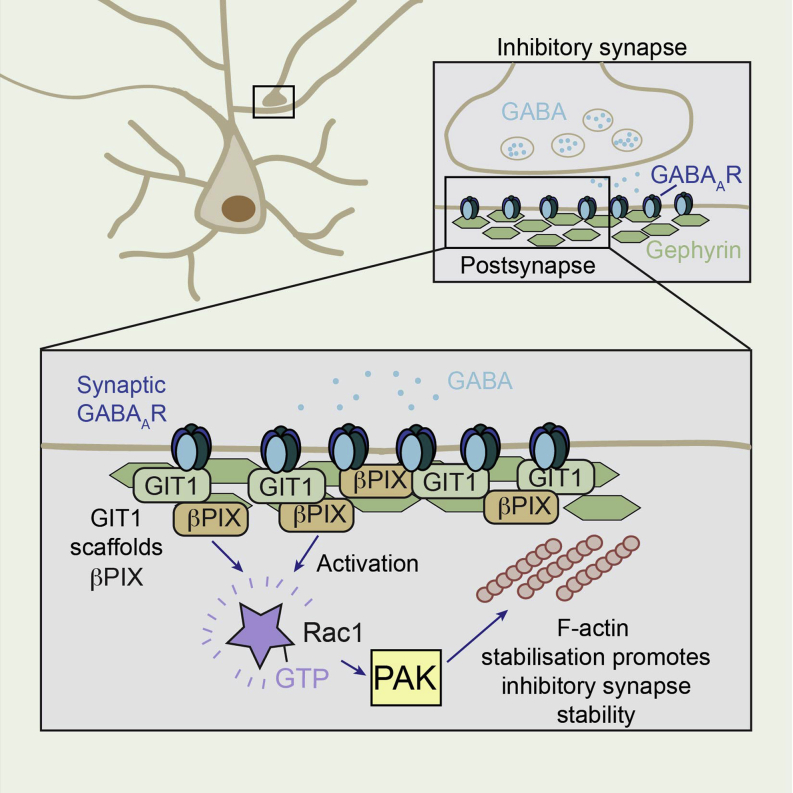
Graphical Abstract
Highlights
-
•
GIT1 and βPIX are present at inhibitory synapses and complex with GABAARs
-
•
GIT1 and βPIX are important for GABAAR clustering and inhibitory transmission
-
•
Rac1 and PAK activity is required for stabilization of GABAARs at synapses
-
•
A GIT1/βPIX/Rac1/PAK pathway is required for inhibitory synaptic transmission
Clustering of GABAA receptors at inhibitory synapses is important for maintaining the correct balance of excitation and inhibition in the brain. Smith et al. reveal a signaling mechanism at inhibitory synapses involving the scaffold GIT1, which anchors βPIX to the inhibitory synaptic site and activates Rac1 and PAK, thereby stabilizing F-actin. This signaling pathway underlies the stabilization of synaptic GABAA receptors and therefore contributes to efficient inhibitory synaptic transmission in the brain.
Introduction
GABAA receptors (GABAARs) are essential mediators of inhibitory neurotransmission in the central nervous system and are critical for maintaining the correct balance of excitation and inhibition in the brain (Smith and Kittler, 2010). GABAergic synapses undergo extensive synaptic plasticity that alters the strength and efficacy of synaptic inhibition (Luscher et al., 2011a). Inhibitory synapse strength can be rapidly controlled by changing the number of GABAARs in the postsynaptic domain, which is achieved by receptor insertion into and removal from the plasma membrane at extrasynaptic sites and by dynamic movements of GABAARs to and from the synapse via lateral diffusion in the plasma membrane (Arancibia-Cárcamo et al., 2009; Bannai et al., 2009; Muir et al., 2010; Smith et al., 2012; Twelvetrees et al., 2010). However, the molecular mechanisms and regulatory signaling pathways that locally control GABAAR surface levels and synaptic stability remain unclear.
The stabilization of synaptic GABAARs opposite GABAergic presynaptic terminals is crucial for efficient synaptic inhibition, circuit excitability, and animal behavior (Blundell et al., 2009; Crestani et al., 1999; Papadopoulos et al., 2007). GABAAR clustering is mediated by a complex inhibitory postsynaptic density, the major constituent of which is the hexameric scaffold, gephyrin (Fritschy et al., 2008). However, in the absence of gephyrin, subsets of inhibitory synapses remain (Essrich et al., 1998; Kneussel et al., 1999), suggesting the existence of other inhibitory synaptic scaffold molecules. The inhibitory postsynaptic specialization also contains key adhesion molecules such as neuroligin 2 and Slitrk3 (Takahashi et al., 2012; Varoqueaux et al., 2004), in addition to cytoskeletal-associated proteins, which together contribute to controlling the formation and stabilization of GABAergic synapses. Interestingly, several filamentous actin (F-actin) regulatory proteins have been associated with the inhibitory postsynaptic density and gephyrin (Luscher et al., 2011a), suggesting a potential role for the actin cytoskeleton at inhibitory synapses. However, little is known regarding the regulatory signaling scaffolds that can act locally to coordinate cytoskeletal dynamics to tune GABAAR synaptic stability and synaptic inhibition.
The Rho family of small GTPases—Rho, Rac, and Cdc42—and their regulators play essential roles in modulating actin dynamics and are increasingly implicated in synaptic pathology and neurological dysfunction. The activation state of small GTPases is determined by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), which together control GTP-GDP exchange and thereby promote GTPase activation and inactivation, respectively (Nobes and Hall, 1999). Local regulation of GTPase signaling can be further controlled by subcellular compartmentalization of GEFs and GAPs determined by protein scaffolds (Kiraly et al., 2010). Currently the key GTPases, regulatory GEFs and signaling scaffolds acting to regulate GABAAR trafficking and inhibitory transmission are poorly understood.
In this study, we have identified a GIT1/βPIX/Rac1/PAK signaling complex that is important for maintaining GABAAR surface clusters and synaptic inhibition in neurons. Using a combination of imaging, biochemical, and electrophysiological approaches, we show that the signaling scaffold protein GIT1 (G protein-coupled receptor kinase-interacting protein 1), which interacts with the Rac1 GEF βPIX, forms complexes with GABAARs and is essential for normal GABAAR clustering. Furthermore, we demonstrate that downstream Rac1 activity is also crucial to maintain inhibitory synapse stability and works in concert with the key Rac1 effector and actin-regulator, PAK. We find that Rac1 activity stabilizes GABAARs at inhibitory synapses while disrupting GIT1, Rac1 or PAK all lead to impaired inhibitory transmission. Thus GIT1 and βPIX, in complex with GABAARs, play a key role in locally coordinating Rac1 and downstream effector activity to regulate GABAAR surface stability and inhibitory synapse strength.
Results
The GIT1/βPIX Complex Is Localized at Inhibitory Synapses and Forms Complexes with Synaptic GABAARs
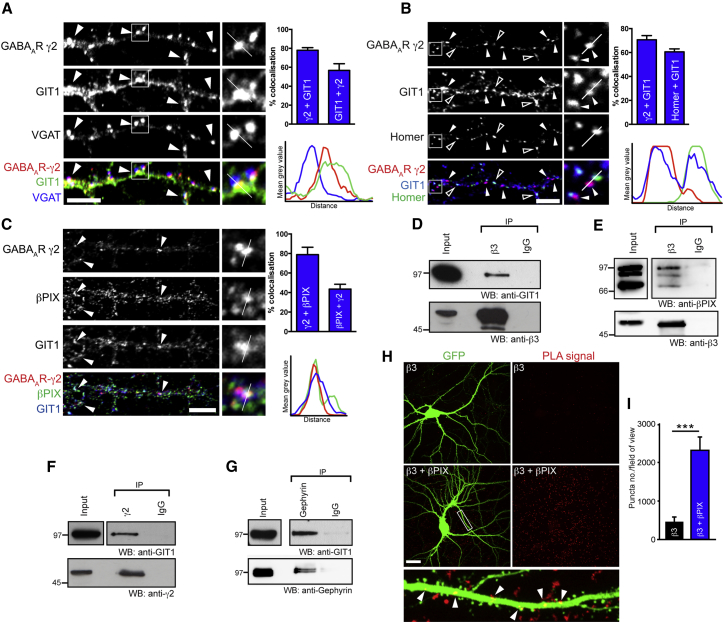
The signaling scaffolds that regulate postsynaptic GABAAR stability in neurons remain largely unknown. GIT1 is a signaling scaffold that can recruit βPIX, a GEF for the small GTPase Rac1, to locally control the activation of Rac1 (Zhang et al., 2005). GIT1 has been localized to synaptic sites in neurons but its potential association with GABAARs and role in regulating signaling in the inhibitory postsynaptic domain remains unstudied. We hypothesized that GIT1 and βPIX might control Rac1 signaling at inhibitory synapses and are important for GABAAR clustering and inhibitory synaptic transmission. We initially determined if GIT1 and βPIX were localized at inhibitory synapses using immunocytochemistry and confocal laser scanning microscopy (CLSM) of hippocampal neurons. Neurons labeled with antibodies to GIT1, VGAT (Vesicular GABA transporter) to label inhibitory presynaptic terminals, and the γ2 GABAAR subunit to label synaptic GABAAR clusters showed GIT1 was distributed along dendrites and exhibited colocalization with synaptic GABAAR clusters (Figure 1A; Figure S1A). Approximately 55% of GIT1 localized at inhibitory synapses, whereas ∼78% of synaptic GABAAR clusters colocalized with GIT1. In addition, GIT1 is known to be at excitatory synapses (Zhang et al., 2003), which we confirm by showing that the excitatory synapse marker, homer, demonstrates ∼60% colocalization with GIT1 puncta (Figure 1B). GIT1 and βPIX are known to form supramolecular signaling platforms and are consistently found in a tight signaling complex in many cell types (Premont et al., 2004; Schlenker and Rittinger, 2009). We therefore imaged neurons labeled with GIT1, βPIX, and γ2 GABAAR antibodies, showing that ∼79% of inhibitory synapses colocalized with βPIX and thereby verifying the localization of both GIT1 and βPIX at inhibitory synapses (Figure 1C). Interestingly, GIT1 localizes with synaptic GABAARs (γ2, β3, α2 subunits, Figures S1B and S1C) but showed little overlap with extrasynaptic GABAARs (δ subunits, Figures S1D and S1E).
Figure 1.
GABAARs Form Complexes with GIT1 and βPIX in Neurons
(A–C) CLSM of neurons labeled with antibodies to GABAAR-γ2 (red) and (A) VGAT (blue) and GIT1 (green), (B) GIT1 (blue) and homer (green) or (C) βPIX (green) and GIT1 (blue). Arrowheads, colocalization; scale bar represents 5 μm. Bar graphs summarize colocalization quantification (n = 5–10 cells). Example line scans through clusters show localization of GIT1 to inhibitory synapses (A and C) and excitatory synapses (B). Values are mean ± SEM.
(D–G) Western blots of coimmunoprecipitation assays of GABAARs (β3 and γ2 subunits) and gephyrin, with GIT1 and βPIX from rat brain homogenate (WB, western blot; IP, immunoprecipitation).
(H and I) Proximity ligation assay of neurons with antibodies to GABAAR-β3 and βPIX in situ, (n = 3). Scale bar represents 20 μm. Values are mean ± SEM.
See also Figure S1.
We also demonstrated that GABAAR β3 and γ2 subunit antibodies readily coimmunoprecipitate GIT1 or βPIX from rat brain lysate as analyzed by western blotting (Figures 1D–1F), confirming that GIT1 and βPIX can form native complexes with synaptic GABAARs in vivo. GIT1 did not interact in a complex with extrasynaptic δ subunits (Figure S1F). We also found exogenous FLAG-GIT1 to interact with the intracellular domain of the GABAAR-β3 subunit, by COS7 cell pull-down assays (Figure S1G), supporting GIT1’s interaction with GABAARs. Coimmunoprecipitation of GIT1 with the inhibitory postsynaptic scaffold gephyrin confirmed this postsynaptic localization (Figure 1G), although our data suggest that this interaction is indirect, as demonstrated by lack of coimmunoprecipitations in transfected COS7 cells (Figure S1H). As an alternative approach for further validating GABAAR and βPIX complexes in neuronal dendrites, we performed proximity ligation assays (PLAs), which provide valuable information about native protein interactions in situ (Ko et al., 2012). Using PLAs, we demonstrate that βPIX complexes with GABAARs in neuronal dendrites (Figures 1H and 1I). Furthermore, we show that GIT1 and gephyrin also interact in situ via PLAs (Figures S1J and S1K), providing additional evidence to support an inhibitory postsynaptic and close association for these proteins in dendrites.
GIT1, βPIX, and F-Actin Regulation Are Important for Maintaining Surface GABAAR Levels
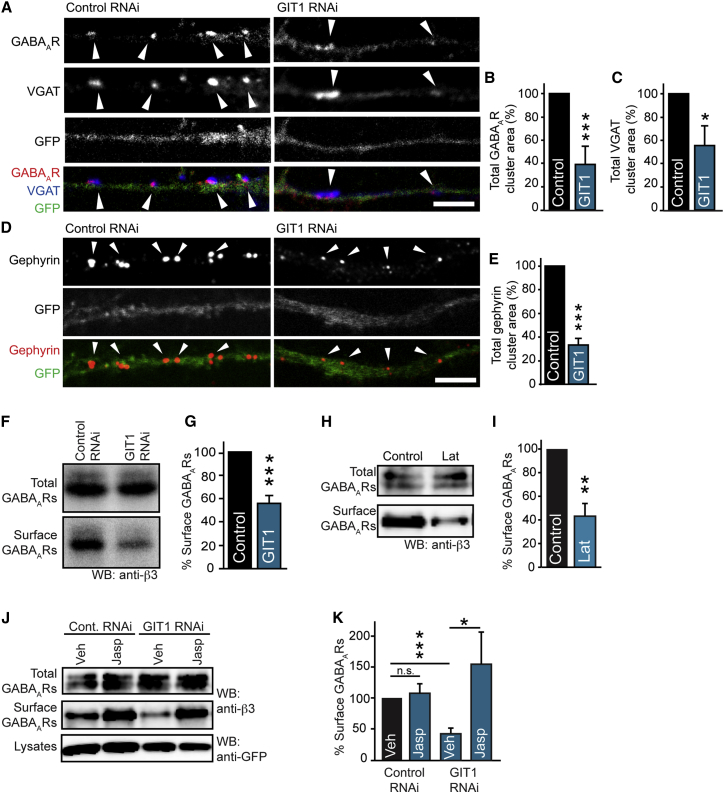
To investigate the role of GIT1 at inhibitory synapses, we utilized RNAi to knock down its protein expression in neurons. RNAi caused a significant reduction of GIT1 expression levels in addition to causing a small reduction in dendrite length as previously shown (Figures S2A–S2D; Menon et al., 2010). To determine the consequences of GIT1 knockdown on inhibitory synapse and surface GABAAR cluster area, we performed immunocytochemistry and CLSM of neurons expressing GIT1 or control RNAi constructs, using an extracellular γ2 subunit antibody to label surface GABAARs and antibodies to VGAT to identify inhibitory synapses. GIT1 knockdown neurons exhibited a significant decrease in surface GABAAR and VGAT cluster area compared to control (Figures 2A–2C), suggesting a possible role for GIT1 in maintaining the integrity of inhibitory pre- and postsynaptic domains in neuronal dendrites. Importantly, this effect could be rescued by coexpression of RNAi-resistant human GIT1 (hGIT1; Figures S2E and S2F). GIT1 knockdown also caused a large decrease in gephyrin cluster area (Figures 2D and 2E), suggesting that GIT1 is important for maintaining both GABAAR clusters and the gephyrin scaffold in neurons. This was further confirmed by surface biotinylation assays, which revealed that surface GABAAR levels were reduced in GIT1 knockdown neurons compared with control (Figures 2F and 2G).
Figure 2.
GIT1 Knockdown Alters GABAAR Surface Stability
(A) Confocal images of GIT1 or control RNAi-transfected neurons (green) labeled with antibodies to GABAAR-γ2 (red) and to VGAT (blue). Arrowheads, GABAAR clusters. Scale bar represents 5 μm.
(B and C) Bar graphs of GABAAR and VGAT cluster area showing a reduction to 39.3% ± 13.6% and 56.7% ± 13.3% of control (∗∗∗p = 0.0008 and ∗p = 0.03, n = 3, ten cells). Values are mean ± SEM.
(D) Confocal images of control and GIT1 RNAi-transfected neurons (green) immunostained with antibodies to gephyrin (red). Arrowheads, gephyrin clusters. Scale bar represents 5 μm.
(E) Bar-graph of gephyrin cluster area showing a 67.0% ± 5.6% decrease compared with control (∗∗∗p = 0.0003, n = 5, 20 cells). Values are mean ± SEM.
(F and G) Expression of GIT1 RNAi reduces surface expression of GABAARs to 55.9% ± 6.0% of control as assayed by surface biotinylation and western blotting with anti-GABAAR-β3 (∗∗∗p = 2.6 × 10−5, n = 5). Values are mean ± SEM.
(H) Surface biotinylations of neurons treated with 3 μM latrunculin-A for 30 min and analyzed by western blotting with anti-GABAAR-β3.
(I) Bar graph showing reduction in surface GABAARs on treatment with latrunculin-A (∗∗p = 0.004, n = 3). Values are mean ± SEM.
(J) Surface biotinylations of control or GIT1 RNAi neurons treated with 2 μM jasplakinolide or vehicle and analyzed by western blotting with GABAAR-β3 antibodies. Lysates were probed with GFP antibodies to show transfection (lower panel).
(K) Summary bar graphs showing a 57.2% ± 5.8% reduction in surface GABAARs in neurons transfected with GIT1 RNAi (∗∗∗p = 0.0002, n = 5); however, treatment with jasplakinolide prevented this reduction (∗p = 0.047, n = 5). Values are mean ± SEM.
See also Figure S2.
Considering its role in other cell types, we hypothesized that GIT1 may be important for localizing F-actin regulatory pathways to inhibitory synapses. Therefore, we sought to determine whether surface GABAARs were sensitive to short-term disruption of the actin cytoskeleton by treating neurons with latrunculin-A, an inhibitor of actin polymerization (Renner et al., 2009). We found that a 30 min application of 3 μM latrunculin-A to neurons caused a significant decrease in surface GABAARs (Figures 2H and 2I), with no effect on extrasynaptic GABAAR populations (Figures S2G and S2H), suggesting that actin polymerization does indeed play an important role in the maintenance of inhibitory synapses. We then asked whether the inhibitory synapse effects we observed upon knockdown of GIT1 were due to altered F-actin regulation. We therefore treated control or GIT1 RNAi-expressing neurons with the F-actin-stabilizing drug, jasplakinolide (Hering et al., 2003), prior to surface biotinylation and western blot analysis (Figure 2J). As predicted, GIT1 RNAi caused a significant loss of surface GABAARs compared to control, which was restored by treatment with jasplakinolide (Figures 2J and 2K). This suggests that the decrease in surface GABAARs observed in GIT1-deficient neurons is caused by impaired F-actin regulation, and points toward a mechanism involving actin-regulatory proteins.
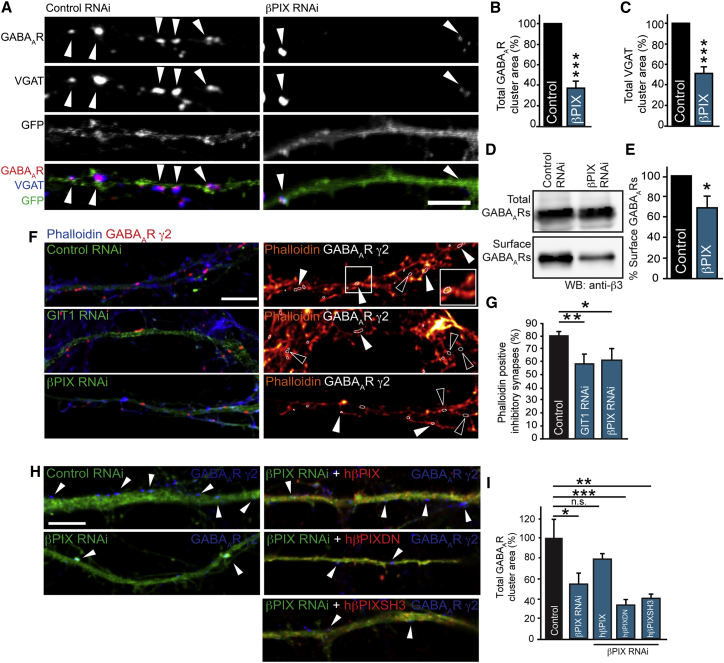
GIT1’s primary binding partner βPIX is one such actin-regulatory protein and is a strong candidate to collaborate with GIT1 in mediating actin regulation at inhibitory synapses. To test this hypothesis, we utilized RNAi to βPIX, which caused a significant reduction of βPIX expression levels (Figures S3A and S3B). βPIX knockdown in neurons caused a similar effect to that of GIT1 RNAi, reducing both surface GABAARs and VGAT cluster area (Figures 3A–3C). Surface biotinylation assays revealed the same phenotype, with βPIX knockdown neurons exhibiting reduced surface GABAAR levels compared with control cells (Figures 3D and 3E). We found GIT1 or βPIX knockdown had no effect on AMPA receptor clustering or extrasynaptic δ- containing GABAARs (Figures S3C–S3F), suggesting that this protein complex is important for synaptic GABAAR clustering only. We then sought to determine whether GIT1 and βPIX are important in controlling actin regulation at inhibitory synapses. We treated control neurons with phalloidin to label F-actin and found that ∼80% of inhibitory synapses were positive for F-actin. GIT1 and βPIX knockdown caused a significant decrease in the percentage of inhibitory synapses containing F-actin (Figures 3F and 3G), suggesting that GIT1 and βPIX have an important role in controlling F-actin at inhibitory synapses. The effect of βPIX knockdown on surface GABAAR clusters was rescued by RNAi resistant human βPIX (hβPIX) showing the specificity of the RNAi. In contrast, versions of hβPIX that no longer have GEF activity for Rac1 (hβPIX-DN), or that contain a mutation of the βPIX SH3 domain (which is critical for coupling to a downstream effector, PAK [Hoelz et al., 2006]), were unable to rescue βPIX knockdown induced GABAAR declustering (Figures 3H and 3I). This suggests that the ability of βPIX to activate Rac1 and interact with PAK is essential for its role in maintaining inhibitory synapses and supports the idea that there exists a key actin regulatory mechanism controlling inhibitory synapse maintenance.
Figure 3.
The Rac1 Activator βPIX Is Essential for GABAAR Surface Stability
(A) Confocal images of control and βPIX RNAi-transfected neurons (green), labeled with antibodies to GABAAR-γ2 (red) and to VGAT (blue). Arrowheads, GABAAR clusters. Scale bar represents 5 μm.
(B and C) Bar graphs of GABAAR and VGAT cluster area showing a reduction to 37.5% ± 5.9% and 49.2% ± 5.4% of control on knockdown of βPIX (∗∗∗p = 0.0009 in B, ∗∗∗p = 0.0006 in C, n = 5, 18 cells). Values are mean ± SEM.
(D and E) βPIX RNAi reduces surface expression of GABAARs to 65.3% ± 13.0% of control as assayed by surface biotinylation of neurons and western blotting with anti-GABAAR-β3 (∗p = 0.04, n = 4). Values are mean ± SEM.
(F and G) Neurons transfected with control, GIT1 and βPIX RNAi were labeled with antibodies to the GABAAR-γ2 (red) and Alexa-647-conjugated Phalloidin to label F-actin. Scale bar represents 5 μm. (G) The percentage of synaptic phalloidin-positive GABAAR clusters were reduced in GIT1 and βPIX knockdown neurons compared with control (∗p = 0.028, ∗∗p = 0.005, n = 3, seven to nine cells). Values are mean ± SEM.
(H and I) Coexpression of RNAi resistant βPIX (hβPIX) with βPIX RNAi rescues the effect of the βPIX knockdown surface GABAAR clusters (n = 4, 18–22 cells, one-way ANOVA ∗∗∗p = 0.0001). However, dominant-negative (hβPIXDN) or SH3 domain mutants (hβPIXSH3) are unable to rescue these effects. Values are mean ± SEM. Scale bar represents 5 μm.
See also Figure S3.
Rac1 Activity Maintains Surface GABAAR Levels
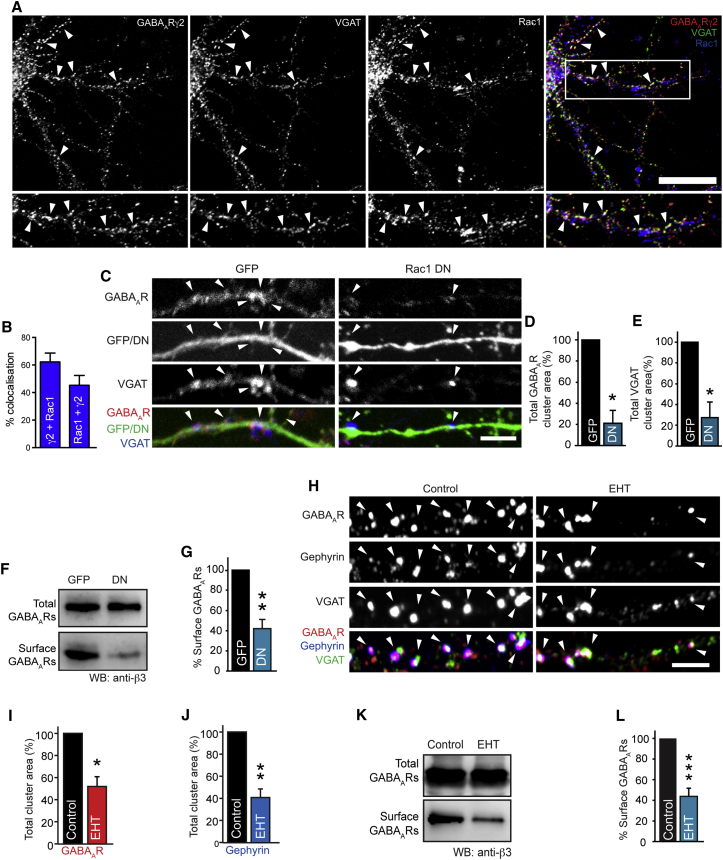
GIT1 can anchor βPIX, which directly interacts with the small GTPase Rac1 and mediates its activation (ten Klooster et al., 2006). Because we could not rescue the effects of βPIX knockdown with a version of βPIX with impaired GEF activity toward Rac1, we hypothesized that Rac1 might be responsible for the changes in GABAAR clustering in GIT1 or βPIX knockdown neurons. We verified that Rac1 was localized to synaptic GABAAR clusters in neurons using immunocytochemistry with antibodies to Rac1, the γ2 GABAAR subunit and VGAT, followed by CLSM (Figure 4A). We found that ∼45% of Rac1 colocalized with synaptic GABAAR clusters along the dendritic shaft (Figure 4B), suggesting that Rac1-positive signaling complexes may influence synaptic GABAAR trafficking or function.
Figure 4.
Rac1 Is Localized at Inhibitory Synapses and Maintains Surface GABAAR Stability
(A) Neurons were labeled with antibodies to Rac1 (blue), GABAAR-γ2 (red), and VGAT (green). Scale bar represents 10 μm. Rac1 colocalizes (white) with synaptic GABAAR clusters and VGAT in neurons (arrowheads).
(B) Bar graph of colocalization analysis of GABAAR-γ2 and Rac1 in neurons. Values are mean ± SEM.
(C) Confocal images of GFP or Rac1 dominant-negative (DN) -expressing neurons (green) immunostained with antibodies to the GABAAR-γ2 (red) and VGAT (blue). Scale bar represents 5 μm.
(D and E) Summary bar graphs showing expression of Rac1 DN causes a decrease in surface GABAAR and VGAT cluster area to 20.92% ± 12.2% and 27.28% ± 13.5% of control (∗p = 0.02 in D, ∗p = 0.03 in E, n = 3, eight cells). Values are mean ± SEM.
(F and G) Neurons expressing GFP or Rac1 DN were surface biotinylated and analyzed by western blotting with anti-GABAAR-β3, revealing a significant decrease in surface GABAARs to 43.8% ± 9.0% of control (∗∗p = 0.005, n = 4). Values are mean ± SEM.
(H) Confocal images of neurons were treated with EHT and labeled with antibodies to GABAAR-γ2 (red) and VGAT (green) and gephyrin (blue). Scale bar represents 5 μm.
(I and J) Analysis of cluster area reveals decreased GABAAR (to 49.9% ± 8.2% of control, ∗∗p = 0.006, n = 4, 17 cells) and gephyrin (to 39.7% ± 7.7% of control, ∗∗p = 0.001, n = 4, 17 cells) cluster area. Values are mean ± SEM.
(K and L) Surface biotinylation of neurons treated with 100 μM EHT or vehicle for 1 hr and analyzed by western blotting with anti-GABAAR-β3 demonstrates a 62.1% ± 6.7% decrease in surface GABAARs (∗∗∗p = 9.0 × 10−5, n = 7). Values are mean ± SEM.
To test whether Rac1 activity has a role in maintaining synaptic GABAAR clustering, we utilized a dominant-negative Rac1 mutant (Nobes and Hall, 1999) (Rac1-DN) to block Rac1 activation in neurons. Immunostaining and CLSM of hippocampal neurons transfected with GFP or Rac1-DN revealed a decrease in surface GABAAR cluster area and VGAT area in neurons expressing Rac1-DN compared with control neurons (Figures 4C–4E). In addition, surface biotinylation assays revealed that blocking Rac1 activation with the Rac1-DN caused a decrease in GABAAR surface levels (Figures 4F and 4G).
As an alternative approach, we determined if acute short-term inhibition of Rac1 would cause similar effects on GABAAR surface levels. To achieve this, we incubated neurons for 1 hr with the Rac1 inhibitor EHT 1864 (EHT; Shutes et al., 2007), followed by immunocytochemistry and CLSM. Similar to the results with Rac1-DN, analysis of these neurons showed that acute inhibition of Rac1 activity reduced the area of GABAAR and gephyrin clusters (Figures 4H–4J). Moreover, surface biotinylation assays with neurons treated with EHT caused a decrease in surface GABAARs (Figures 4K and 4L), indicating that Rac1 contributes to GABAergic synapse stability in neurons.
PAK Activation Is Important for GABAAR Surface Stability
We then wanted to explore the mechanism downstream of active Rac1 leading to the stabilization of GABAAR clusters. PAK is a major effector of Rac1 that modulates F-actin to stabilize essential cellular structures (Kreis and Barnier, 2009). We hypothesized that Rac1 might mediate its action at inhibitory synapses by activating PAK, which is supported by our observation that an SH3-domain mutant of βPIX (which can no longer couple to PAK) is unable to rescue the effects of βPIX RNAi on GABAAR clusters (Figures 3H and 3I).
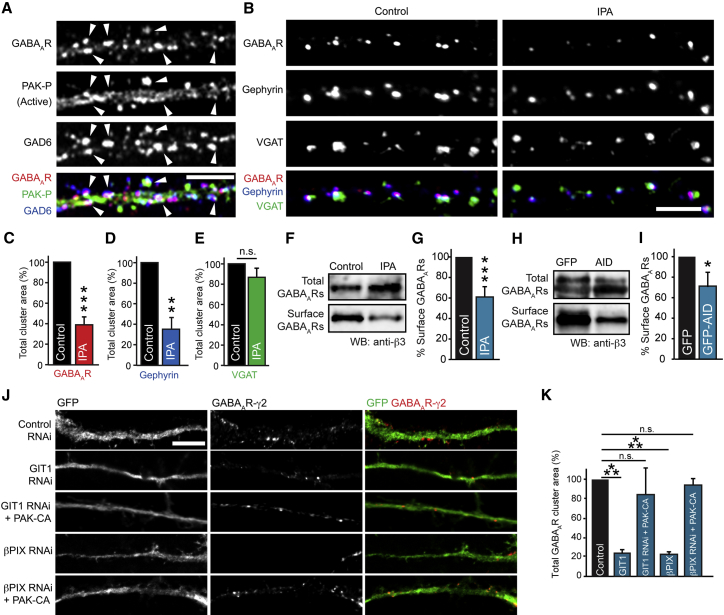
Active PAK is autophosphorylated; therefore, we tested whether we could detect phospho-PAK at inhibitory synaptic sites by performing immunocytochemistry and CLSM of neurons labeled with antibodies to GABAAR γ2 subunit, the inhibitory presynaptic marker GAD6 and phospho-PAK (Figure 5A). Active PAK was found clustered along dendrites and colocalized with both synaptic GABAAR clusters and GAD6, confirming its presence at inhibitory synapses. To determine whether PAK activity is important for maintaining GABAAR and gephyrin cluster stability, we incubated hippocampal neurons with IPA (Deacon et al., 2008), a specific PAK inhibitor and assessed GABAAR γ2, gephyrin and VGAT cluster area. Treatment with IPA caused a substantial decrease in GABAAR and gephyrin cluster area, with little effect on VGAT cluster area (Figures 5B–5E). Surface biotinylation assays showed a decrease in surface GABAAR expression in neurons treated with IPA compared with vehicle-treated neurons (Figures 5F and 5G), showing that PAK activity is required for maintaining surface GABAARs in neurons. To further verify a role for PAK in controlling GABAAR surface stability, we utilized the autoinhibitory domain (AID) of PAK fused to GFP, which has widely been used to inhibit PAK activity in culture by blocking its autophosphorylation (Kreis and Barnier, 2009). Surface biotinylation of neurons transfected with GFP or GFP-PAK-AID revealed that GFP-PAK-AID expression caused reduced surface GABAAR expression compared with control (Figures 5H and 5I). To confirm that PAK acts downstream of GIT1 and βPIX at inhibitory synapses, we performed rescue experiments with neurons cotransfected with GIT1 or βPIX RNAi and a constitutively active (CA) mutant of PAK. Cotransfection with PAK-CA effectively prevented the depletion of GABAAR clusters observed with GIT1 or βPIX RNAi alone (Figures 5J and 5K), suggesting that PAK acts downstream of GIT1 and βPIX to control inhibitory synapse maintenance in neurons. Together, these data suggest that a GIT1/βPIX/Rac1/PAK signaling pathway plays an important role in stabilizing GABAAR and gephyrin clusters and the maintenance of inhibitory synapses.
Figure 5.
PAK Activation Is Important for GABAAR Surface Stability
(A) Neurons labeled with antibodies to phospho-PAK (PAK-P; green), GABAAR-γ2 (red) and the inhibitory presynaptic marker, GAD6 (blue). Scale bar represents 5 μm. Arrowheads, colocalization.
(B) Neurons labeled with antibodies to the GABAAR-γ2 (red), gephyrin (blue), and VGAT (green) treated with IPA or vehicle. Scale bar represents 5 μm.
(C–E) Summary bar graphs showing that inhibition of PAK with IPA causes a 60.8% ± 7.2% decrease in GABAAR cluster area, a 65.0% ± 9.3% decrease in gephyrin cluster area (∗∗∗p = 0.0009, ∗∗p = 0.003, n = 3, 10–12 cells), and no change in VGAT cluster area. Values are mean ± SEM.
(F and G) Surface biotinylation of neurons treated with IPA or vehicle, followed by western blotting with anti-GABAAR-β3, demonstrates a 41.0% ± 8.0% decrease in surface GABAARs (∗∗∗p = 0.0003, n = 7). Values are mean ± SEM.
(H and I) Surface biotinylation of neurons expressing GFP or GFP-PAK-AID reveals a 28.8% ± 12.0% decrease in surface GABAARs (∗p = 0.03, n = 6). Values are mean ± SEM.
(J) Expression of constitutively active PAK (PAK-CA) rescues the effects of GIT1/βPIX knockdown on surface GABAARs. Representative images of transfected neurons (green) labeled with antibodies to the GABAAR-γ2 (red). Scale bar represents 5 μm.
(K) Summary bar graphs of average GABAAR cluster area showing significant decreases in surface GABAARs in GIT1 and βPIX knockdown neurons, but not knockdown neurons cotransfected with PAK-CA (∗∗∗p = 0.0003, n = 3, 9–15 cells). Values are mean ± SEM.
Inhibitory Synaptic Transmission Is Dependent on the Activity of GIT1/βPIX, Rac1, and PAK
Our results suggest that components of a signaling pathway involving GIT1, βPIX, Rac1, and PAK are critical for stabilizing surface and synaptic GABAARs and maintaining GABAergic synapse integrity in neurons. We then asked whether this pathway directly affects GABAergic transmission in neurons. Whole-cell patch-clamp recordings were performed to measure inhibitory synaptic transmission in neurons expressing the GIT1 RNAi, βPIX RNAi or GFP-PAK AID constructs. Analysis of spontaneous IPSC (sIPSC) traces from these cells revealed that GIT1 or βPIX knockdown, or inhibition of PAK all caused a considerable decrease in sIPSC amplitude compared to control neurons (Figures S4A–S4E). These reduced amplitudes can be seen in representative sIPSC traces (Figure S4A) and the leftward shift to smaller amplitudes in cumulative probability plots (Figures S4B and S4D). Analysis of these data showed there was no significant change in the sIPSC frequency (Figures S4C and S4E).
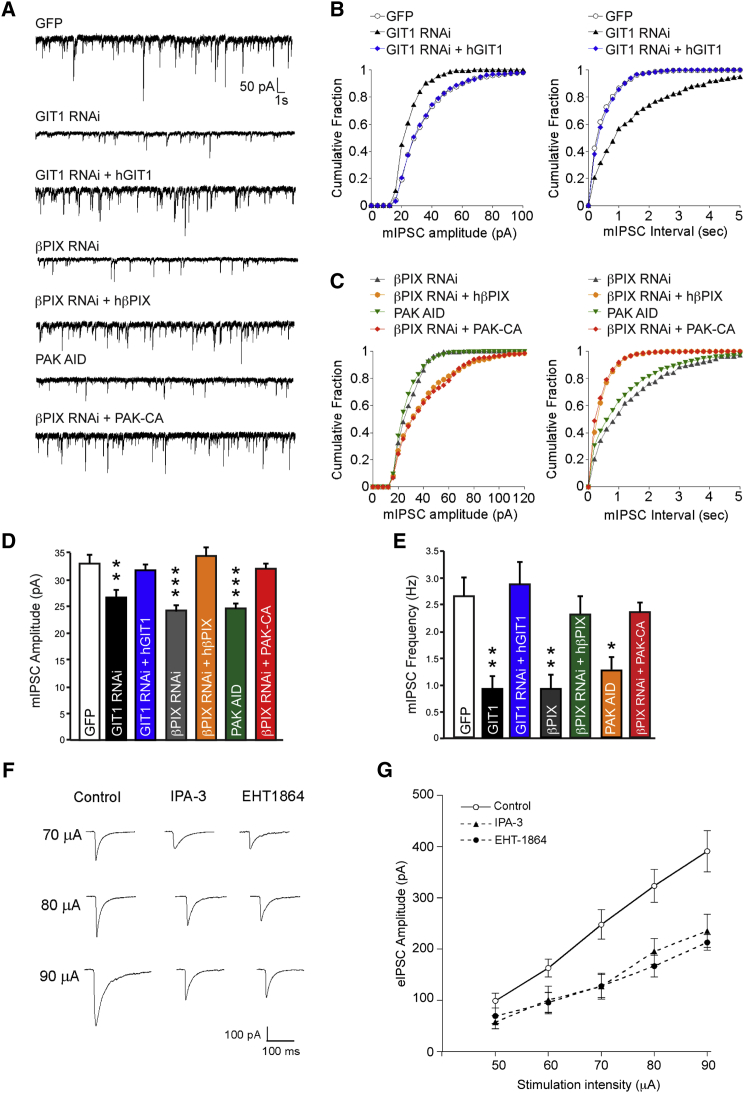
To further explore the impact of inhibiting the GIT1 signaling pathway, we measured the impact on miniature IPSCs (mIPSCs). We recorded mIPSCs from neurons transfected with control or GIT1 RNAi (Figures 6A–6E). Analysis of traces from these neurons showed that knockdown of GIT1 caused a significant decrease in both mIPSC amplitude and frequency, which could be rescued with RNAi resistant hGIT1, again showing the specificity of the RNAi knockdown. We also recorded mIPSCs from neurons transfected with βPIX RNAi and PAK-AID, analysis of which demonstrated a similar reduction in mIPSC amplitude and frequency as that of GIT1 RNAi-expressing neurons (Figures 6A, and 6C–6E). The effects of the βPIX RNAi were successfully rescued by coexpression with hβPIX, and also by PAK-CA, suggesting that PAK indeed mediates the effects of βPIX on GABAergic synaptic transmission. Importantly, there was no significant difference between the decay time constants of the events recorded from neurons expressing GIT1 RNAi, βPIX RNAi, or PAK-AID compared with control neurons, indicating that the receptor properties are unaltered (Figure S4F). In addition, βPIX RNAi expression had no significant effect on miniature excitatory postsynaptic currents (Figures S4G–S4I), suggesting that inhibition of this pathway under these conditions has specific effects on inhibitory synaptic transmission.
Figure 6.
A GIT1/βPIX/Rac1/PAK Signaling Pathway Is Crucial for GABAergic Synaptic Transmission
(A) Representative traces of mIPSCs recorded from transfected neurons.
(B) Cumulative distribution plots of mIPSC shows that neurons transfected with GIT1 RNAi have smaller event amplitudes and larger interevent intervals in comparison to control and GIT1 RNAi + hGIT1-transfected neurons.
(C) Cumulative distribution plots of mIPSC of event amplitude and mIPSC interval for neurons expressing βPIX RNAi, PAK1-DN, βPIX RNAi + hβPIX or βPIX RNAi + PAK-CA.
(D and E) Summary bar graphs of average amplitude and frequency (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, GFP control, n = 27 cells; GIT1 RNAi, n = 12 cells; GIT1 RNAi + hGIT1, n = 16 cells; βPIX RNAi, n = 17 cells; GFP-PAK-AID, n = 19 cells; βPIX RNAi + PAK-CA, n = 15 cells). Values are mean ± SEM.
(F) Representative traces of eIPSCs in brain slices with or without treatment with either EHT or IPA to inhibit Rac1 or PAK, respectively.
(G) Input-output curves of GABAAR-IPSCs evoked by a series of stimulus intensities in slices incubated with EHT or IPA (control, n = 15 cells; EHT, n = 9; IPA, n = 8 cells). Values are mean ± SEM.
See also Figure S4.
To determine the impact of inhibiting this pathway on GABAergic transmission in an intact network, we also performed patch-clamp recordings from pyramidal neurons in cortical slices. Slices were incubated with either IPA or EHT to inhibit PAK or Rac1, respectively (Figure 6F). Analysis of the strength of inhibition with input/output curves of evoked IPSCs (eIPSCs) generated by a series of stimulus intensities showed that inhibition of the Rac1-PAK pathway in brain slices caused a substantial depression of the input/output eIPSC curves (40%–50% reduction for IPA and 30%–50% reduction for EHT; Figures 6F and 6G). This indicates that Rac1 and PAK are indeed critical to maintain GABAergic synaptic transmission in the brain. Together, these electrophysiological experiments reveal that inhibiting the GIT1/βPIX/Rac1/PAK pathway in neurons not only reduces surface GABAAR cluster and gephyrin cluster area, but also leads to reduced inhibitory synaptic transmission.
Discussion
Clustering of GABAARs at inhibitory synapses is imperative for correct synaptic inhibition in the brain and is tightly controlled by components of the inhibitory postsynaptic density. Reduced GABAAR synaptic clustering equates to reduced inhibition in neuronal circuits and subsequent disruption of excitatory/inhibitory (E/I) balance, producing defects in information processing at the network level. Our results describe a signaling complex localized to the inhibitory postsynaptic domain that is crucial for correct inhibitory neurotransmission and the maintenance of GABAergic synaptic transmission. We show surface GABAAR clusters are maintained by a GIT1/βPIX/Rac1/PAK signaling complex that modulates F-actin, thereby stabilizing the inhibitory postsynaptic density and synaptic GABAARs (Figure S4J).
We demonstrate that GIT1 interacts with synaptic GABAAR subunits and is localized at inhibitory postsynaptic sites, suggesting that it is intimately involved with the inhibitory postsynapse and its function. Indeed, the interaction between GIT1 and GABAARs places it in the exact location to scaffold βPIX at the inhibitory synapse where it could locally activate Rac1 signaling (Zhang et al., 2003). Consistent with this, we also show that βPIX is located at inhibitory synapses and interacts in a complex with GABAARs. GEFs such as βPIX are essential signaling coordinators that localize and regulate small GTPase signaling at specific sites within the cell (Kiraly et al., 2010). Therefore, our data showing the presence of βPIX and Rac1 at the inhibitory synaptic site, combined with the effects of βPIX knockdown and Rac1 inhibition on GABAAR clustering and inhibitory synaptic transmission, strongly suggest that a Rac1 signaling pathway is important for maintaining synaptic inhibition. Our results also point to a scaffolding role for GIT1 at the inhibitory synapse, potentially as an additional scaffolding protein to increase the stability of gephyrin and GABAAR surface clusters. GIT1 and βPIX are also shown to be important for excitatory synapse function, acting via a Rac1/PAK/actin pathway (Zhang et al., 2005), in an activity-dependent manner (Saneyoshi et al., 2008). Our findings are supportive of similar scaffolding mechanisms at inhibitory synapses, and suggest that the GIT1/βPIX signaling pathway may represent a key coordinator of actin pathways at synapses. Indeed, this is in agreement with the role of this GIT1/βPIX signaling module at regions of cell-cell contact in multiple cell types (Hoefen and Berk, 2006). Further study will now be required to define how the GIT1/βPIX complex may coordinate potential crosstalk between excitatory and inhibitory synapses.
The number of GABAARs at the neuronal surface and synaptic sites directly correlates with the strength of inhibitory synaptic transmission; therefore, the modulation of GABAAR synaptic accumulation is a key mechanism for plasticity of inhibitory synapses. Here, we show that GIT1 or βPIX knockdown causes reduced GABAAR clustering and a decrease in the number of GABAARs at the neuronal surface, and this effect is mimicked by inhibition of Rac1 or PAK in neurons. Indeed, disruption of GABAAR clustering by βPIX knockdown cannot be rescued by βPIX mutants lacking GEF activity for Rac1 or the ability to couple to PAK, implicating PAK as a downstream effector. Importantly, the effects of RNAi mediated knockdown of either GIT1 or βPIX on GABAAR clustering is rescued by active PAK. These biochemical and imaging data are supported by electrophysiological data, which show that knockdown of GIT1 or βPIX, or inhibition of Rac1 or PAK, causes reduced GABAergic currents in neurons, suggesting the reduction in GABAAR clusters does indeed correlate with reduced inhibition. In addition to these postsynaptic effects, we also observe reductions in VGAT clustering and mIPSC frequency, suggestive of additional presynaptic effects of GIT1/βPIX knockdown. In our imaging and electrophysiological experiments, we analyze GIT1/βPIX knockdown in the postsynaptic neuron, suggesting the presynaptic effects we observe are due to destabilization of the presynaptic GABAergic synaptic bouton concurrent with the loss of postsynaptic receptors, scaffolds, and adhesion molecules. Previously, disruption of gephyrin has been shown to reduce GABAAR clusters postsynaptically, accompanied by a loss of presynaptic GABAergic innervation (Marchionni et al., 2009; Yu et al., 2007). Similarly, loss of the γ2 subunit (as we demonstrate here occurs upon disruption of GIT1/βPIX) causes loss of both postsynaptic clustering and presynaptic innervation (Li et al., 2005). It is becoming clear that crosstalk between the pre- and postsynaptic sites of inhibitory synapses is essential for their plasticity, demonstrated by the observation that inhibitory pre- and postsynaptic structures are highly mobile and can move as a single entity (Dobie and Craig, 2011). Because our RNAi experiments are targeting the postsynaptic cell, our results are consistent with alteration of the GIT1/βPIX complex disrupting inhibitory postsynaptic domains, which also causes subsequent disruption of presynaptic innervation.
The GIT1/βPIX/Rac1/PAK pathway we have presented here documents a signaling pathway that links GABAAR stability to the actin cytoskeleton via a GTPase signaling cascade. By treating neurons with latrunculin-A for 30 min, we show that an intact actin cytoskeleton is important for GABAAR stability. This is in contrast with earlier findings showing no effect of latrunculin-A on GABAAR clustering (Allison et al., 2000); however, this study differed in both age of neurons and length of treatment (24 hr). We conclude that, although actin is not structurally required at inhibitory synapses as in dendritic spine heads, it is emerging that actin is required for the integrity of the inhibitory postsynaptic site (Charrier et al., 2006) and for postsynaptic scaffold mobility in general (Kerr and Blanpied, 2012). Gephyrin also interacts with collybistin (Kins et al., 2000), a GEF for the small GTPase Cdc42. The role of collybistin in region-specific inhibitory synapse formation has been investigated (Papadopoulos and Soykan, 2011), although it is still unclear whether Cdc42 activity is required for gephyrin clustering with several studies suggesting that collybistin functions independently of its GEF activity (Reddy-Alla et al., 2010). Thus, other Rho GTPase signaling and scaffolding mechanisms are likely to be present at inhibitory synapses. In agreement with this, we show that βPIX and Rac1 activity is required for inhibitory synapse function, by maintaining GABAAR surface levels at synapses, gephyrin clustering, and GABAergic currents.
GIT1 knockout (KO) mice have been investigated in the context of neuronal function in two independent reports. Reduced dendritic spine density and dendrite length in the hippocampal CA1 region have been reported in one GIT1 KO model, in addition to impaired performance in learning tasks (Menon et al., 2010). In a second study, investigators also observed memory and learning impairments, with increased hyperactivity and reduced inhibitory presynaptic input in CA1 (Won et al., 2011). Here, we demonstrate the effects of acute GIT1 knockdown and the short-term effects of inhibiting the GIT/βPIX/Rac1/PAK pathway on GABAAR clustering and synaptic inhibition and find reduced clustering of essential inhibitory synapse components including gephyrin. We therefore attribute the differences in our findings and those of Won et al., to the use of global KO strategies, which cause GIT1 knockdown in all cell types throughout development, which is in contrast to the short-term RNAi targeting and pharmacological treatment that we employ here.
Reduced inhibition due to depletion of GABAARs from the neuronal cell surface can alter the E/I balance of neuronal circuits, causing disrupted information processing, which may lead to altered animal behavior (Blundell et al., 2009; Crestani et al., 1999; Papadopoulos et al., 2007; Tretter et al., 2009; Yizhar et al., 2011). Deficits in GABAergic neurotransmission leading to an altered E/I balance have also been implicated in multiple neuropsychiatric disorders including depression (Luscher et al., 2011b), bipolar disorder (Craddock et al., 2010), schizophrenia (Charych et al., 2009), and attention deficit hyperactivity disorder (ADHD; Won et al., 2011). Therefore, identifying the molecular mechanisms that are essential for maintaining inhibitory synaptic transmission in the brain is also critical to understanding the development of these neuropsychiatric disorders, where it may become necessary to readdress pathological alterations in E/I balance. Our findings showing that a Rac1 signaling pathway is important for regulating inhibitory synaptic transmission may shed light on the molecular mechanisms underlying mental illness. Indeed, many of the proteins involved in the GTPase signaling pathway we describe here have been directly linked to mental disorders (Allen et al., 1998; Boda et al., 2004; Govek et al., 2004; Won et al., 2011). Altered PAK signaling due to mutations in the PAK3 gene has been linked to patients with mental retardation, and PAK signaling is additionally implicated in models of fragile-X syndrome and schizophrenia (Chen et al., 2010; Hayashi et al., 2007; Hayashi-Takagi et al., 2014), making it an important molecule in the synaptic pathology of psychiatric disorders. In addition, GIT1 was recently shown to harbor a single nucleotide polymorphism causing reduced GIT1 expression that is strongly linked to ADHD in humans (Won et al., 2011). Our results suggest how a postsynaptic GIT1 signaling complex plays a key role in controlling synaptic inhibition, by stabilizing GABAARs at the inhibitory synaptic site, and may be an important locus for altered animal behavior and psychiatric and cognitive deficits.
We have characterized a GIT1/βPIX/Rac1/PAK signaling pathway that controls GABAAR clustering from a molecular and physiological viewpoint. We found that GIT1, βPIX, Rac1, and PAK are all essential to maintain surface GABAAR clusters and inhibitory currents in neurons, therefore making this signaling pathway an important regulator of inhibitory signaling in the brain.
Experimental Procedures
Details regarding antibodies, immunocytochemistry and analysis, coimmunoprecipitations, biotinylations, and cDNA cloning are included in the Supplemental Experimental Procedures.
Neuronal Cell Culture
All experimental procedures were carried out in accordance with institutional animal welfare guidelines and the UK Animals (Scientific Procedures) Act 1986. Cultures of cortical and hippocampal neurons were prepared from E18 Sprague-Dawley rat embryos as described previously (Pathania et al., 2014; Twelvetrees et al., 2010); see also the Supplemental Experimental Procedures.
Electrophysiology
Standard voltage-clamp techniques were used for whole-cell recordings of spontaneous IPSC in cultured neurons (Twelvetrees et al., 2010). Electrodes were filled with the following internal solution (in mM): 100 CsCl, 30 N-methyl-D-glucamine, 10 HEPES, 1 MgCl2, 5 EGTA, 2 QX314, 12 phosphocreatine, 5 MgATP, 0.5 Na2GTP, 0.2 leupeptin (pH 7.2–7.3), and 270–280 mOsm. The external solution consisted of the following (in mM): 130 NaCl, 26 NaHCO3, 3 KCl, 5 MgCl2, 1.25 NaH2PO4, 1 CaCl2, and 10 glucose (pH 7.4), 300 mOsm. Neurons were held at −70 mV. CNQX (20 μM) and APV (50 μM) were added to block AMPA and NMDA receptors. Mini IPSC recordings were performed in the presence of 0.01 mM TTX. Data were analyzed with Kaleidagraph (Albeck Software) and Mini Analysis program (Synaptosoft).
To measure GABAergic transmission in cortical slices, we used standard whole-cell recording techniques (Yuen et al., 2011). Pyramidal neurons at layer V of prefrontal cortex were used for recordings. Slices were incubated at room temperature (20°C –22°C) in artificial CSF (ACSF) bubbled with 95% O2, 5% CO2, and then slices were treated with various agents for 1 hr before recordings.
Proximity Ligation Assay
The in situ proximity ligation assay (PLA) was used according to the manufacturer’s instructions (Olink Bioscience). Neurons were fixed in 4% PFA/30% sucrose, blocked (10% horse serum, 0.5% BSA, and 0.2% Triton X-100, 10 min at room temperature), and incubated with primary antibodies. For control PLA, a single antibody was applied. Cells were washed in 1 × PBS and then incubated with secondary antibodies conjugated to oligonucleotides. Ligation and amplification reactions were conducted at 37°C, before mounting and visualization with CLSM.
Author Contributions
K.R.S. performed and analyzed most of the biochemistry and confocal imaging experiments, led the project, and wrote the paper. E.C.D. performed molecular biology and performed and analyzed some of the biochemistry and confocal imaging experiments. M.P. performed and analyzed the PLA. V.V. performed molecular biology. J.W. and X.L. performed and analyzed electrophysiology experiments. Z.Y. supervised and advised on electrophysiology experiments. J.T.K. supervised the project and wrote the paper. E.C.D. and J.W. contributed equally to this work.
Acknowledgments
This work was funded by the UK Medical Research Council (MRC; G0802377) to J.T.K. E.C.D. is on the MRC LMCB PhD Programme, and V.V. is on the four-year PhD Programme in Neuroscience at UCL. We thank Aaron Jaffe for providing constructs and members of J.T.K.’s lab for critical reading of the manuscript.
Published: October 2, 2014
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.08.061.
Supplemental Information
References
- Allen K.M., Gleeson J.G., Bagrodia S., Partington M.W., MacMillan J.C., Cerione R.A., Mulley J.C., Walsh C.A. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat. Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- Allison D.W., Chervin A.S., Gelfand V.I., Craig A.M. Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules. J. Neurosci. 2000;20:4545–4554. doi: 10.1523/JNEUROSCI.20-12-04545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia-Cárcamo I.L., Yuen E.Y., Muir J., Lumb M.J., Michels G., Saliba R.S., Smart T.G., Yan Z., Kittler J.T., Moss S.J. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc. Natl. Acad. Sci. USA. 2009;106:17552–17557. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H., Lévi S., Schweizer C., Inoue T., Launey T., Racine V., Sibarita J.-B., Mikoshiba K., Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Blundell J., Tabuchi K., Bolliger M.F., Blaiss C.A., Brose N., Liu X., Südhof T.C., Powell C.M. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda B., Alberi S., Nikonenko I., Node-Langlois R., Jourdain P., Moosmayer M., Parisi-Jourdain L., Muller D. The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J. Neurosci. 2004;24:10816–10825. doi: 10.1523/JNEUROSCI.2931-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier C., Ehrensperger M.V., Dahan M., Lévi S., Triller A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. J. Neurosci. 2006;26:8502–8511. doi: 10.1523/JNEUROSCI.1758-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych E.I., Liu F., Moss S.J., Brandon N.J. GABA(A) receptors and their associated proteins: implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology. 2009;57:481–495. doi: 10.1016/j.neuropharm.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.Y., Rex C.S., Babayan A.H., Kramár E.A., Lynch G., Gall C.M., Lauterborn J.C. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J. Neurosci. 2010;30:10977–10984. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N., Jones L., Jones I.R., Kirov G., Green E.K., Grozeva D., Moskvina V., Nikolov I., Hamshere M.L., Vukcevic D., Wellcome Trust Case Control Consortium (WTCCC) Strong genetic evidence for a selective influence of GABAA receptors on a component of the bipolar disorder phenotype. Mol. Psychiatry. 2010;15:146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F., Lorez M., Baer K., Essrich C., Benke D., Laurent J.P., Belzung C., Fritschy J.M., Lüscher B., Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Deacon S.W., Beeser A., Fukui J.A., Rennefahrt U.E., Myers C., Chernoff J., Peterson J.R. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie F.A., Craig A.M. Inhibitory synapse dynamics: coordinated presynaptic and postsynaptic mobility and the major contribution of recycled vesicles to new synapse formation. J. Neurosci. 2011;31:10481–10493. doi: 10.1523/JNEUROSCI.6023-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C., Lorez M., Benson J.A., Fritschy J.-M., Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fritschy J.M., Harvey R.J., Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Govek E.-E., Newey S.E., Akerman C.J., Cross J.R., Van der Veken L., Van Aelst L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat. Neurosci. 2004;7:364–372. doi: 10.1038/nn1210. [DOI] [PubMed] [Google Scholar]
- Hayashi M.L., Rao B.S., Seo J.S., Choi H.S., Dolan B.M., Choi S.Y., Chattarji S., Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc. Natl. Acad. Sci. USA. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A., Araki Y., Nakamura M., Vollrath B., Duron S.G., Yan Z., Kasai H., Huganir R.L., Campbell D.A., Sawa A. PAKs inhibitors ameliorate schizophrenia-associated dendritic spine deterioration in vitro and in vivo during late adolescence. Proc. Natl. Acad. Sci. USA. 2014;111:6461–6466. doi: 10.1073/pnas.1321109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H., Lin C.C., Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefen R.J., Berk B.C. The multifunctional GIT family of proteins. J. Cell Sci. 2006;119:1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- Hoelz A., Janz J.M., Lawrie S.D., Corwin B., Lee A., Sakmar T.P. Crystal structure of the SH3 domain of betaPIX in complex with a high affinity peptide from PAK2. J. Mol. Biol. 2006;358:509–522. doi: 10.1016/j.jmb.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Kerr J.M., Blanpied T.A. Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J. Neurosci. 2012;32:658–673. doi: 10.1523/JNEUROSCI.2927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kins S., Betz H., Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat. Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Kiraly D.D., Eipper-Mains J.E., Mains R.E., Eipper B.A. Synaptic plasticity, a symphony in GEF. ACS Chem Neurosci. 2010;1:348–365. doi: 10.1021/cn100012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M., Brandstätter J.H., Laube B., Stahl S., Müller U., Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J. Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.J., Isozaki K., Kim I., Lee J.H., Cho H.J., Sohn S.Y., Oh S.R., Park S., Kim D.G., Kim C.H., Roche K.W. PKC phosphorylation regulates mGluR5 trafficking by enhancing binding of Siah-1A. J. Neurosci. 2012;32:16391–16401. doi: 10.1523/JNEUROSCI.1964-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis P., Barnier J.V. PAK signalling in neuronal physiology. Cell. Signal. 2009;21:384–393. doi: 10.1016/j.cellsig.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Li R.W., Yu W., Christie S., Miralles C.P., Bai J., Loturco J.J., De Blas A.L. Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J. Neurochem. 2005;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Luscher B., Fuchs T., Kilpatrick C.L. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B., Shen Q., Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni I., Kasap Z., Mozrzymas J.W., Sieghart W., Cherubini E., Zacchi P. New insights on the role of gephyrin in regulating both phasic and tonic GABAergic inhibition in rat hippocampal neurons in culture. Neuroscience. 2009;164:552–562. doi: 10.1016/j.neuroscience.2009.07.063. [DOI] [PubMed] [Google Scholar]
- Menon P., Deane R., Sagare A., Lane S.M., Zarcone T.J., O’Dell M.R., Yan C., Zlokovic B.V., Berk B.C. Impaired spine formation and learning in GPCR kinase 2 interacting protein-1 (GIT1) knockout mice. Brain Res. 2010;1317:218–226. doi: 10.1016/j.brainres.2009.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J., Arancibia-Carcamo I.L., MacAskill A.F., Smith K.R., Griffin L.D., Kittler J.T. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the γ2 subunit. Proc. Natl. Acad. Sci. USA. 2010;107:16679–16684. doi: 10.1073/pnas.1000589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos T., Soykan T. The role of collybistin in gephyrin clustering at inhibitory synapses: facts and open questions. Front Cell Neurosci. 2011;5:11. doi: 10.3389/fncel.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos T., Korte M., Eulenburg V., Kubota H., Retiounskaia M., Harvey R.J., Harvey K., O’Sullivan G.A., Laube B., Hülsmann S. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania M., Davenport E.C., Muir J., Sheehan D.F., López-Doménech G., Kittler J.T. The autism and schizophrenia associated gene CYFIP1 is critical for the maintenance of dendritic complexity and the stabilization of mature spines. Transcult. Psychiatry. 2014;4:e374. doi: 10.1038/tp.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont R.T., Perry S.J., Schmalzigaug R., Roseman J.T., Xing Y., Claing A. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell. Signal. 2004;16:1001–1011. doi: 10.1016/j.cellsig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Reddy-Alla S., Schmitt B., Birkenfeld J., Eulenburg V., Dutertre S., Böhringer C., Götz M., Betz H., Papadopoulos T. PH-domain-driven targeting of collybistin but not Cdc42 activation is required for synaptic gephyrin clustering. Eur. J. Neurosci. 2010;31:1173–1184. doi: 10.1111/j.1460-9568.2010.07149.x. [DOI] [PubMed] [Google Scholar]
- Renner M., Choquet D., Triller A. Control of the postsynaptic membrane viscosity. J. Neurosci. 2009;29:2926–2937. doi: 10.1523/JNEUROSCI.4445-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T., Wayman G., Fortin D., Davare M., Hoshi N., Nozaki N., Natsume T., Soderling T.R. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker O., Rittinger K. Structures of dimeric GIT1 and trimeric beta-PIX and implications for GIT-PIX complex assembly. J. Mol. Biol. 2009;386:280–289. doi: 10.1016/j.jmb.2008.12.050. [DOI] [PubMed] [Google Scholar]
- Shutes A., Onesto C., Picard V., Leblond B., Schweighoffer F., Der C.J. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J. Biol. Chem. 2007;282:35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- Smith K.R., Kittler J.T. The cell biology of synaptic inhibition in health and disease. Curr. Opin. Neurobiol. 2010;20:550–556. doi: 10.1016/j.conb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Smith K.R., Muir J., Rao Y., Browarski M., Gruenig M.C., Sheehan D.F., Haucke V., Kittler J.T. Stabilization of GABA(A) receptors at endocytic zones is mediated by an AP2 binding motif within the GABA(A) receptor β3 subunit. J. Neurosci. 2012;32:2485–2498. doi: 10.1523/JNEUROSCI.1622-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Katayama K., Sohya K., Miyamoto H., Prasad T., Matsumoto Y., Ota M., Yasuda H., Tsumoto T., Aruga J., Craig A.M. Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nat. Neurosci. 2012;15:389–398. doi: 10.1038/nn.3040. S1–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster J.P., Jaffer Z.M., Chernoff J., Hordijk P.L. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J. Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V., Revilla-Sanchez R., Houston C., Terunuma M., Havekes R., Florian C., Jurd R., Vithlani M., Michels G., Couve A. Deficits in spatial memory correlate with modified gamma-aminobutyric acid type A receptor tyrosine phosphorylation in the hippocampus. Proc. Natl. Acad. Sci. USA. 2009;106:20039–20044. doi: 10.1073/pnas.0908840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twelvetrees A.E., Yuen E.Y., Arancibia-Carcamo I.L., MacAskill A.F., Rostaing P., Lumb M.J., Humbert S., Triller A., Saudou F., Yan Z., Kittler J.T. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F., Jamain S., Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Won H., Mah W., Kim E., Kim J.W., Hahm E.K., Kim M.H., Cho S., Kim J., Jang H., Cho S.C. GIT1 is associated with ADHD in humans and ADHD-like behaviors in mice. Nat. Med. 2011;17:566–572. doi: 10.1038/nm.2330. [DOI] [PubMed] [Google Scholar]
- Yizhar O., Fenno L.E., Prigge M., Schneider F., Davidson T.J., O’Shea D.J., Sohal V.S., Goshen I., Finkelstein J., Paz J.T. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Jiang M., Miralles C.P., Li R.-W., Chen G., de Blas A.L. Gephyrin clustering is required for the stability of GABAergic synapses. Mol. Cell. Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E.Y., Liu W., Karatsoreos I.N., Ren Y., Feng J., McEwen B.S., Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol. Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Webb D.J., Asmussen H., Horwitz A.F. Synapse formation is regulated by the signaling adaptor GIT1. J. Cell Biol. 2003;161:131–142. doi: 10.1083/jcb.200211002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Webb D.J., Asmussen H., Niu S., Horwitz A.F. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 2005;25:3379–3388. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.