Abstract
Child maltreatment increases the risk for psychiatric disorders throughout childhood and into adulthood. One negative outcome of child maltreatment can be a disorder of emotional functioning, reactive attachment disorder (RAD), where the child displays wary, watchful, and emotionally withdrawn behaviours. Despite its clinical importance, little is known about the potential neurobiological consequences of RAD. The aim of this study was to elucidate whether RAD was associated with alterations in grey matter volume (GMV). High-resolution magnetic resonance imaging datasets were obtained for children and adolescents with RAD (n = 21; mean age = 12.76 years) and typically developing (TD) control subjects (n = 22; mean age = 12.95 years). Using a whole-brain voxel-based morphometry approach, structural images were analysed controlling for age, gender, full scale intelligence quotient, and total brain volume. The GMV was significantly reduced by 20.6% in the left primary visual cortex (Brodmann area 17) of the RAD group compared to the TD group (p = .038, family-wise error-corrected cluster level). This GMV reduction was related to an internalising problem measure of the Strength and Difficulties Questionnaire. The visual cortex has been viewed as part of the neurocircuit regulating the stress response to emotional visual images. Combined with previous studies of adults with childhood maltreatment, early adverse experience (e.g. sensory deprivation) may affect the development of the primary visual system, reflecting in the size of the visual cortex in children and adolescents with RAD. These visual cortex GMV abnormalities may also be associated with the visual emotion regulation impairments of RAD, leading to an increased risk for later psychopathology.
Keywords: Child maltreatment, Reactive attachment disorder, Voxel-based morphometry, Grey matter volume, Visual cortex, Emotional regulation impairments
Highlights
-
•
We examined grey matter alterations in reactive attachment disorder (RAD).
-
•
Grey matter volume (GMV) was analysed using whole-brain voxel-based morphometry.
-
•
Reduced visual cortex GMV was shown in RAD children compared to control children.
-
•
Visual cortex GMV of RAD children was related to their internalising problems.
-
•
The structural alterations in RAD may be due to emotion regulation impairments.
1. Introduction
Child maltreatment increases the risk for psychiatric disorders throughout childhood and into adulthood [1,2]. Maltreatment encompasses a spectrum of abusive actions (sexual, physical, or emotional abuse) or lack of actions (physical or emotional neglect) by the parent or other caregivers. A psychiatric disorder associated with early life abuse and neglect is reactive attachment disorder (RAD), where the child displays wary, watchful, and emotionally withdrawn behaviours, according to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5 [3]). Because of emotional dampening, RAD closely resembles internalising disorders with depressive and anxiety symptoms. In populations of maltreated children in foster care, 19.4–40.0% had signs of RAD based on the DSM-IV criteria [4,5], in which RAD (the inhibited type of RAD) and disinhibited social engagement disorder (DSED; the disinhibited type of RAD) were not completely independent. Even in a general population, the prevalence of RAD based on the DSM-IV criteria has been reported in 1.4% of children and children with RAD are more likely to have multiple comorbidities with other disorders, such as attention deficit hyperactivity disorder (ADHD; 52%), post-traumatic stress disorder (PTSD; 19%), and autism spectrum disorder (ASD; 14%) [6,7]. Despite its high prevalence and clinical importance, there have been very few investigations into the possible neurobiological consequences of RAD.
Of the childhood psychiatric disorders, RAD, which is a negative outcome of child maltreatment, remains one of the least understood phenotype. While most previous studies into RAD have been conducted among Romanian orphans and are limited to children younger than 6 years (for reviews [8,9]), a few studies have recently demonstrated that RAD symptoms are reliably identifiable in children older than 6 years [6,10–12]. Historically, RAD (or attachment disorders) was initially defined as a psychiatric disorder in the DSM-III [13] and associated with attachment insecurity as first proposed in Bowlby's attachment theory [14,15]. Now, it is doubted that RAD is closely associated with attachment insecurity in terms of classic attachment theory [10]. Although RAD and DSED were defined as two subtypes (inhibited and disinhibited types) of the same disorder in the DSM-IV criteria, they are now divided into two distinct disorders based on the DSM-5 criteria. According to the DSM-5, children with RAD exhibit wary, watchful, and emotionally withdrawn behaviours, whereas children with DSED display clingy and indiscriminately friendly behaviours even in their interactions with unfamiliar adults. It may also co-occur with ASD, intellectual developmental disorder, or depressive disorders. Although the presence of ASD is considered an exclusionary condition for diagnosing RAD in the DSM-5, the clinical differential diagnosis is complicated by clinical correlates of RAD with ‘quasi-autism’ [8]. To enhance further our understanding of RAD, it is of particular importance to investigate the nature of the neurobiological mechanisms underlying the behavioural problems of RAD.
Previous neuroimaging studies using structural magnetic resonance imaging (sMRI) techniques have revealed that exposure to early adversity is strongly associated with alterations in brain structure, such as in the grey matter (GM) and white matter (WM) (for reviews [16–19]). According to Teicher and Samson [19], many of the identified neuroanatomical abnormalities are interconnected, and are components of a neurocircuit regulating the stress response to emotional stimuli that includes the thalamus [20], visual or auditory sensory cortex [21–23], medial prefrontal cortex [24–27], hippocampus [28–30], and amygdala [29,31–33]. While the majority of sMRI studies predominantly focus on a priori hypothesised regions and thus provide a constrained characterisation of anatomy, studies into child maltreatment using an unbiased whole-brain analysis approach have been increasingly conducted and have reported similar results as shown in region-of-interest (ROI) studies [16,17]. As for other regions not commonly examined in ROI studies, structural abnormalities in the thalamus and sensory cortex have been revealed for the first time by using such an unbiased whole-brain analysis approach. This approach is helpful to understand the unknown neurobiological abnormalities in RAD associated with child maltreatment.
The aim of this study was to identify structural alterations in regional GM volume (GMV) in maltreated children with RAD using voxel-based morphometry (VBM) as an unbiased whole-brain analysis approach. We sought to assess further whether alterations in regional GMV correlated with psychiatric symptom measures. We hypothesised that RAD, as a negative outcome of child maltreatment, would be associated with alterations in the brain regions that are part of a neurocircuit regulating the stress response to emotional stimuli. Given the general pattern of findings in the related fields [19], we predicted that subjects with RAD, compared to controls without RAD, would be associated with reduced GMV in the thalamus, visual occipital cortex, and prefrontal cortex, and with increased GMV in the auditory temporal cortex. There would not be differences in GMV in the amygdala and hippocampus, especially in the child samples.
2. Material and methods
2.1. Ethics statement
The study protocol, approved by the Ethics Committees of the University of Fukui, Japan (Assurance no. FU23–43), was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies of the Ministry of Health, Labour and Welfare of Japan. All children and a parent or director of child welfare facilities gave written informed assent and consent for participation in this study. This study is registered with the University Hospital Medical Information Network (UMIN000014655).
2.2. Subjects
Twenty-one right-handed medication-naive 10- to 17-year-old Japanese children (mean age = 12.76 years) with a clinical diagnosis of RAD were recruited from the Department of Child and Adolescent Psychological Medicine at the University of Fukui Hospital from August 2013 to December 2014. The diagnosis of RAD was assessed by licensed child and adolescent psychiatrists (2nd, 6th, and 8th authors) according to the DSM-5 criteria [3]. To exclude other psychiatric conditions (e.g. PTSD, anxiety disorder, and depression), subjects were administered the Mini-International Neuropsychiatric Interview for Children and Adolescents (MINI-KID [34]) and an assessment module of DSM-IV ADHD taken from the Schedule of Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic version (K-SADS-E [35]) by two licensed paediatric-psychological clinicians (2nd and 3rd authors). All of the children had experienced physical, emotional abuse, and/or neglect early in life prior to coming into care. The children were living within a stable placement (in a child welfare facility) even though they were not living with biological parents (for information about child welfare services in Japan [36]). The control subjects, which included 22 typically developing (TD) Japanese children (mean age = 12.95 years) with no history of maltreatment, were recruited from local schools, matched on age, gender, and handedness. All children had normal or corrected vision and normal hearing. There was no difference in the proportion of subjects with corrected vision (for myopia) between the TD (23%) and RAD (24%) groups.
Children were excluded if they had a full scale intelligence quotient (FSIQ) <70 on the Wechsler Intelligence Scale for Children (WISC [37,38]) or the Wechsler Adult Intelligence Scale [39], and left-handedness according to the Edinburgh Handedness Inventory [40]. They were also excluded if they had any history of substance abuse, recent substance use, head trauma with loss of consciousness, significant foetal exposure to alcohol or drugs, perinatal or neonatal complications, neurological disorders, or medical conditions that might adversely affect growth and development.
2.3. Psychiatric symptom measures
The Depression Self-Rating Scale for Children (DSRSC; [41]), an 18-item self-report measure, was used to measure depressive symptoms. The Trauma Symptom Checklist for Children (TSCC; [42]), a 54-item self-report measure, was used to evaluate post-traumatic symptoms and other relevant symptoms found in some traumatised children (anger, anxiety, depression, post-trauma stress, dissociation, and sexual concerns). Parents or caregivers in the facilities also completed the Strength and Difficulties Questionnaire (SDQ; [43,44]), a 25-item questionnaire, to assess children's internalising and externalising behaviour problems, as well as prosocial behaviour tendencies. The SDQ internalising behaviour problems are found to be associated with a measure of anxiety symptoms in a sample of children with anxiety disorder [45], which resembles RAD due to emotional regulation impairments. A positive association between the SDQ and Relationship Problems Questionnaire (RPQ) scores has been previously reported [12,46]. The ADHD Rating Scale (ADHD-RS; [47]), an 18-item questionnaire, was used to evaluate inattentive and hyperactive/impulsive symptoms. Symptoms of ADHD have been consistently associated with DSED, but not RAD [8,48]. The Autism Spectrum Quotient (AQ [49]), a 50-item questionnaire, was completed by a parent or caregiver to evaluate ASD traits, such as social skills, attention switching, attention to detail, communication, and imagination. The presence of ASD is an exclusionary condition for the diagnosis of RAD [3].
2.4. MRI acquisition
All subjects were scanned on a 3-Tesla MR scanner (Discovery MR 750; General Electric Medical Systems, Milwaukee, WI, USA) with a 32-channel head coil. High-resolution structural whole-brain images were acquired by a 3D T1-weighted fast spoiled gradient recalled imaging sequence (repetition time (TR) =6.38 ms; echo time (TE) =1.99 ms; flip angle (FA) =11°; field of view (FOV) =256 mm; 256 × 256 matrix; 172 slices; voxel dimension = 1.0 × 1.0 × 1.0 mm).
2.5. Voxel-based morphometry
The VBM was performed using Statistical Parametric Mapping (SPM) version 12 software (Wellcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB R2014a (MathWorks, Natick, MA, USA). As a fully automated whole-brain morphometric technique, VBM detects regional structural differences between groups on a voxel-by-voxel basis [50]. The T1-weighted images were preprocessed using the VBM approach with modulation [51], where the images were first segmented into GM, WM, cerebrospinal fluid, and skull/scalp compartments. The Diffeomorphic Anatomical Registration through an Exponentiated Lie algebra (DARTEL) algorithm was applied to the segmented brain tissues to generate a study-specific template and to achieve an accurate inter-subject registration with improved realignment of smaller inner structures. The segmented GM images were spatially normalised into Montréal Neurological Institute (MNI) space and were written out with an isotropic voxel resolution of 1.5 mm3. Any volume change induced by normalisation was adjusted via a modulation algorithm. The normalised modulated GM images were smoothed by a Gaussian kernel of 8 mm full width at half maximum (FWHM).
2.6. Statistical analysis
Regional differences in GM volume (GMV) between groups were analysed in SPM 12 using two-sample t-test models. Potential confounding effects of age, gender, FSIQ, and total brain volume (calculated as the sum of GMV and WM volume (WMV)) were modelled, and variances attributable to them were excluded from the analyses. A GM majority optimal threshold mask, based on a study-specific sample, was applied to the analyses to eliminate voxels of non-GM for the GMV-analyses [52]. The resulting set of voxel values used for comparison generated a statistical parametric map of t-statistic, SPM{t}, that was transformed to a unit normal distribution (SPM{Z}). The statistical threshold was set at p < 0.05 with correction for multiple comparisons at cluster level (height threshold of Z > 3.09) because of the increased sensitivity of clusters to detect spatially extended signal changes [53]. Potential problems relating to non-isotropic smoothness, which can invalidate cluster level comparisons, were corrected by adjusting cluster size from the resel per voxel image [53]. The anatomical localisation of significant clusters was investigated with the Automated Anatomical Labeling (AAL) and Brodmann area (BA) atlases implemented in the MRIcron software package [54].
Significant clinical differences between the TD and RAD groups complicated the exploration of post hoc functional correlates between the VBM-identified cluster in the statistical models (controlling for age, gender, FSIQ, and total brain volume) and psychiatric symptom measures. Hence, multiple regression analyses were carried out to further inspect which psychiatric symptom measures could significantly predict GMV in the identified cluster. To this end, the eigenvariates, representing linearly transformed estimates of GMV, were extracted from the identified cluster in the statistical models. In the multiple regression analyses, several psychiatric symptom measures showing significant between-group differences were treated as independent variables, and the GMV estimates in the relevant cluster for the entire sample and each group were treated as dependent variables. The multiple regression analyses were performed using the Statistical Package for the Social Sciences 22 software (SPSS, Chicago, IL, USA). Bonferroni adjustment for multiple regressions was applied when multiple regressions were made on the measures.
3. Results
3.1. Demographic and questionnaire data
The results are listed in Table 1. The two groups were matched in age, gender, and handedness. For between-group comparison of FSIQ and psychiatric symptom measures, we used analysis of covariance (ANCOVA) with age and gender as covariates. In comparison with the TD group, the RAD group showed lower FSIQ (F[1, 39] = 8.78, p < .01) and higher levels of psychiatric symptom measures: depressive symptoms (DSRSC; F[1, 38] = 9.86, p < .01); post-traumatic symptoms, and other relevant symptoms (TSCC), such as anxiety (F[1, 39] = 6.14, p < .05); depression (F[1, 39] = 8.80, p < .01); anger (F[1, 39] = 8.93, p < .01); post-trauma stress (F[1, 39] = 10.53, p < .01); and dissociation (F[1, 39] = 11.34, p < .01). As for the parent- or caregiver-rating questionnaire for psychiatric symptoms, similar differences (TD < RAD) were found in measures of SDQ internalising (F[1, 39] = 15.72, p < .001) and externalising problems (F[1, 39] = 12.13, p < .01), ADHD symptoms (ADHD-RS total, F[1, 38] = 10.86, p < .01; inattentive, F[1, 38] = 12.64, p < .01; hyperactive/impulsive, F[1, 38] = 4.83, p < .05), and ASD traits (AQ total, F[1, 37] = 7.28, p < .05; attention switching, F[1, 37] = 15.61, p < .001; communication, F[1, 37] = 9.98, p < .01). No subject met the criteria for bipolar disorder, ADHD, or ASD as all of the scores were under the threshold for the clinical criteria.
Table 1.
Demographic and clinical characteristics of TD and RAD groups.
| Measures |
Group |
Statistics |
||||
|---|---|---|---|---|---|---|
| TD | RAD | F-value | p-Value | |||
| Subjects (n) | 22 | 21 | ||||
| Age (years) | 12.95 | (2.15) | 12.76 | (1.89) | 0.10 | .76 |
| Gender (%, female/male) | 55/45 | 62/38 | (Fisher) | .76 | ||
| Handedness (%, left/right) | 0/100 | 0/100 | (Fisher) | − | ||
| Wechsler Intelligence Scale for Children | ||||||
| Full scale IQa | 101.14 | (8.39) | 92.14 | (10.92) | 8.78 | <.01 |
| Depression self-rating scale for childrenb | 8.00 | (4.45) | 14.71 | (8.44) | 9.86 | <.01 |
| Trauma symptom checklist for children | ||||||
| Anxiety | 39.27 | (4.82) | 46.95 | (14.21) | 6.14 | <.05 |
| Depression | 37.77 | (3.02) | 45.00 | (11.72) | 8.80 | <.01 |
| Anger | 36.95 | (2.42) | 43.10 | (8.95) | 8.93 | <.01 |
| Post-trauma stress | 37.50 | (3.33) | 45.76 | (11.60) | 10.53 | <.01 |
| Dissociation | 37.68 | (2.75) | 46.29 | (11.94) | 11.34 | <.01 |
| Sexual concerns | 38.91 | (2.47) | 40.71 | (7.97) | 1.32 | .26 |
| Strengths and difficulties questionnaire | ||||||
| Internalising problems | 2.00 | (1.72) | 5.24 | (3.33) | 15.72 | <.001 |
| Externalising problems | 4.05 | (2.06) | 7.00 | (3.67) | 12.13 | <.01 |
| Prosocial | 6.05 | (2.52) | 5.38 | (2.33) | 1.08 | .30 |
| ADHD rating scalec | ||||||
| Total | 2.23 | (2.29) | 9.45 | (9.66) | 10.86 | <.01 |
| Inattentive | 1.77 | (1.72) | 6.95 | (6.42) | 12.64 | <.01 |
| Hyperactive/impulsive | 0.45 | (0.96) | 2.50 | (4.02) | 4.83 | <.05 |
| Autism spectrum quotientd | ||||||
| Total | 12.32 | (4.77) | 16.95 | (7.05) | 7.28 | <.05 |
| Social skills | 3.50 | (2.48) | 3.47 | (2.63) | <.01 | .97 |
| Attention switching | 2.05 | (1.05) | 3.89 | (1.79) | 15.61 | <.001 |
| Attention to detail | 3.41 | (1.92) | 3.37 | (2.17) | <.01 | .99 |
| Communication | 1.23 | (1.45) | 3.00 | (2.62) | 9.98 | <.01 |
| Imagination | 2.14 | (1.46) | 3.21 | (1.91) | 3.84 | .06 |
| Maltreatment type (n) | ||||||
| Emotional abuse | − | 11 | ||||
| Neglect | − | 15 | ||||
| Physical abuse | − | 7 | ||||
| Sexual abuse | − | 2 | ||||
Numbers in parentheses represent standard deviations. Group differences were tested using ANCOVA with age and gender as covariates. aFull scale IQ data were based on WISC (n = 42) and WAIS (n = 1). Some subjects' data were not available due to missing values in specific questionnaires: bDepression self-rating scale (TD, 1 subject), cADHD rating scale (RAD, 1 subject), and dautism spectrum quotient (RAD, 2 subjects).
3.2. Structural MRI data
We estimated absolute global volumes, that is, GMV (789.06 ± 73.19 ml and 749.88 ± 58.97 ml), WMV (396.21 ± 48.93 ml and 378.13 ± 31.43 ml), and total brain volume (1185.28 ± 113.40 ml and 1128.01 ± 85.29 ml) in the TD and RAD groups, respectively. No differences between the two groups were found for each global volume.
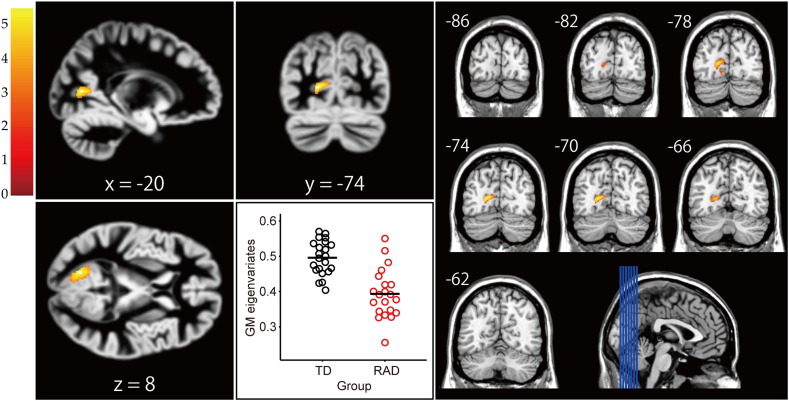
A whole-brain analysis with FWE correction at cluster level was conducted to examine regional differences in GMV between the two groups. The RAD group, in comparison with the TD group, showed reduced GMV in the left primary visual cortex (BA17; MNI coordinates, x = −20, y = −74, z = 8; cluster size = 644 voxels, p = .038, FWE-corrected cluster level; Fig. 1). A 20.6% average reduction of GMV was found in the identified regions of the RAD group compared with the TD group. To confirm the trend of the occipital GMV reduction further, we used a lower criterion for statistical significance (marginal significance level). The left inferior occipito-temporal cortex (i.e. fusiform gyrus; BA19/37; MNI coordinates, x = −30, y = −47, z = −11; cluster size = 310 voxels) exhibited a marginally significant decrease in GMV in the RAD group relative to the TD group (p = .072, FWE-corrected cluster-level). Overall, reduced GMV in the RAD group was shown in the left side of the occipital (visual) cortex extending anteriorly toward the inferior occipito-temporal cortex.
Fig. 1.
Structural differences in regional GMV between the TD and RAD groups. The RAD group showed significantly reduced GMV in the left primary visual cortex (BA 17) compared to the TD group (p = .038, FWE-corrected cluster level). Colour scales represent t-values.
We then performed multiple regression analyses to further characterise the relationship between psychiatric symptom measures and GMV in the left visual cortex identified by the whole-brain analysis. The GM eigenvariates (i.e. linearly transformed estimates of GMV) from the identified cluster were extracted and used for regression analyses. As indicated in Table 2, the analysis showed that the psychiatric symptom measures for SDQ internalising problems (β = −0.96, t = −3.86, p < .05) were a significant predictor for the left visual cortex GMV estimates in the RAD group, which explained approximately 55% of the variance of the GMV (adjusted R2 = .55, F[10, 8] = 3.16, p < .10). Except for the SDQ internalising problems, there were no significant predictors for the visual cortex GMV estimates. Such a relationship between the measures and the GMV estimates was not seen in the entire sample or the TD group.
Table 2.
Multiple regression analysis of the left visual cortex GMV predictors of psychiatric symptom measures.
| Predictors |
All subjects |
TD |
RAD |
|||
|---|---|---|---|---|---|---|
| Beta | t-Value | Beta | t-Value | Beta | t-Value | |
| Depression self-rating scale for children | −0.23 | −1.51 | 0.12 | 0.41 | −0.32 | −1.73 |
| Trauma symptom checklist for children | ||||||
| Anxiety | −0.14 | −0.49 | −0.81 | −1.02 | −0.91 | −2.55 |
| Depression | 0.29 | 0.91 | 1.36 | 2.00 | −0.10 | −0.25 |
| Anger | −0.33 | −1.10 | 0.26 | 0.79 | −0.40 | −1.04 |
| Post-trauma stress | 0.03 | 0.07 | 0.39 | 1.05 | 1.32 | 2.05 |
| Dissociation | −0.07 | −0.22 | −0.73 | −1.31 | −0.17 | −0.50 |
| Strengths and difficulties questionnaire | ||||||
| Internalising problems | −0.57 | −2.85† | 0.84 | 1.74 | −0.96 | −3.86* |
| Externalising problems | 0.10 | 0.51 | −0.68 | −1.38 | 0.41 | 1.87 |
| ADHD rating scale | ||||||
| Total | −0.15 | −0.93 | −0.58 | −2.29 | 0.07 | 0.36 |
| Autism spectrum quotient | ||||||
| Total | 0.15 | 0.89 | −0.02 | −0.10 | 0.40 | 1.79 |
| Adjusted R2 | 0.32* | 0.15 | 0.55† | |||
The statistical threshold is set at corrected *p < .05. †p < .10 with the Bonferroni adjustment for multiple regressions. For the analysis, three subjects' data were not available due to missing values in specific questionnaires (TD, 1 subject; RAD, 2 subjects).
Furthermore, additional correlation analyses were conducted to examine whether the left visual cortex GMV of the RAD group was associated with the WISC factor index scores indicating children's cognitive abilities, such as verbal comprehension, perceptual reasoning, working memory, and processing speed. There was no significant association of the GMV estimates with each WISC factor index score (all ps > .46). Alterations in the visual cortex GMV of the RAD group were not associated with cognitive ability measures.
4. Discussion
The present study provides the first evidence demonstrating that children and adolescents with RAD exhibit structural abnormalities in the left primary visual cortex (BA17). A smaller visual cortex GMV was observed in the RAD group compared to the TD group. Further analysis exhibited a negative correlation of the visual cortex GMV of the RAD group with SDQ internalising problems, but not with other psychiatric symptom measures (e.g. SDQ externalising problems) or cognitive ability measures (WISC factor index scores).
Our results are consistent with previous findings reported in adults with histories of childhood maltreatment [21,22,55]. Although many studies into the effects of early adversity adopted a constrained ROI approach and did not report results for the visual cortex, some studies using an unbiased whole-brain approach have found a smaller visual cortex GMV in young adults with episodes of childhood sexual abuse [21] or witnessing domestic violence [22]. In an earlier study [55], reduced occipital GMV was also associated with a priori history of child abuse but not with victims of intimate partner violence. Reportedly, the visual cortices that process and convey the adverse sensory input of childhood maltreatment, such as sexual abuse and witnessing domestic violence, appear to be specifically modified by this experience [21,22]. These findings fit with preclinical studies showing that the visual cortex is a highly plastic structure [56]. Hence, early adverse experiences (e.g. sensory deprivation) may affect the development of the primary visual system, reflecting in the size of the visual cortex in children and adolescents with RAD. These structural abnormalities may also be associated with the behavioural problems of RAD.
The visual cortex has been viewed as part of a neurocircuit that regulates the stress response to emotional visual images [19]. Through the inferior longitudinal fasciculus (ILF), the primary visual cortex and limbic system (e.g. amygdala, hippocampus) are anatomically connected [57,58]. The ILF, (i.e. visual-limbic pathway) subserves emotional functions specific to the visual modality. Based on this anatomical connection (i.e. the ILF), the primary visual cortex conveys emotional signals (e.g. fearful sights) to the amygdala and receives feedback signals from the amygdala [59,60]. In functional MRI studies, a connectivity analysis has revealed that the effect of the amygdala on behaviour to emotional visual stimuli was mediated through backward projections from the amygdala to the visual cortex [61,62]. Furthermore, this visual-limbic pathway seems to be negatively affected by exposure to early life stress. In a recent sMRI study using a diffusion tensor imaging (DTI) technique, a reduction in the WM tract integrity of the left ILF interconnecting the visual cortex to the limbic system was reported in a sample of young adults who witnessed interparental violence during childhood [63] and of children with exposure to maltreatment [64]. Alterations in the development of the left visual cortex connected via the ILF may play a critical role in the occurrence of RAD where the child displays emotionally withdrawn behaviours.
That the GMV abnormalities may play a critical role in RAD symptoms is also supported by the present results of the multiple regression analysis that showed an association between the visual cortex GMV of the RAD subjects and their internalising problems (SDQ internalising) when incorporating other relevant factors (e.g. SDQ externalising) into the multiple regression model. As addressed in the Introduction section, RAD is considered in the DSM-5 to resemble internalising disorders with depressive and anxiety symptoms. Because of emotional dampening in RAD, it is also reasonable to consider a potential convergence between RAD symptoms and internalising problems [8]. While the SDQ internalising problems are likely to reflect inhibited RAD symptoms, they seem to be associated with other questionnaire scores for RAD symptoms. Subscales of the SDQ internalising measure (emotional and peer problems) have been associated with the inhibited behaviour subscale of the RPQ as a useful but limited instrument for screening inhibited and disinhibited RAD symptoms [12].
Why the left-sided hemisphere of the visual cortex was affected is an interesting question. Given that RAD patients show reduced or absent expression of positive emotions during routine interactions with caregivers [3,8], we propose that the reduction of GMV in the left visual cortex identified here may be associated with a malfunction in the neurocircuit regulating positive emotional visual images (e.g. a smiling face). In line with our proposal, it has been reported that children exposed to child adversity have difficulty recognising positive emotional expressions [65]. Indeed, the processing of positive emotion has been proposed to be associated with the left hemisphere, although hemispheric lateralisation in emotion processing remains controversial between the valence hypothesis and the right hemisphere hypothesis (e.g. [66]). According to the valence hypothesis, which is the currently leading hypothesis in emotion processing [67–69], the left hemisphere is specialised for positive emotions and the right is dominant for negative emotions. More recently, behavioural studies using a divided visual field paradigm have found support for this hypothesis, showing that positive emotions were better recognised when presented in the right visual field and negative emotions were better identified when presented in the left visual field (e.g. [70]). A neurophysiological study using event-related potentials has also found that processing of the mouths of happy faces (i.e. analytical or part-based processing of positive emotional expressions) enhanced left-sided occipito-temporal activity during the early visual processing phase [71]. Combined with a previous DTI study showing that the left-sided ILF WM abnormalities were associated with early adversity [63], we suggest that the left-sided visual cortex GM abnormalities may lead to a difficulty with regulating positive emotions in RAD.
Only one brain region, the left occipital visual cortex, was affected by the symptoms of RAD in the present sMRI study. However, this does not mean that other regions of the neurocircuit that regulates emotion were not associated with child maltreatment and other psychiatric disorders. The related prefrontal and temporal cortices seem to be associated more with PTSD than RAD, which are both incorporated into the same category of trauma- and stressor-related disorders in the DSM-5. As suggested by a quantitative meta-analysis of GMV changes in PTSD [72], the prefrontal and temporal cortices were likely to be ‘disease-related’ and disconnected from ‘stress-related’ regions including the occipital cortex. Moreover, in child sMRI studies using unbiased whole-brain morphometric comparisons, alterations in the left thalamus have been involved in the association between child maltreatment and the occurrence of generalized anxiety disorder [20]. Although generalized anxiety disorder resembles RAD due to emotional regulation impairments [3], the neural vulnerability markers of these psychiatric disorders may differ regardless of the histories of child maltreatment. For the limbic system (hippocampus and amygdala), there appears to be differences in maltreatment effects on brain structure between studies of children and adults, with GMV alterations being previously observed in adults but not in children [19,25]. These findings suggest that maltreatment has differentiated effects on brain structure across the development and reorganisation processes. Another possibility may be tied to an association between specific maltreatment type and abnormalities in the limbic system. In a recent sMRI study, the GMV reduction in the limbic system was associated with children exposed to only a single type of maltreatment (physical abuse, neglect) but not to multiple complex types of maltreatment [29]. Furthermore, it should be noted that ADHD has been associated with altered occipital GMV [73]. As ADHD symptoms have not been associated with RAD, but have been with DSED [8,48], there seems to be partially overlapping structural abnormalities in these psychiatric disorders. In this study, however, no subject had a history of ADHD. Thus, the reduced occipital GMV of the RAD group identified here cannot be explained by specific cognitive dysfunctions based on ADHD, such as visual attentional dysfunction in ADHD related to the occipital visual cortex [74]. Although we have discussed this finding in terms of a potential cause and effect mechanism, it must be emphasised that our evidence only supports an association between emotional dysfunction and current symptoms of RAD.
Some limitations of the present study should be noted. First, the main limitation is the relatively small patient group. Although a sample size of at least twenty in each group is desirable for reliability in between-group comparisons [16], studies involving a larger number of subjects are essential to generalise our results. Second, whole-brain analysis approaches are limited by the need to adjust for multiple comparisons to minimise the risk of detecting chance-related differences. Consequently, only the most robust differences in brain structure tend to emerge. Third, the present study used a cross-sectional design that precludes the identification of causal links between RAD and the GMV differences. Longitudinal studies are needed to investigate how the structural differences associated with RAD change with intervention programs aimed at diminishing the signs of RAD. Other cross-sectional designs adopting different comparison groups of maltreated children without RAD would also be helpful to better understand the neurobiological consequences of RAD. Fourth, as seen in Table 1, there were substantial IQ differences in our study, with the RAD group showing a lower FSIQ compared to the TD group as previously reported in child maltreatment studies [75,76]. However, we used statistical methods to control for these confounds and adjusted for the FSIQ in the VBM analysis. Fifth, the subjects' pubertal stages were not assessed in this study. While chronological age has been associated with pubertal stage, recent evidence from studies of the developing brain has suggested that pubertal stage might play a more important role in adolescent brain development than chronological age (e.g. [77]). Future studies are needed to elucidate whether pubertal stage has a differentiated effect on brain development across the samples. Finally, there is a potential limitation in the diagnosis of RAD. As previously suggested [6,8,78], a child may be given only a ‘suspected’ diagnosis of RAD because the diagnosis is not absolutely clear. A more robust diagnosis needs to be based on ecological observation of the child's interaction activity in a local school or community, as well as on clinical interview and questionnaire data [6]. It is also suggested that the phenotype of RAD in the DSM-5 may be further encompassed within a broader phenotype [78]. Nevertheless, this study sheds light on the neural underpinning of emotional dysregulation as a core symptom of RAD.
5. Conclusions
We provide novel evidence that structural abnormalities in the left occipital visual cortex were associated with children and adolescents with RAD. Furthermore, the internalising problems of RAD correlated with the identified visual cortex GMV. Combined with previous studies of adults with childhood maltreatment, early adverse experience (e.g. sensory deprivation) may affect the development of the primary visual system, reflecting in the size of the visual cortex in children and adolescents with RAD. These visual cortex GMV abnormalities may also be associated with the visual emotion regulation impairments of RAD, leading to an increased risk for later psychopathology.
Acknowledgements
This work was supported, in part, by Grants-In-Aid for Scientific Research B # 24300149 (A. T.) and Challenging Exploratory research #25560386 (A. T.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This work was also partially supported by a Grant-in-Aid for Scientific Research #230201 (A. T.) from the Japan–U.S. Brain Research Cooperative Program. The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Edwards V.J., Holden G.W., Felitti V.J., Anda R.F. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am. J. Psychiatry. 2003;160(8):1453–1460. doi: 10.1176/appi.ajp.160.8.1453. 12900308 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert R., Widom C.S., Browne K., Fergusson D., Webb E., Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68–81. doi: 10.1016/S0140-6736(08)61706-7. 19056114 [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . fifth edition (DSM-v) American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 4.Lehmann S., Havik O.E., Havik T., Heiervang E.R. Mental disorders in foster children: a study of prevalence, comorbidity and risk factors. Child Adolesc. Psychiatry Ment. Health. 2013;7(1):39. doi: 10.1186/1753-2000-7-39. 24256809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeanah C.H., Scheeringa M., Boris N.W., Heller S.S., Smyke A.T., Trapani J. Reactive attachment disorder in maltreated toddlers. Child Abus. Negl. 2004;28(8):877–888. doi: 10.1016/j.chiabu.2004.01.010. 15350771 [DOI] [PubMed] [Google Scholar]
- 6.Minnis H., Macmillan S., Pritchett R., Young D., Wallace B., Butcher J., Sim F., Baynham K., Davidson C., Gillberg C. Prevalence of reactive attachment disorder in a deprived population. Br. J. Psychiatry. 2013;202(5):342–346. doi: 10.1192/bjp.bp.112.114074. 23580380 [DOI] [PubMed] [Google Scholar]
- 7.Pritchett R., Pritchett J., Marshall E., Davidson C., Minnis H. Reactive attachment disorder in the general population: a hidden ESSENCE disorder. Scientific. World J. 2013;2013:818157. doi: 10.1155/2013/818157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeanah C.H., Gleason M.M. Annual research review: attachment disorders in early childhood — clinical presentation, causes, correlates, and treatment. J. Child Psychol. Psychiatry. 2015;56(3):207–222. doi: 10.1111/jcpp.12347. 25359236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zilberstein K. Clarifying core characteristics of attachment disorders: a review of current research and theory. Am. J. Orthopsychiatr. 2006;76(1):55–64. doi: 10.1037/0002-9432.76.1.55. 16569127 [DOI] [PubMed] [Google Scholar]
- 10.Minnis H., Green J., O'Connor T.G., Liew A., Glaser D., Taylor E., Follan M., Young D., Barnes J., Gillberg C., Pelosi A., Arthur J., Burston A., Connolly B., Sadiq F.A. An exploratory study of the association between reactive attachment disorder and attachment narratives in early school-age children. J. Child Psychol. Psychiatry. 2009;50(8):931–942. doi: 10.1111/j.1469-7610.2009.02075.x. 19344386 [DOI] [PubMed] [Google Scholar]
- 11.Minnis H., Reekie J., Young D., O'Connor T.O.M., Ronald A., Gray A., Plomin R. Genetic, environmental and gender influences on attachment disorder behaviours. Br. J. Psychiatry. 2007;190:490–495. doi: 10.1192/bjp.bp.105.019745. 17541108 [DOI] [PubMed] [Google Scholar]
- 12.Vervoort E., De Schipper J.C., Bosmans G., Verschueren K. Screening symptoms of reactive attachment disorder: evidence for measurement invariance and convergent validity. Int. J. Methods Psychiatr. Res. 2013;22(3):256–265. doi: 10.1002/mpr.1395. 24022942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychiatric Association . third edition. American Psychiatric Association; Washington, DC: 1980. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 14.Bowlby J. Basic Books; New York: 1973. Attachment and Loss, Volume Two: Separation, Anxiety and Anger. [Google Scholar]
- 15.Bowlby J. The making and breaking of affectional bonds: I. Aetiology and psychopathology in the light of attachment theory. Br. J. Psychiatry. 1977;130:201–210. doi: 10.1192/bjp.130.3.201. 843768 [DOI] [PubMed] [Google Scholar]
- 16.Hart H., Rubia K. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. 22457645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim L., Radua J., Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am. J. Psychiatry. 2014;171(8):854–863. doi: 10.1176/appi.ajp.2014.13101427. 24781447 [DOI] [PubMed] [Google Scholar]
- 18.McCrory E., De Brito S.A., Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front. Psychiatry. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. 21847382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teicher M.H., Samson J.A. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry. 2013;170(10):1114–1133. doi: 10.1176/appi.ajp.2013.12070957. 23982148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao M., Yang F., Zhang Y., He Z., Song M., Jiang T., Li Z., Lu S., Wu W., Su L., Li L. Childhood maltreatment is associated with larger left thalamic gray matter volume in adolescents with generalized anxiety disorder. PLOS One. 2013;8(8):e71898. doi: 10.1371/journal.pone.0071898. 23951265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomoda A., Navalta C.P., Polcari A., Sadato N., Teicher M.H. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol. Psychiatry. 2009;66(7):642–648. doi: 10.1016/j.biopsych.2009.04.021. 19560122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomoda A., Polcari A., Anderson C.M., Teicher M.H. Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLOS One. 2012;7(12):e52528. doi: 10.1371/journal.pone.0052528. 23300699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomoda A., Sheu Y.-S., Rabi K., Suzuki H., Navalta C.P., Polcari A., Teicher M.H. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage. 2011;54(Suppl. 1):S280–S286. doi: 10.1016/j.neuroimage.2010.05.027. 20483374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrion V.G., Weems C.F., Watson C., Eliez S., Menon V., Reiss A.L. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 2009;172(3) doi: 10.1016/j.pscychresns.2008.07.008. 19349151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Brito S.A., Viding E., Sebastian C.L., Kelly P.A., Mechelli A., Maris H., McCrory E.J. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J. Child Psychol. Psychiatry. 2013;54(1):105–112. doi: 10.1111/j.1469-7610.2012.02597.x. 22880630 [DOI] [PubMed] [Google Scholar]
- 26.Tomoda A., Suzuki H., Rabi K., Sheu Y.-S., Polcari A., Teicher M.H. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 2009;47(Suppl. 2):T66–T71. doi: 10.1016/j.neuroimage.2009.03.005. 19285558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Harmelen A.-L., van Tol M.-J., van der Wee N.J.A., Veltman D.J., Aleman A., Spinhoven P., van Buchem M.A., Zitman F.G., Penninx B.W.J.H., Elzinga B.M. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol. Psychiatry. 2010;68(9):832–838. doi: 10.1016/j.biopsych.2010.06.011. 20692648 [DOI] [PubMed] [Google Scholar]
- 28.Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. 22112927 [DOI] [PubMed] [Google Scholar]
- 29.Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., Shirtcliff E.A., Pollak S.D., Davidson R.J. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. 24993057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U. S. A. 2012;109(9):E563–E572. doi: 10.1073/pnas.1115396109. 22331913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edmiston E.E., Wang F., Mazure C.M., Guiney J., Sinha R., Mayes L.C., Blumberg H.P. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch. Pediatr. Adolesc. Med. 2011;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. 22147775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta M.A., Golembo N.I., Nosarti C., Colvert E., Mota A., Williams S.C.R., Rutter M., Sonuga-Barke E.J.S. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees Study Pilot. J. Child Psychol. Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. 19457047 [DOI] [PubMed] [Google Scholar]
- 33.Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T., Millner A., Galvan A., Davidson M.C., Eigsti I.-M., Thomas K.M., Freed P.J., Booma E.S., Gunnar M.R., Altemus M., Aronson J., Casey B.J. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. 20121862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheehan D.V., Sheehan K.H., Shytle R.D., Janavs J., Bannon Y., Rogers J.E., Milo K.M., Stock S.L., Wilkinson B. Reliability and validity of the Mini International neuropsychiatric interview for children and adolescents (MINI-KID) J. Clin. Psychiatry. 2010;71(3):313–326. doi: 10.4088/JCP.09m05305whi. 20331933 [DOI] [PubMed] [Google Scholar]
- 35.Orvaschel H., Puig-Antich J. fifth edition. Nova Southeastern University; Fort Lauderdale, FL: 1994. Schedule for Affective Disorders and Schizophrenia for School-Age Children — Epidemiologic Version (K-SADS-E) [Google Scholar]
- 36.Suzuki H., Tomoda A. Roles of attachment and self-esteem: impact of early life stress on depressive symptoms among Japanese institutionalized children. B.M.C. Psychiatry. 2015;15:8. doi: 10.1186/s12888-015-0385-1. 25651759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wechsler D. third edition. Psychological Corporation; San Antonio, TX: 1991. Wechsler Intelligence Scale for Children. [Google Scholar]
- 38.Wechsler D. fourth edition. Psychological Corporation; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- 39.Wechsler D. third edition. Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- 40.Oldfield R.C. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- 41.Birleson P. The validity of depressive disorder in childhood and the development of a self-rating scale: a research report. J. Child Psychol. Psychiatry. 1981;22(1):73–88. doi: 10.1111/j.1469-7610.1981.tb00533.x. 7451588 [DOI] [PubMed] [Google Scholar]
- 42.Briere J. Psychological Assessment Resources; Odessa, FL: 1996. Trauma Symptom Checklist for Children (TSCC) [Google Scholar]
- 43.Goodman A., Lamping D.L., Ploubidis G.B. When to use broader internalising and externalising subscales instead of the hypothesised five subscales on the Strengths and Difficulties Questionnaire (SDQ): data from British parents, teachers and children. J. Abnorm. Child Psychol. 2010;38(8):1179–1191. doi: 10.1007/s10802-010-9434-x. 20623175 [DOI] [PubMed] [Google Scholar]
- 44.Goodman R. The strengths and difficulties questionnaire: a research note. J. Child Psychol. Psychiatry. 1997;38(5):581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. 9255702 [DOI] [PubMed] [Google Scholar]
- 45.Arendt K., Hougaard E., Thastum M. Psychometric properties of the child and parent versions of Spence children's Anxiety Scale in a Danish community and clinical sample. J. Anxiety Disord. 2014;28(8):947–956. doi: 10.1016/j.janxdis.2014.09.021. 25445085 [DOI] [PubMed] [Google Scholar]
- 46.Kočovská E., Puckering C., Follan M., Smillie M., Gorski C., Barnes J., Wilson P., Young D., Lidstone E., Pritchett R., Hockaday H., Minnis H. Neurodevelopmental problems in maltreated children referred with indiscriminate friendliness. Res. Dev. Disabil. 2012;33(5):1560–1565. doi: 10.1016/j.ridd.2012.02.016. 22522215 [DOI] [PubMed] [Google Scholar]
- 47.DuPaul G.J., Power T.J., Anastopoulos A., Reid D. ADHD. Guilford Press; New York: 1998. Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. [Google Scholar]
- 48.Gleason M.M., Fox N.A., Drury S., Smyke A., Egger H.L., Nelson C.A., III, Gregas M.C., Zeanah C.H. Validity of evidence-derived criteria for reactive attachment disorder: indiscriminately social/disinhibited and emotionally withdrawn/inhibited types. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(3):216–231.e3. doi: 10.1016/j.jaac.2010.12.012. 21334562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baron-Cohen S., Hoekstra R.A., Knickmeyer R., Wheelwright S. The autism-spectrum quotient (AQ) — adolescent version. J. Autism Dev. Disord. 2006;36(3):343–350. doi: 10.1007/s10803-006-0073-6. 16552625 [DOI] [PubMed] [Google Scholar]
- 50.Ashburner J., Friston K.J. Voxel-based morphometry: the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. 10860804 [DOI] [PubMed] [Google Scholar]
- 51.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. 17761438 [DOI] [PubMed] [Google Scholar]
- 52.Ridgway G.R., Omar R., Ourselin S., Hill D.L., Warren J.D., Fox N.C. Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage. 2009;44(1):99–111. doi: 10.1016/j.neuroimage.2008.08.045. 18848632 [DOI] [PubMed] [Google Scholar]
- 53.Hayasaka S., Phan K.L., Liberzon I., Worsley K.J., Nichols T.E. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22(2):676–687. doi: 10.1016/j.neuroimage.2004.01.041. 15193596 [DOI] [PubMed] [Google Scholar]
- 54.Rorden C., Karnath H.-O., Bonilha L. Improving lesion-symptom mapping. J. Cogn. Neurosci. 2007;19(7):1081–1088. doi: 10.1162/jocn.2007.19.7.1081. 17583985 [DOI] [PubMed] [Google Scholar]
- 55.Fennema-Notestine C., Stein M.B., Kennedy C.M., Archibald S.L., Jernigan T.L. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol. Psychiatry. 2002;52(11):1089–1101. doi: 10.1016/s0006-3223(02)01413-0. 12460692 [DOI] [PubMed] [Google Scholar]
- 56.Hubel D.H., Wiesel T.N. Early exploration of the visual cortex. Neuron. 1998;20(3):401–412. doi: 10.1016/s0896-6273(00)80984-8. 9539118 [DOI] [PubMed] [Google Scholar]
- 57.Amaral D.G., Behniea H., Kelly J.L. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118(4):1099–1120. doi: 10.1016/s0306-4522(02)01001-1. 12732254 [DOI] [PubMed] [Google Scholar]
- 58.Catani M., Jones D.K., Donato R., ffytche D.H. Occipito-temporal connections in the human brain. Brain. 2003;126(9):2093–2107. doi: 10.1093/brain/awg203. 12821517 [DOI] [PubMed] [Google Scholar]
- 59.Adolphs R. Emotional vision. Nat. Neurosci. 2004;7(11):1167–1168. doi: 10.1038/nn1104-1167. 15508009 [DOI] [PubMed] [Google Scholar]
- 60.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. (Regul. Ed.) 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. 16289871 [DOI] [PubMed] [Google Scholar]
- 61.Lim S.-L., Padmala S., Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc. Natl. Acad. Sci. U. S. A. 2009;106(39):16841–16846. doi: 10.1073/pnas.0904551106. 19805383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pessoa L., Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat. Rev. Neurosci. 2010;11(11):773–783. doi: 10.1038/nrn2920. 20959860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi J., Jeong B., Polcari A., Rohan M.L., Teicher M.H. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage. 2012;59(2):1071–1079. doi: 10.1016/j.neuroimage.2011.09.033. 21985907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang H., Gundapuneedi T., Rao U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology. 2012;37(12):2693–2701. doi: 10.1038/npp.2012.133. 22850736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koizumi M., Takagishi H. The relationship between child maltreatment and emotion recognition. PLOS One. 2014;9(1):e86093. doi: 10.1371/journal.pone.0086093. 24465891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prete G., Laeng B., Fabri M., Foschi N., Tommasi L. Right hemisphere or valence hypothesis, or both? The processing of hybrid faces in the intact and callosotomized brain. Neuropsychologia. 2015;68:94–106. doi: 10.1016/j.neuropsychologia.2015.01.002. 25575451 [DOI] [PubMed] [Google Scholar]
- 67.Ahern G.L., Schwartz G.E. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia. 1985;23(6):745–755. doi: 10.1016/0028-3932(85)90081-8. 4080136 [DOI] [PubMed] [Google Scholar]
- 68.Davidson R.J., Ekman P., Saron C.D., Senulis J.A., Friesen W.V. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology I. J. Pers. Soc. Psychol. 1990;58(2):330–341. 2319445 [PubMed] [Google Scholar]
- 69.Silberman E.K., Weingartner H. Hemispheric lateralization of functions related to emotion. Brain Cogn. 1986;5(3):322–353. doi: 10.1016/0278-2626(86)90035-7. 3530287 [DOI] [PubMed] [Google Scholar]
- 70.Jansari A., Rodway P., Goncalves S. Identifying facial emotions: valence specific effects and an exploration of the effects of viewer gender. Brain Cogn. 2011;76(3):415–423. doi: 10.1016/j.bandc.2011.03.009. 21514027 [DOI] [PubMed] [Google Scholar]
- 71.Calvo M.G., Beltrán D. Brain lateralization of holistic versus analytic processing of emotional facial expressions. Neuroimage. 2014;92:237–247. doi: 10.1016/j.neuroimage.2014.01.048. 24495810 [DOI] [PubMed] [Google Scholar]
- 72.Li L., Wu M., Liao Y., Ouyang L., Du M., Lei D., Chen L., Yao L., Huang X., Gong Q. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neurosci. Biobehav. Rev. 2014;43:163–172. doi: 10.1016/j.neubiorev.2014.04.003. 24769403 [DOI] [PubMed] [Google Scholar]
- 73.Proal E., Reiss P.T., Klein R.G. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch. Gen. Psychiatry. 2011;68(11):1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. 22065528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castellanos F.X., Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn. Sci. (Regul. Ed.) 2012;16(1):17–26. doi: 10.1016/j.tics.2011.11.007. 22169776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Bellis M.D., Woolley D.P., Hooper S.R. Neuropsychological findings in pediatric maltreatment: relationship of PTSD, dissociative symptoms, and abuse/neglect indices to neurocognitive outcomes. Child Maltreat. 2013;18(3):171–183. doi: 10.1177/1077559513497420. 23886642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smyke A.T., Zeanah C.H., Gleason M.M., Drury S.S., Fox N.A., Nelson C.A., Guthrie D. A randomized controlled trial comparing foster care and institutional care for children with signs of reactive attachment disorder. Am. J. Psychiatry. 2012;169(5):508–514. doi: 10.1176/appi.ajp.2011.11050748. 22764361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blakemore S.-J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. 20496383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kay C., Green J. Reactive attachment disorder following early maltreatment: systematic evidence beyond the institution. J. Abnorm. Child Psychol. 2013;41(4):571–581. doi: 10.1007/s10802-012-9705-9. 23250477 [DOI] [PubMed] [Google Scholar]