Abstract
Control of colorectal cancer needs to be tailored to its etiology. Tumor promotion mechanisms in colitis-associated colon cancer differ somewhat from the mechanisms involved in hereditary and sporadic colorectal cancer. Unlike sporadic or inherited tumors, some experimental models show that colitis-associated colon tumors do not require cyclooxygenase (COX) expression for progression, and non-steroidal anti-inflammatory drugs (NSAIDs) which prevent sporadic or inherited colon cancer do not prevent colitis-associated colon cancer. We report that myeloperoxidase (MPO), an ancestor of the COX isoenzymes, is a determinant of colitis-associated colon tumors in ApcMin/+ mice. During experimentally induced colitis, inhibition of MPO by resorcinol dampened colon tumor development. Conversely, in the bowels of ApcMin/+ mice without colitis, resorcinol administration or ‘knockout’ of MPO gene coincided with a slight, but discernible increase in colon tumor incidence. Acrolein, a by-product of MPO catalysis, formed a covalent adduct with the phosphatase tensin homolog (PTEN) tumor suppressor and enhanced the activity of the Akt kinase proto-oncogene in vitro and in vivo. Thus, MPO may be an important determinant of diet and inflammation on colon cancer risk via its effect on endogenous exposure to oxidants and acrolein. We propose a hypothetical model to explain an apparent dichotomy between colon tumor occurrence and MPO inhibition in inflamed versus non-inflamed colons.
Abbreviations: Apc, adenomatous polyposis coli; BME, β-mercaptoethanol; COX, cyclooxygenase; DTT, dithiothreitol; DSS, dextran sodium sulfate; ECL, enhanced chemiluminescence; EPO, eosinophil peroxidase; FBS, fetal bovine serum; HRP, horse radish peroxidase; MBTH, 3-methyl-2-benzothiazolinone hydrazone hydrochloride; MEM, modified Eagle's medium; MPO, myeloperoxidase; NSAIDs, non-steroidal anti-inflammatory drugs; PBS, phosphate buffered saline; PI3, phosphatidylinositol; PTEN, phosphatase and tensin homolog on chromosome 10; PVDF, polyvinylidene difluoride; WT, wild type
Keywords: Inflammation, Colitis, Colon cancer, Myeloperoxidase
Graphical Abstract
Highlights
-
•
Myeloperoxidase is a determinant of colitis-associated colon tumors in ApcMin/+ mice.
-
•
Inhibition of MPO by resorcinol dampened colitis-associated colon tumor occurrence. Acrolein is a by-product of MPO catalysis.
-
•
Acrolein forms a covalent adduct with the phosphatase tensin homolog tumor suppressor.
-
•
Acrolein adducted PTEN enhances the activity of the Akt kinase proto-oncogene.
-
•
MPO may have an effect on endogenous exposure to oxidants and acrolein. MPO may be an important determinant of diet and inflammation on colon cancer risk.
1. Introduction
Chronic ulcerative colitis is an independent risk factor for colorectal cancer [1,2]. Consistent with epidemiological observations in humans, experimentally-induced colitis increases colon tumor progession in the ApcMin/+ mouse, a model of intestinal cancer [3–7]. While the molecular pathways of inherited, sporadic and colitis-associated colon tumor progression overlap, they are not identical [8,9]. Tumor formation in some mouse models of colitis-associated colon cancer does not require cyclooxygenase (COX) expression [10]. In a study using COX-1 and COX-2 ‘knockout’ mice, investigators concluded that the mechanism of colorectal tumor promotion in colitis-associated cancer differs from the mechanism of tumor promotion for hereditary and sporadic colorectal cancer [10]. However, the exact role of prostaglandins in colitis associated colorectal cancer models varies with the model [11]. Additionally, the pharmacological rationale for colon cancer prevention with non-steroidal anti-inflammatory drugs (NSAIDs) does not apply to colitis-associated tumors [12,13]. In fact, NSAIDs aggravate inflammation and malignant progression in rodent models of colitis-associated tumors [14–16], albeit with some exceptions [17]. One NSAID, 5-aminosalicylic acid, can maintain remission of ulcerative colitis, which ought to prevent colitis-associated colon cancer. However, that hypothesis is unproven despite many attempts at validation [12,18].
Myeloperoxidase (MPO), an ancestor of COX enzymes [19], helps gut associated lymphoid tissue defend against harmful enteric microbes, while tolerating harmless commensal bacteria and dietary antigens. Because MPO activity correlates with the severity of experimentally induced colitis [20] and its expression is an indicator of colon cancer risk [21] we investigated its influence on colon tumor development in ApcMin/+ mice. We report that elevated MPO activity in the inflamed bowels of ApcMin/+ mice correlated with greater colon tumor occurrence; inhibition of MPO activity in inflamed colons of ApcMin/+ mice partly suppressed colon tumor occurrence. Conversely, tumors were absent or rare in non-inflamed colons with low, basal MPO activity in ApcMin/+ mice. Unexpectedly, either pharmacological or genetic suppression of basal MPO activity correlated with a small, but discernible rise in colon tumors in ApcMin/+ without colitis. Thus, the relationship between MPO activity and colon tumor occurrence in ApcMin/+ mice varies with the status of inflammation in the gut. Our mechanistic experiments found that a carcinogenic by-product of MPO catalysis, acrolein, formed a protein adduct with phosphatase tensin homolog (PTEN) in colonocytes isolated from the inflamed bowel of ApcMin/+ mice. Modification of the PTEN tumor suppressor coincided with activation of the Akt kinase proto-oncogene, which favors cell growth and survival. Since acrolein can originate endogenously from MPO mediated oxidation of threonine or serine [22] this mechanism may contribute to complex effects of diet on inflammation and colon cancer risk.
2. Materials and methods
2.1. Reagents
The following were used: resorcinol, acrolein, L-threonine, hydrogen peroxide (H2O2) and 3-methyl-2-benzothiazolinone hydrazone hydrochloride monohydrate (MBTH) (Sigma Aldrich, St. Louis, MO); recombinant MPO (Athens Research & Technology, Athens, GA); myeloperoxidase activity EnzChek® assay kits; complete protease inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN); lysis buffer A, 0.6% Igepal CA-630 in PBS (Promega, Madison, WI); wortmannin, primary antibodies against PTEN, Akt, phospho-(Ser473)Akt (Cell Signaling Technologies, Danvers, MA); rabbit polyclonal antibodies to PTEN (Upstate, Lake Placid, NY); anti-acrolein adduct antibody (Cosmo Bio Co. Ltd., Tokyo, Japan); horseradish peroxidase-conjugated (HRP) secondary antibodies and protein A/G PLUS-Agarose (Santa Cruz Biotechnology; Santa Cruz, CA); polyvinylidene difluoride (PVDF) membranes and Western Lightning chemiluminescence reagents (Perkin-Elmer, Waltham, MA); and dextran sodium sulfate, MW 36,000–50,000 (MP Biomedicals, Solon, OH). Lysis buffer B was 50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, 0.2% deoxycholic acid sodium salt, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 5 μg/ml leupeptin, and 5 μg/ml pepstatin A. Authenticated HCT116 colon cancer cells and MCF-7 breast cancer cells were from ATCC (Manassas, VA).
2.2. Mice experiments and husbandry
All mice experiment protocols (Fig. 1A) were approved by the University of Utah Institutional Animal Care and Use Committee. Equal numbers of male and female mice were used throughout the study. ApcMin/+ mice (C57BL/6J-ApcMin/J), and their corresponding C57BL/6J wild type littermates were obtained from Jackson Laboratories, Bar Harbor, ME. MPO ‘knockout’ mice (B6.129×1-Mpotm1Lus/J) procured from Jackson Laboratory were bred on a C57BL/6J background through at least 10 generations. A strain is considered fully congenic after ten generations of backcrossing (N10). Transgenic mice (ApcMin/+/MPO−/−) on a C57Bl/6J background were identified by PCR amplification of genomic DNA isolated from mouse tails using salt precipitation. All mice, kept in standard housing conditions, and fed 8656 Teklad sterilizable rodent diet (Harlan Laboratories, Denver, CO). Mice were sacrificed by CO2 asphyxiation.
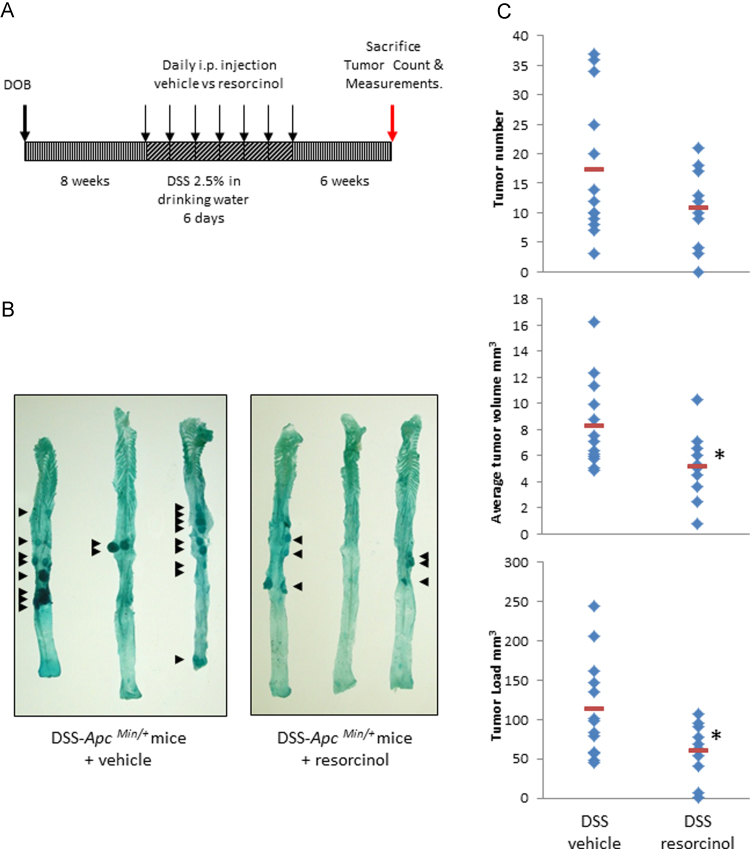
Fig. 1.
Myeloperoxidase and colitis-associated colon tumors in ApcMin/+ Mice. Panel A: protocol for induction of colitis with dextran sodium sulfate (DSS) and modulation of MPO activity with resorcinol in ApcMin/+ mice. Panel B: colitis-associated tumors (arrows) in representative colons from DSS-ApcMin/+ mice treated with vehicle or resorcinol. Panel C: graphs of colon tumor numbers, volumes and loads in colons from DSS-ApcMin/+ mice treated with vehicle (n=13) or resorcinol (n=10).
2.3. DSS induced colitis
At 8 weeks of age ApcMin/+ or wild type mice were given drinking water containing 2.5% (w/v) DSS for 7 days. The occurrence and severity of colitis was measured by quantifying the MPO activity, a proxy for colitis, in the intestinal mucosa of mice [20]. Both peroxidase and chlorination activity were quantified, with chlorination being a more specific index of MPO activity. We used EnzChek® MPO activity assay kits, following the manufacturer's protocol. Briefly, the assay measures selective cleavage of 3′-(p-aminophenyl) by hypochlorite (−OCl) to yield fluorescein. Stock reagents include 3′-(p-aminophenyl) fluorescein (5 mM in dimethyformamide) and 5 mM H2O2 prepared fresh daily in phosphate-buffered saline (PBS), pH 7.2. The substrate-cofactor working solution is a combination of 20 μM 3′-(p-aminophenyl) fluorescein and 20 μM H2O2 in PBS, pH 7.2. Experimental samples (50 μL of tissue lysate) or MPO standards were added to 96-well microplates suitable for measuring fluorescence. MPO catalysis was initiated by adding substrate-cofactor working solution (50 μL) at 25 °C. Plates were covered with tin foil to protect from ambient light. The fluorescence intensity of fluorescein formed by MPO activity in each sample was measured continuously using excitation at 485 nm and emission at 530 nm. MPO activity is proportional to the rate of change of fluorescence in the linear portion of the curve, typically around 30 min after starting the reaction. Reaction rates were calculated after correction using a negative control (boiled samples plus MPO inhibitor). MPO levels were calibrated using serially diluted (1:2) MPO standard solutions ranging from 0 to 200 ng/ml. When resorcinol was used as an inhibitor it was added simultaneously with the substrate co-factor solution.
2.4. Tumor studies
ApcMin/+ mice were divided into four groups. Starting at 8 weeks of age two groups of ApcMin/+ mice were treated with 2.5% (w/v) DSS for 7 days, while the remaining two groups were given water. The MPO inhibitor resorcinol (1.25 mg/kg) or saline vehicles (0.20 ml) were injected once daily, i.p., for the duration of the DSS treatment. Mice were allowed to mature a further 6 weeks before being sacrificed. In addition, two separate groups of ApcMin/+ and ApcMin/+ x MPO−/− double mutant mice were matured to the same age as above and sacrificed to compare the effect of pharmacological inhibition of MPO by resorcinol [23] versus a genetic ‘knockout’ of MPO in a non-colitis background.
2.5. Tissue harvesting and tumor assessment
The small intestine and colon were dissected longitudinally and washed with ice-cold PBS. They were then fixed for 4 h in 10% formalin in PBS, and stored in 70% ethanol at 4 °C. The intestine and colons were stained with methylene blue, and then polyps and colon tumors were counted with the aid of a dissecting microscope. The counting studies were independently performed by two individuals who had no knowledge of the genotype and/or type of treatment used for each mouse. Colon tumors were photographed, the largest and smallest diameters were recorded, and tumor volumes were calculated according to the equation (volume (mm3)=π/6×largest diameter×smallest diameter2). Tumor load was calculated by summing all measured tumor volumes in a mouse.
2.6. Generation of acrolein in situ by MPO
2-Hydroxy-propenal and acrolein (2-propenal) were generated by incubating 1.5 μg MPO (10 nM), 150 mM chloride and 200 μM L-threonine in 1.0 ml of 0.05 M phosphate buffer, pH 7.0, at 37 °C [22]. The reaction was initiated by the addition of 200 μM H2O2. For some experiments incubation times were varied, MPO was omitted, or the MPO inhibitor resorcinol (0–100 μM) was added. Formation of 2-hydroxy-propenal and acrolein were quantified spectrophotometrically by measuring the absorbance of their MBTH derivative at 598 nm. The assay was calibrated with acrolein standards. Briefly, a sample of the MPO reaction mixture (200 μl) was incubated for 20 min, 25 °C with 568 μl of sodium phosphate buffer (5 mM, pH 7.0), 25 μl HCl (6 N), and 132 μl of MBTH (155 mM). The derivative was then reacted with 75 μl FeCl3 (370 mM) and incubated for 10 min at 25 °C. The MBTH derivative was stable for at least 5 h.
2.7. Cell culture
HCT116 cells were grown in modified Eagle's medium (MEM) with 10% fetal bovine serum (FBS), 100 units of penicillin/streptomycin, 2 mM L-glutamine, and 1 mM pyruvate. MCF-7 cells were grown in MEM with 10% v/v FBS, 2 mM L-glutamine, 1.5 g/l NaHCO3, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 0.01 mg/ml bovine insulin, and 0.01 mg/ml gentamicin.
2.8. MPO dependent modulation of PTEN–Akt signaling
HCT 116 and MCF-7 cells were grown to ~80% confluency; culture media was removed and replaced with 2 ml of 0.05 M phosphate buffer, pH 7.0, MPO, chloride and threonine as detailed above. Acrolein formation was initiated with 200 μM H2O2 before addition to cells. Cells were exposed to the reaction products at 37 °C for intervals from 0 to 30 min. For some experiments MPO was omitted, or resorcinol (0–100 μM) was added to modulate MPO dependent generation of reactive aldehydes. A phosphatidyl-inositol-3-kinase (PI3-K) inhibitor, wortmannin, was used as a control to modulate Akt activation. Treated cells were lysed in 250 mM sucrose, 50 mM Tris pH 7.4, 5 mM MgCl2, 1 mM EGTA, 1× complete protease inhibitor, 2 mM NaF and 2 mM sodium orthovanadate. Lysates from treated cells (15 µg protein) were fractionated on SDS-10% PAGE and proteins were transferred to polyvinyldifluoride membranes for immunoblot analysis of Akt and phospho-(S473) Akt as described [24,25].
2.9. Detection of acrolein-PTEN adducts in colonocytes from inflamed and non-inflamed colons of mice
After treatment of ApcMin/+ mice with either vehicle or DSS, ±resorcinol, (Fig. 1A) colons were removed. Colon crypts, and the colonocytes within, were isolated as described [26]. Longitudinally dissected colons were incubated in PBS containing 3 mM EDTA and 50 μM DTT for 90 min at 4 °C. The colons were then washed and vigorously shaken in ice-cold PBS 3–4 times to release intact colon crypts and wash away any leukocytes. Colon crypts and their colonocytes were enriched via centrifugation at 40g for 10 min at 4 °C, and then reconstituted in ice-cold cell lysis buffer.
Colon crypts were lysed in buffer B and their PTEN protein was isolated by immunoprecipitation. Briefly, lysates were pre-cleared by incubation with 2 μg of mouse IgG and 20 μl of protein G PLUS agarose at 4 °C for 30 min and centrifuged at 1000g at 4 °C for 5 min. Aliquots containing equivalent amounts of total cellular protein were immunoprecipitated using 1 μg of mouse monoclonal anti-PTEN by incubation at 4 °C for 2 h. Immune complexes were precipitated by incubation with 20 μl of protein G PLUS agarose at 4 °C overnight and collected by centrifugation. Immunoprecipitates were washed four times with lysis buffer B, resuspended in Laemmli buffer, separated on a 10% SDS-polyacrylamide gel, and transferred to PVDF membranes. After blocking with 5% powered milk in 10 mM Tris–HCl, pH 7.5, 100 mM NaCl, and 0.1% Tween 20, acrolein-modified PTEN was detected with the monoclonal antibody mAb5F6 (1:5000 dilution) [27] followed by horseradish peroxidase-conjugated mouse anti-goat IgG (1:10,000 dilution) followed by detection with ECL Plus®. Blots were stripped using buffer containing 62.5 mM Tris–HCl, pH 6.7, 100 mM 2-mercaptoethanol, 2% SDS, at 55 °C for 30 min, and reprobed with the rabbit polyclonal antibodies to PTEN and visualized with HRP-conjugated secondary antibody followed by ECL Plus®.
2.10. Western immunoblotting
Samples were dissolved in 50 µl of Laemmli loading buffer, 0.5% BME and heated at 95 °C for 10 min. Samples (15–30 µg protein) were fractionated by SDS-PAGE and transferred to polyvinyldifluoride membranes. Membranes were blocked with 5% w/v nonfat dry milk in tris buffered saline with Tween 20, and then incubated for 16 h at 4 °C with primary antibodies, followed by HRP-conjugated secondary antibody (1:5000). Antigen–antibody complexes were detected with Western Lightning ECL reagents. The intensity of chemiluminescent protein-antibody complexes was quantified with a Kodak Image Station 440. Bar graphs depict the mean±standard error from densitometric analyses of separate experiments.
2.11. Statistical analysis
Data was analyzed using the Microsoft Excel statistical package. A two-tail homoscedastic or heteroscedastic unpaired Student's t-test was used. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Leukocyte MPO activity in DSS-induced colitis correlates with colon tumor occurrence in ApcMin/+ mice
At 10–12 weeks after birth, ApcMin/+ mice fed a standard diet and normal drinking water have intestinal polyps, but few or no colon tumors [28]. In our experiments 42% of ApcMin/+ control mice were free of any colon tumors and the others had only 1.3 colon tumors per mouse (range 1–3 colon tumors/mouse).
ApcMin/+ mice exposed to 2.5% w/v DSS in drinking water have increased colon tumor occurrence [29]. In our protocol all mice developed colon tumors, secondary to their colitis (100% incidence). The colon tumor number rose 13-fold to a mean=17.3 tumors per colon, range 3–37; with a median tumor volume=8.3 mm3, range 4.9–16.2 mm3 and an average tumor load=112.8 mm3, range 44.9–245 mm3. Leukocyte MPO activity in the colon, a proxy for colitis [22], rose 2.5-fold in DSS-treated mice compared to controls given normal drinking water.
Resorcinol, administered once daily (1.25 mg/kg, i.p.) for 7 days [Fig. 1A], inhibited MPO activity by ~50% on day 1 and 3, and 75% on day 6 in ApcMin/+ mice with DSS colitis [Table 1]. On day 6, leukocyte MPO activity in the mucosa of mice treated with DSS plus resorcinol was comparable to basal MPO activity in the mucosa from ApcMin/+ mice given normal drinking water. Corresponding with its suppression of MPO activity in the inflamed gut, resorcinol lessened colitis-associated colon tumor development [Fig. 1B and C]. Resorcinol reduced incidence by 10% and tumor multiplicity and tumor volume by 38%. Resorcinol-treated mice had a mean tumor number=10.7, range 0–21, and a median tumor volume=5.2 mm3, range 0.8–10.3 mm3. As mentioned above, the vehicle control mice had a comparative mean tumor number=17.3, range 3–37, and a median tumor volume=8.3 mm3, range 4.9–16.2 mm3. Resorcinol also reduced tumor load by 46%. Resorcinol-treated mice had an average tumor load=60.7 mm3, range 0.8–106.6 mm3; while the control mice had an average tumor load=112.8 mm3, range 44.9–245 mm3. In these same mice, resorcinol reduced the number of polyps in the small intestine, by 20%, but this was not statistically significant.
Table 1.
MPO activity* in colon crypts from Apc Min/+ mice treated with DSS±resorcinol.
| DSS control | DSS+resorcinol | % MPO inhibition | |
|---|---|---|---|
| Day 1 | 53.7±8.1 (n=3) | 29.0±4.3 (n=3) | 46.0±8.0 |
| Day 3 | 59.9±7.5 (n=3) | 33.1±2.2 (n=3) | 44.7±3.6 |
| Day 6 | 36.8±8.4 (n=6) | 9.0±2.1 (n=6) | 75.5±5.7 |
Mean±std. dev. (pg MPO/μg protein).
3.2. MPO activity in the non-inflamed gut of ApcMin/+ mice correlates inversely with colon tumors
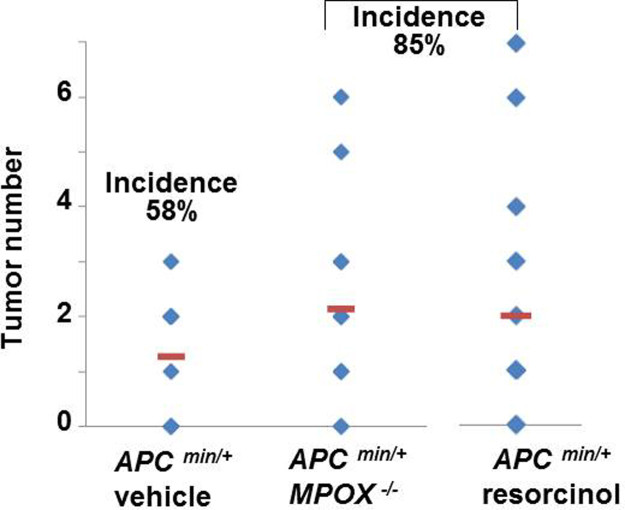
ApcMin/+ mice fed a standard diet and normal drinking water-with no DSS-had few or no colon tumors [Fig. 2, upper panel]. Administration of resorcinol (1.25 mg/kg) to these mice was associated with a small but discernible increase in colon tumor incidence from 58% during vehicle treatment, to 85% during resorcinol treatment. The number of tumors rose from a mean=1.3 (range 0–3) in mice treated with normal drinking water, to a mean=2 (range 0–7) in corresponding ApcMin/+ mice treated with resorcinol [Fig. 2, upper panel]. Exposure to resorcinol did not alter the tumor volume or tumor load in these mice under our protocol (not statistically different, p>0.05) [Fig. 2, middle and lower panels]. We draw attention to the fact that fewer tumors occur in non-inflamed colons (Fig. 2), compared to inflamed colons (Fig. 1).
Fig. 2.
Effects of resorcinol and genetic ‘knockout’ of myeloperoxidase on inherited colon tumors in ApcMin/+ mice without DSS-colitis. Tumor numbers in colons from ApcMin/+ mice treated with vehicle (n=15); ApcMin/+/MPO−/− treated with vehicle (n=16) or ApcMin/+ mice treated with resorcinol (n=14) administered at times and doses shown in Fig. 1A. Incidence of tumor occurrence was 58%, 85% and 85%, respectively.
Results in Fig. 2 imply that resorcinol may have impeded any role that MPO has in anti-tumor host-defense processes under basal conditions in the colon of ApcMin/+ mice. Alternatively, resorcinol, or its metabolites, in ApcMin/+ mice may have been carcinogenic. To address this issue we examined colon tumor formation in ApcMin/+/MPO−/− mice fed a standard diet and normal drinking water. In these mice genetic ‘knockout’ of the MPO gene heightened colon tumor incidence and number to the same extent (85%) as pharmacological suppression of MPO by resorcinol [Fig. 2]. Like resorcinol, ‘knockout’ of the MPO gene only altered tumor incidence and number, but not the tumor volume or tumor load in ApcMin/+ mice in our protocol (p>0.05).
3.3. Acrolein, a by-product of threonine and MPO catalysis, forms an adduct with PTEN, thereby enhancing Akt kinase proto-oncogene signaling
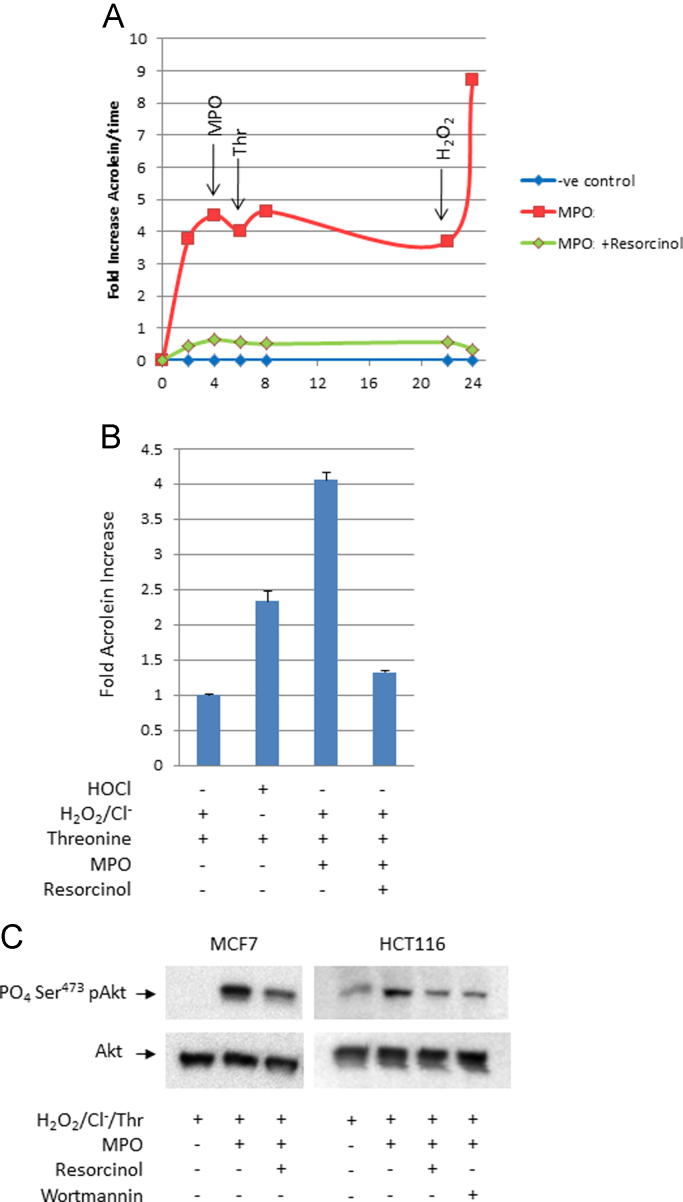
Reactive lipid-enals and -enones generated by COX or lipoxygenase enzymes during inflammation can irreversibly modify the PTEN tumor suppressor, inactivate its inositol phosphatase activity, and thereby nullify its restraint of Akt proto-oncogene signaling [24,25]. MPO can generate acrolein, a prototypical-enal carcinogen [30], from hydroxyl-amino acids at sites of inflammation [22] [Fig. 3A and B]. When HCT 116 or MCF 7 cells were exposed in situ to acrolein generated by MPO, threonine, hydrogen peroxide, and chloride ions, the cells accumulated higher amounts of phospho-Ser473Akt, an index of Akt kinase activation. The MPO inhibitor, resorcinol, suppressed the formation of cellular phospho-Ser473-Akt [Fig. 3C] to about the same extent as 0.5 μM wortmannin, an inhibitor of phosphatidylinositol-3-kinase.
Fig. 3.
Myeloperoxidase enhances cellular Akt kinase activation via acrolein generation in vitro. Panel A. Time-course of acrolein generation via MPO-mediated formation of threonine chloramine. Active MPO+substrate  ; active MPO+substrate+resorcinol
; active MPO+substrate+resorcinol  ; substrate alone
; substrate alone  . Arrows indicate supplemental addition of MPO, threonine, or H2O2 to determine the relationship between their consumption and acrolein production: depletion of H2O2 limited acrolein generation. Panel B. Relative levels of acrolein generated via chemical (HOCl) versus MPO mediated formation of threonine chloramine. These data correspond with experiments shown in panel C. Panel C. Immunochemical analysis of phospho-Ser473 Akt, a biomarker of cellular Akt activation in HCT 116 and MCF 7 cells exposed to acrolein via MPO-mediated formation of threonine chloramine±resorcinol and wortmannin.
. Arrows indicate supplemental addition of MPO, threonine, or H2O2 to determine the relationship between their consumption and acrolein production: depletion of H2O2 limited acrolein generation. Panel B. Relative levels of acrolein generated via chemical (HOCl) versus MPO mediated formation of threonine chloramine. These data correspond with experiments shown in panel C. Panel C. Immunochemical analysis of phospho-Ser473 Akt, a biomarker of cellular Akt activation in HCT 116 and MCF 7 cells exposed to acrolein via MPO-mediated formation of threonine chloramine±resorcinol and wortmannin.
During DSS-induced colitis, inflammation may expose colonocytes of ApcMin/+ mice to the processes depicted in Fig. 3. Consistent with this mechanistic hypothesis, colon crypts isolated from inflamed bowels of ApcMin/+ mice contained PTEN protein that was post-translationally modified by acrolein [Fig. 4A]. Formation of acrolein-PTEN adducts in colonocytes also corresponded with Akt kinase activation (increased phospho-Ser473-Akt levels) and with the MPO activity in the samples [Fig. 4B].
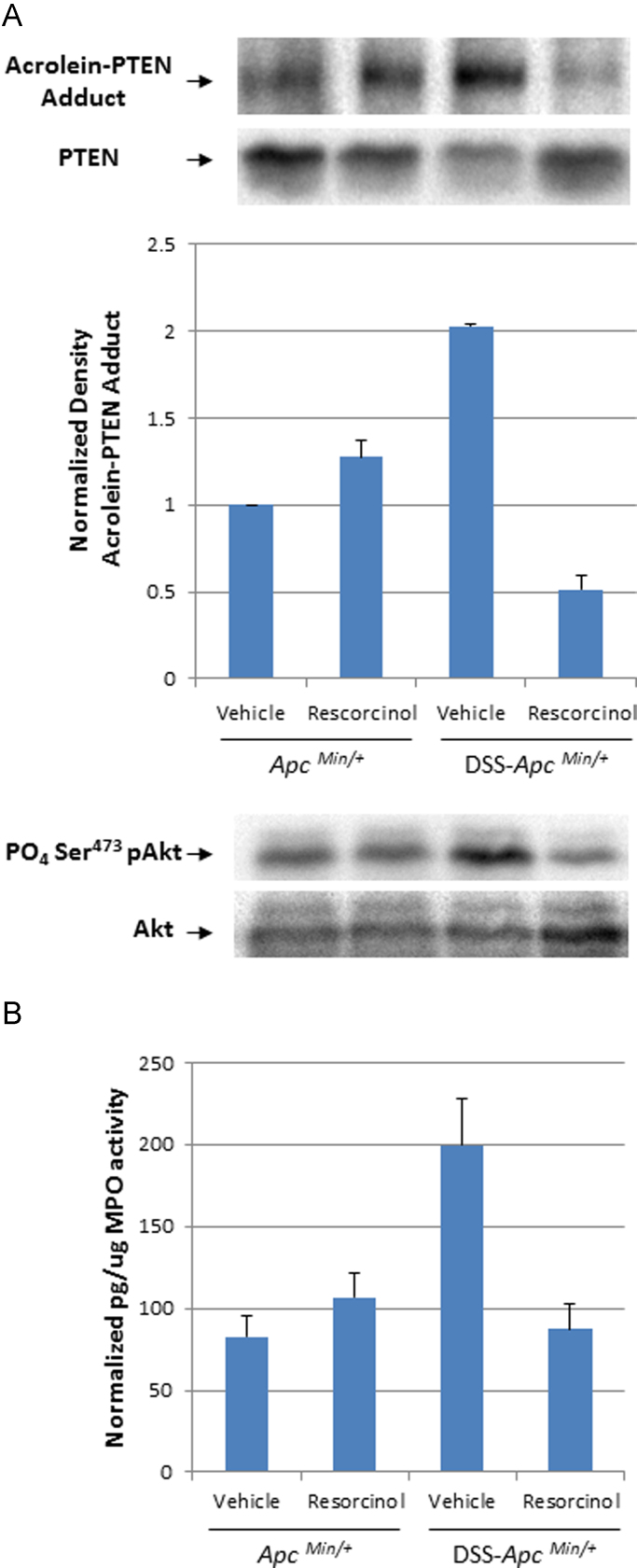
Fig. 4.
Post-translational modification of the PTEN tumor suppressor and activation of cellular Akt kinase activation in colonocytes isolated from ApcMin/+ mice. Panel A. Immunoblot and bar graph of acrolein-PTEN adducts in the total PTEN content isolated from colonocytes of ApcMin/+ mice or DSS-ApcMin/+ mice treated with vehicle or resorcinol, respectively. The lower immunoblot depicts phospho-Ser473 Akt, a biomarker of cellular Akt activation in the same samples of colonic mucosa. Panel B. MPO activity in the mucosa of ApcMin/+ mice or DSS-ApcMin/+ treated with vehicle or resorcinol, respectively.
4. Discussion
Colon tumor occurrence correlates with high MPO activity found in DSS-inflamed colons of ApcMin/+ mice. MPO can generate oxidants that cause DNA damage, and mutagenesis [30]; it can also generate acrolein as an oxidative by-product from unsaturated fats, serine, or threonine [31]. Acrolein–protein adducts in colon tissue are associated with the transition from benign to malignant colon tumors in humans [32]; however, little is known about the identity of proteins that form acrolein adducts, or their role in the etiology of colon tumor progression. In colonocytes isolated from ApcMin/+ with inflamed colons we found that PTEN, a prominent intestinal tumor suppressor, formed a protein adduct with acrolein. PTEN modification coincided with Akt proto-oncogene activation in these colonocytes. Reactive nitrogen species also reportedly oxidize PTEN and disable its restraint of Akt kinase signaling [33]. Thus, MPO metabolites or by-products may augment colitis-associated colon tumor occurrence via genetic and energetic mechanisms [34]. Genetically, oxidants and acrolein have mutagenic potential [30,35]. Energetically, oxidants and acrolein enhance anabolic signaling via the PI3-kinase–PTEN–Akt kinase axis. While speculative, this latter mechanism can account for the increased size of colon tumors reported in the DSS-ApcMin/+ model [3,5].
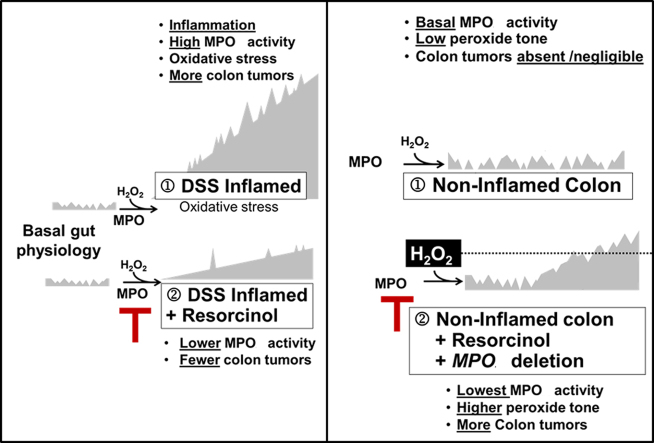
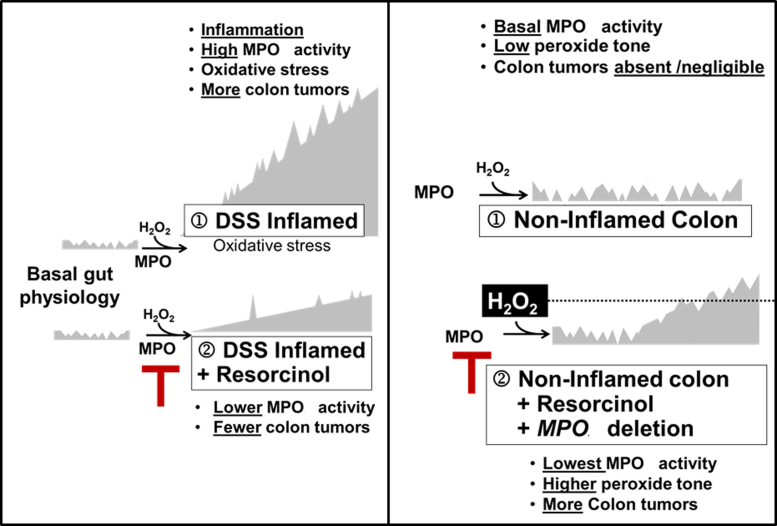
Colon tumors are absent or rare in non-inflamed colons of ApcMin/+ mice with physiologically basal MPO activity. Under these conditions, inhibition or genetic deletion of MPO coincided with a small but discernible rise in colon tumors. Thus, there is a dichotomy between MPO activity and colon tumor occurrence in ApcMin/+ mice. During DSS-induced colitis, high MPO activity favors colon tumor development; inhibition of MPO during DSS-colitis partly prevents colon tumors. However, under basal conditions – without DSS-colitis – inhibition or deletion of MPO activity favors colon tumor occurrence, suggesting that ApcMin/+ mice may rely on basal MPO activity for mucosal host defense against tumors [36]. The occurrence of colon tumors that coincided with inhibition of basal MPO activity may relate to the disposition of H2O2, its substrate. Ordinarily, consumption of H2O2 by MPO may help restrain peroxide ‘tone’ below the level needed for optimal COX activity [37]. Accordingly, inhibition or deletion of MPO activity in non-inflamed bowels may allow H2O2 to accumulate and reach levels supporting COX-mediated or H2O2-mediated colon tumor progression [38]. Fig. 5 depicts a speculative model to explain the apparent dichotomy between tumor occurrence and inhibition of MPO activity in inflamed versus non-inflamed colons. A similar dichotomy exists in humans. On one hand, MPO protein levels correlate directly with colorectal cancer progression [21], and colon tumors have elevated MPO activity compared to non-inflamed colonic mucosa [39,40]. On the other hand, malignancies have been associated with human MPO deficiency and MPO deficient neutrophils fail to destroy malignant cells [41,42].
Fig. 5.
Legend. Hypothetical model of the relationship between MPO activity and colon tumor occurrence in inflamed colon (left) and non-inflamed colon (right).
Resorcinol causes irreversible, H2O2-dependent loss of peroxidase activity in various heme-containing peroxidases [43], including both MPO and EPO. Strictly speaking, we cannot exclude a contribution from other granulocytes, such as eosinophils, in DSS-induced colitis in ApcMin+ mice. A role for eosinophil peroxidase (EPO) in DSS- induced colitis has been established using EPO ‘knockout' mice (EPO−/−) [23]. Environmental and genetic variation can modify mouse models of disease [44–47], consequently results with (EPO−/−) mice on a 129/Ola/Hsd×129/SvJ background, do not extrapolate seamlessly to ApcMin/+ mice on a C57Bl/6J background. Generally, the involvement of neutrophils in DSS colitis in C57/Bl6J mice including ApcMin/+ mice is well established [3–7]. It should be stressed that the investigators studying the role of eosinophils in DSS colitis were, themselves, unable to exclude a contribution from neutrophils [23].
Consistent with gut's dual role as a digestive organ and a lymphoid organ, diet and inflammation can modify colon cancer risk in complex ways. To interpret these complexities Bruce et al. [34] have proposed an intriguing model that integrates two separate mechanisms: (1) disruption of cellular energetic signaling processes, leading to growth and proliferation; (2) focal loss of epithelial barriers, inflammation and oxidative stress, leading to proliferation and mutation. Our results lend support and refinement to this model. In evaluating cancer risk Swenberg et al. [48] and Wild [49] have championed a comprehensive approach which considers all exposure events – both exogenous and endogenous. This includes electrophilic molecules that are generated in living cells and organs through normal physiology, lifestyle, and nutrition. Our results suggest that MPO is an aspect of the ‘exposome’ [49] that deserves attention in the etiology and control of colorectal cancer [50–52].
Animal experiments
Approved and performed under the University of Utah IACUC Protocol number 09-02001.
Acknowledgments
Kansas City University of Medicine provided funding to FAF for this investigation. This work was also supported by the NCI P30 CA42014.
Footnotes
Financial support: NCI P30 CA42014 and the Kansas City University of Medicine and Biosciences.
Contributor Information
Mazin Al-Salihi, Email: mazin@fulbrightmail.org.
F.A. Fitzpatrick, Email: ffitzpatrick@kcumb.edu.
References
- 1.Rutter M. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Lashner B.A. Colorectal cancer in ulcerative colitis patients: survival curves and surveillance. Clevel. Clin. J. Med. 1994;61:272–275. doi: 10.3949/ccjm.61.4.272. [DOI] [PubMed] [Google Scholar]
- 3.Cooper H. The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium-induced colitis. Gastroenterology. 2001;121:1407–1416. doi: 10.1053/gast.2001.29609. [DOI] [PubMed] [Google Scholar]
- 4.Huang E. Induction of inflammatory bowel disease accelerates adenoma formation in Min+/− mice. Surgery. 2006;139:782–788. doi: 10.1016/j.surg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc(Min/+) mice: inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int. J. Cancer. 2006;118:25–34. doi: 10.1002/ijc.21282. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki R. Dose-dependent promoting effect of dextran sodium sulfate on mouse colon carcinogenesis initiated with azoxymethane. Histol. Histopathol. 2005;20:483–492. doi: 10.14670/HH-20.483. [DOI] [PubMed] [Google Scholar]
- 7.Gerling M. Characterization of chromosomal instability in murine colitis-associated colorectal cancer. PLoS One. 2011;6:e22114. doi: 10.1371/journal.pone.0022114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson I. A comparison of the genetic pathways involved in the pathogenesis of three types of colorectal cancer. J. Pathol. 1998;184:148–152. doi: 10.1002/(SICI)1096-9896(199802)184:2<148::AID-PATH986>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 9.Hussain S. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 10.Ishikawa T., Herschman H.R. Tumor formation in a mouse model of colitis-associated colon cancer does not require COX-1 or COX-2 expression. Carcinogenesis. 2010;31:729–736. doi: 10.1093/carcin/bgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi S.H. Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis. J. Clin. Invest. 2014;124:2472–2482. doi: 10.1172/JCI69672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawk E.T. Chemoprevention in ulcerative colitis: narrowing the gap between clinical practice and research. Ann. Intern. Med. 2001;134:158–160. doi: 10.7326/0003-4819-134-2-200101160-00017. [DOI] [PubMed] [Google Scholar]
- 13.Thun M.J. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 14.Kefalakes H. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: myth or reality? Eur. J. Clin. Pharmacol. 2009;65:963–970. doi: 10.1007/s00228-009-0719-3. [DOI] [PubMed] [Google Scholar]
- 15.Hegazi R.A. Celecoxib and rofecoxib potentiate chronic colitis and premalignant changes in interleukin 10 knockout mice. Inflamm. Bowel Dis. 2001;9:230–236. doi: 10.1097/00054725-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Okayama M. Aggravation by selective COX-1 and COX-2 inhibitors of dextran sulfate sodium (DSS)-induced colon lesions in rats. Dig. Dis. Sci. 2007;52:2095–2103. doi: 10.1007/s10620-006-9597-z. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T. Effects of nimesulide, a cyclooxygenase-2 selective inhibitor, on colitis induced tumors. Inflammopharmacology. 2008;16:36–39. doi: 10.1007/s10787-006-1543-3. [DOI] [PubMed] [Google Scholar]
- 18.Terdiman J.P. The prevention of colitis-related cancer by 5-aminosalicylates: an appealing hypothesis that remains unproven. Am. J. Gastroenterol. 2011;106:737–740. doi: 10.1038/ajg.2011.56. [DOI] [PubMed] [Google Scholar]
- 19.Daiyasu H. Molecular evolution of the myeloperoxidase family. J. Mol. Evol. 2000;51:433–445. doi: 10.1007/s002390010106. [DOI] [PubMed] [Google Scholar]
- 20.Vowinkel T. Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Dig. Dis. Sci. 2004;49:556–564. doi: 10.1023/b:ddas.0000026298.72088.f7. [DOI] [PubMed] [Google Scholar]
- 21.Roncucci L. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2008;17:2291–2297. doi: 10.1158/1055-9965.EPI-08-0224. [DOI] [PubMed] [Google Scholar]
- 22.Anderson M.M. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha, beta-unsaturated aldehydes by phagocytes at sites of inflammation. J. Clin. Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes E. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J. Immunol. 2004;172:5664–5675. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 24.Covey T.M. Alkylation of the tumor suppressor PTEN activates Akt and β-catenin signaling: a mechanism linking inflammation and oxidative stress with cancer. PLoS One. 2010;5:e13545. doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covey T.M. Akt activation by arachidonic acid metabolism occurs via oxidation and inactivation of PTEN tumor suppressor. Oncogene. 2007;26:5784–5792. doi: 10.1038/sj.onc.1210391. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead R.H. Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology. 1999;117:858–865. doi: 10.1016/s0016-5085(99)70344-6. [DOI] [PubMed] [Google Scholar]
- 27.Furuhata A. Thiolation of protein-bound carcinogenic aldehyde. An electrophilic acrolein-lysine adduct that covalently binds to thiols. J. Biol. Chem. 2002;277:27919–27926. doi: 10.1074/jbc.M202794200. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y. Multistep carcinogenesis of the colon in the Apc Min/+ mouse. Cancer Sci. 2007;98:6–10. doi: 10.1111/j.1349-7006.2006.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clapper M.L. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol. Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 30.VanderVeen Evaluation of the mutagenic potential of the principal DNA adduct of acrolein. J. Biol. Chem. 2001;276:9066–9070. doi: 10.1074/jbc.M008900200. [DOI] [PubMed] [Google Scholar]
- 31.Stevens J.F. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarkovic K. Tissue distribution of lipid peroxidation product acrolein in human colon carcinogenesis. Free Radic. Res. 2006;40:543–552. doi: 10.1080/10715760500370048. [DOI] [PubMed] [Google Scholar]
- 33.Yu C.X. Redox regulation of PTEN by S-nitrosothiols. Mol. Pharmacol. 2005;68:847–854. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]
- 34.Bruce W.R. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol. Biomark. Prev. 2000;9:1271–1279. [PubMed] [Google Scholar]
- 35.Marnett L.J. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 36.Revaz V. The importance of mucosal immunity in defense against epithelial cancers. Curr. Opin. Immunol. 2005;17:175–179. doi: 10.1016/j.coi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Smith W.L. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Micromolar concentrations of hydrogen peroxide induce oxidative DNA lesions more efficiently than millimolar concentrations in mammalian cells. Nucl. Acids Res. 2003;31:1790–1795. doi: 10.1093/nar/gkg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haklar G. Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett. 2001;165:219–224. doi: 10.1016/s0304-3835(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 40.Rainis T. Enhanced oxidative stress and leukocyte activation in neoplastic tissues of the colon. Dig. Dis. Sci. 2007;52:526–530. doi: 10.1007/s10620-006-9177-2. [DOI] [PubMed] [Google Scholar]
- 41.Lanza F. Does a relationship exist between neutrophil myeloperoxidase deficiency and the occurrence of neoplasms? J. Clin. Lab. Immunol. 1987;22:175–180. [PubMed] [Google Scholar]
- 42.Lanza F. Clinical manifestation of myeloperoxidase deficiency. J. Mol. Med. 1998;76:676–681. doi: 10.1007/s001090050267. [DOI] [PubMed] [Google Scholar]
- 43.Divi R.L., Doerge. D.R. Mechanism-based inactivation of lactoperoxidase and thyroid peroxidase by resorcinol derivatives. Biochemistry. 1994;33:9668. doi: 10.1021/bi00198a036. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda K. Increase of oxidant-related triglycerides and phosphatidylcholines in serum and small intestinal mucosa during development of intestinal polyp formation in Min mice. Cancer Sci. 2011;102:79–87. doi: 10.1111/j.1349-7006.2010.01754.x. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki R. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- 46.Mangerich A. Chemistry meets biology in colitis-associated carcinogenesis. Free Radic. Res. 2013;47:958–986. doi: 10.3109/10715762.2013.832239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taioli E. Myeloperoxidase G-463A polymorphism and lung cancer: a HuGE Genetic Susceptibility to Environmental Carcinogens pooled analysis. Genet. Med. 2007;9:67–73. doi: 10.1097/gim.0b013e31803068b1. [DOI] [PubMed] [Google Scholar]
- 48.Swenberg J.A. Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology, and risk assessment. Toxicol. Sci. 2011;120(Suppl. 1):S130–S145. doi: 10.1093/toxsci/kfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wild C.P. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 50.Kim Y.S. Dietary modulation of colon cancer risk. J. Nutr. 2007;137:2576S–2579S. doi: 10.1093/jn/137.11.2576S. [DOI] [PubMed] [Google Scholar]
- 51.Seifried H.E. The antioxidant conundrum in cancer. Cancer Res. 2003;63:4295–4298. [PubMed] [Google Scholar]
- 52.Kristal A.R. Nutritional prevention of cancer: new directions for an increasingly complex challenge. J. Natl. Cancer Inst. 2009;101:363–365. doi: 10.1093/jnci/djp029. [DOI] [PubMed] [Google Scholar]