Kwon et al. show that paracrine signaling between SCLC subclones is a critical requirement in the early steps of the metastatic process. Paracrine signaling via Fgf2 and MAPK between these diverged tumor subclones causes enhanced expression of the Pea3 transcription factor, resulting in metastatic dissemination of the neuroendocrine tumor subclones.
Keywords: Fgf2, Pea3, metastasis, small cell lung cancer, tumor heterogeneity
Abstract
Tumor heterogeneity can create a unique symbiotic tumor microenvironment. Earlier, we showed that clonal evolution in mouse small cell lung cancer (SCLC) can result in subclones that, upon cografting, endow the neuroendocrine tumor cells with metastatic potential. We now show that paracrine signaling between SCLC subclones is a critical requirement in the early steps of the metastatic process, such as local invasion and intravasation. We further show evidence that paracrine signaling via fibroblast growth factor 2 (Fgf2) and Mapk between these diverged tumor subclones causes enhanced expression of the Pea3 (polyomavirus enhancer activator 3) transcription factor, resulting in metastatic dissemination of the neuroendocrine tumor subclones. Our data reveal for the first time paracrine signaling between tumor cell subclones in SCLC that results in metastatic spread of SCLC.
Tumor progression is driven by coevolution of neoplastic cells with nontransformed somatic cells such as stromal, vascular endothelial, and immune cells and depends on reciprocal interactions within the tumor microenvironment (Hanahan and Weinberg 2011; Junttila and de Sauvage 2013). There have been increasing efforts to identify the signaling molecules and pathways in these heterogeneous cellular compartments, and many of them show functional roles in multistage tumor development (Hanahan and Weinberg 2011). The existence of intratumor heterogeneity adds further complexity to this phenomenon. As a result of this intratumor heterogeneity, subclones from a single tumor can exhibit different growth properties and metastatic capacities (Liu et al. 2009; Anderson et al. 2011; Notta et al. 2011; Wu et al. 2012). Moreover, genetic variation among tumor subclones allows distinct clones to cope with altered conditions such as exposure to cytotoxic drugs (Burrell and Swanton 2014). Although substantial progress has been made in understanding the coevolutionary interactions and functional roles of the different cell compartments in tumor microenvironments, little is known about the cross-talk between tumor cell subclones and how it affects tumor progression.
Small cell lung cancer (SCLC) represents 13% of all newly diagnosed lung cancer cases, and >90% of patients with SCLC are current or past heavy smokers (van Meerbeeck et al. 2011). SCLC is the most aggressive lung cancer subtype, with neuroendocrine characteristics and centrally located lesions that disseminate early in disease. As a result, SCLC is usually diagnosed after metastatic spread to multiple organs (e.g., the liver, bones, and the brain) has occurred. Chemotherapy, rather than surgery, is the standard treatment of SCLC, and although SCLC tumors often respond well to chemotherapy, tumors invariably relapse, as reflected by poor 5-year survival rates (Jackman and Johnson 2005). The way SCLC is treated strongly limits accessibility to primary patient materials. Therefore, mouse SCLC models closely mimicking the human condition have become an important research tool to study this cancer type (Meuwissen et al. 2003; Kwon and Berns 2013; McFadden et al. 2014). Detailed characterization of mouse SCLC following the inactivation of Rb1 and Trp53 in the lung showed the frequent presence of multiple tumor cell types with divergent marker expression profiles (Calbo et al. 2011). These different cell clones often shared distinct genetic aberrations indicative of their common ancestry. Cultures of these tumors often contained two morphologically different cell types: one growing in suspension with typical SCLC neuroendocrine features (NE cells), and the other proliferating as adherent cultures with a mesenchymal rather than a neuroendocrine marker profile (NonNE cells). Interestingly, both of these phenotypically different cell variants are also found in human SCLC cell lines. Although coculturing NE and NonNE cells showed significant effects on their proliferation capacities, subcutaneously injected NE and NonNE cell mixtures did not show clear accelerated tumor growth. However, grafting of these mixtures did endow the NE cells in the mixture with metastatic capacity, resulting in liver metastases. The presence of NonNE cells in the mixture was required for efficient metastasis, as mice injected with either NE cells alone or NonNE cells alone did not develop liver metastasis. Liver metastases harbored only NE cells. Therefore, mesenchymal-like NonNE cells needed to be present to endow NE cells with metastatic capacity (Calbo et al. 2011). These data clearly illustrate how intratumor heterogeneity can contribute to tumor metastasis. The functional signaling between tumor cells and their microenvironment is increasingly considered as a potential target of cancer therapy (Swartz et al. 2012). Therefore, understanding the paracrine signaling cascades between NE and NonNE cells responsible for the metastatic behavior may help to identify novel drug targets for SCLC. Here we describe a potential signaling cascade between NE and NonNE tumor cells that contributes to metastasis formation in SCLC.
Results and Discussion
NE tumor cells from liver metastases have not acquired autonomous metastatic capacity
Recent mouse and human studies using next-generation sequencing (NGS) have shown that metastases often arise from minor subclones present in the primary tumor tissues (Liu et al. 2009; Yachida et al. 2010; Wu et al. 2012; McFadden et al. 2014). In order to determine whether metastasized tumor cells in our SCLC mouse model have acquired autonomous metastatic potential, we examined whether tumor cells from liver metastases can metastasize from subcutaneous sites without cografted NonNE cells. We chose luciferase-labeled NE cells (C896.04) and NonNE cells (C22.03) expanded from single-cell clones (Calbo et al. 2011). This combination of NE and NonNE cells generated liver metastases in 100% of the subcutaneously injected immunocompromised Balb/c nu/nu mice (nine out of nine mice), and liver metastases were shown to contain only neuroendocrine tumor cells by immunohistochemistry (Calbo et al. 2011; data not shown). Two metastatic liver lesions from two independent mice were expanded in culture as suspensions of small aggregated cells and tested for the expression of neuroendocrine markers (denoted NEMet) (data not shown). Next, we examined whether these NEMet cells exhibited metastatic capability upon subcutaneous grafting with or without the cografting of NonNE cells. All of the mice injected with a mixture of NEMet and NonNE cells showed liver metastases similar to their parental C896.04 cell clone and as observed before (Fig. 1A). The metastatic liver lesions were composed of only NE cells (Fig. 1B). In contrast, mice injected with NEMet cells alone showed strongly reduced metastasis (P < 0.05 in both NEMet #1 and NEMet #2) (Fig. 1A,B; Supplemental Fig. S1A). Moreover, the number of metastatic foci per mouse was substantially decreased (Fig. 1C; Supplemental Fig. S1B). Therefore, NEMet cells continue to depend on signaling from NonNE cells for their metastatic capacity, indicating that the NEMet tumor cells obtained from metastatic sites have not acquired autonomous metastatic potential.
Figure 1.
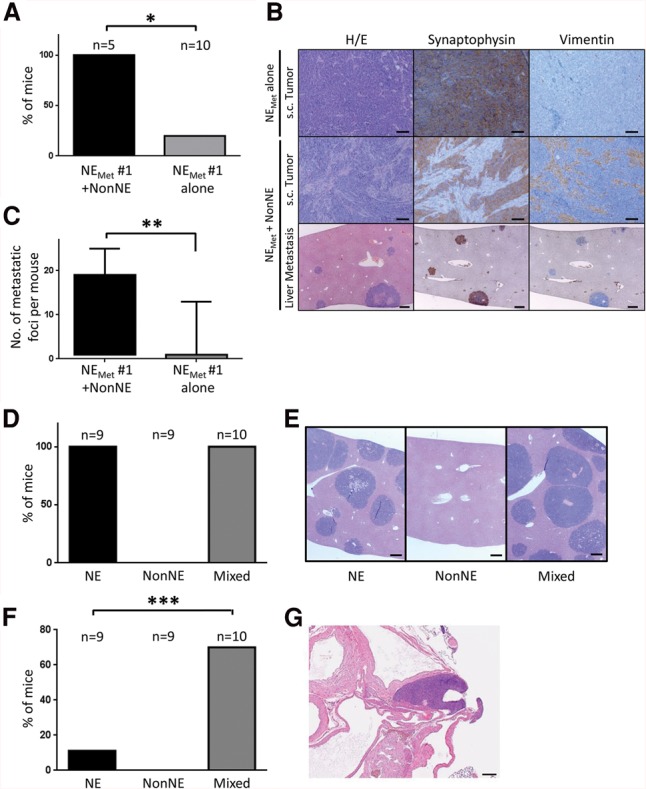
Contribution of NonNE cells to metastasis of NE cells in graft experiments. (A–C) To determine the autonomous metastatic potential of NE cells from metastases, NE cells were established from liver metastases (NEMET cells). NEMET cells were injected subcutaneously into the flank of Balb/c nude mice either as a pure NE cell population or mixed with NonNE cells. (A) The occurrence of metastasis is expressed as the percentage of the number of mice with liver metastases/number of mice in that group. (B) Photomicrographs showing the morphology (H&E staining) and expression of Synaptophysin and Vimentin of transplanted tumors obtained by subcutaneous injection of NEMET cells alone (top panels) and NEMET + NonNE cells (middle panels). The bottom panels show multiple metastatic tumor nodules in the livers of mice injected subcutaneously with NEMET + NonNE cells. Bars: top, middle, 50 µm; bottom, 200 µm. (C) The number of metastatic foci per mouse from each group of mice. Error bars indicate standard deviation (SD). (*) P < 0.005; (**) P < 0.05. (D,E) The supportive role of NonNE cells in metastasis. (D) NE cells and/or NonNE cells were intravenously injected, and liver metastases were evaluated from three independent experiments. (E) Representative micrographs of H&E-stained liver sections. Bars, 200 µm. (F,G) NonNE cells strongly enhance the formation of mediastinal tumors of NE cells. (F) The number of mice showing the mediastinal tumor formation by intravenous injection of both NE and NonNE cells in comparison with injection of only NE cells. (G) Representative micrographs of H&E-stained mediastinal tumor sections. Error bars indicate SD. Bar, 200 µm. (***) P < 0.02.
NonNE cells are dispensable for liver metastasis of NE cells in an intravenous transplantation model
Metastasis is a complex process involving multiple steps, such as invasion, intravasation, survival in the circulation, extravasation, and colonization of distant sites with subsequent outgrowth of secondary tumors (Fidler 2003). During this metastatic process, cells have to survive the harsh conditions imposed by these different microenvironments. This is the reason why the success of a tumor cell to form distant metastasis is very low (Valastyan and Weinberg 2011). To specify the supportive role of NonNE cells in these multiple steps of metastasis, we intravenously injected immunodeficient mice with clonal NE cells, clonal NonNE cells, or a mixture of NE and NonNE cells. All of the mice injected with NE cells showed marked metastases in the liver. Coinjection of NonNE cells did not augment the number or size of the liver metastasis, whereas NonNE cells alone did not show any metastatic spread to the liver (Fig. 1D,E; Supplemental Fig. S1C). However, the intravenous injection of mixtures of NE and NonNE cells did give rise to a substantially higher level of mediastinal metastasis (Fig. 1F,G) and an occasional lung metastasis (we found a single lesion in one of 10 animals, and this tumor contained both NE and NonNE cell types) (Supplemental Fig. S1D), indicating that, in some tissues, colonization is more effective upon injection of the mixture. Nevertheless, the supportive role of NonNE cells for the metastatic spread of NE cells appears most profound in the early steps of the metastatic process, such as local invasion and intravasation. Since we had shown previously that single populations of either NE or NonNE cells as well as the mixed population form tumors in subcutaneous sites (Calbo et al. 2011), we further explored how NonNE cells enhance the invasive capacity of NE cells.
Conditioned medium from NonNE cells induces invasive activity of NE cells
We next tested whether the invasive capacity of NE cells can be modulated by factors secreted by NonNE cells in cell culture. NonNE cells were seeded in the lower chambers of Matrigel-coated modified Boyden chambers 48 h before the assay. NE cells were subsequently placed into the top chamber and allowed to invade through Matrigel for 48 h. NonNE cells did significantly increase the number of invading NE cells as compared with normal culture medium (Fig. 2A; Supplemental Fig. S2A). In contrast, mouse lung fibroblast (MLg) cells did not show any marked influence on invasiveness of NE cells, indicating a specific capacity of NonNE cells in promoting invasion (Fig. 2A; Supplemental Fig. S2A). Since there was no direct contact between NE and NonNE cells in this experiment, secreted factors from NonNE cells have to be responsible for the invasion of the NE cells. Indeed, conditioned medium from NonNE cells was sufficient to promote the invasion of NE cells in a dose-dependent manner (Fig. 2B) while causing a modest decrease in the proliferation rate of NE cells (data not shown). As expected, conditioned medium from NE cells did not affect the invasiveness of NE cells (Supplemental Fig. S2B). In order to gain insight into the underlying factors that promote metastasis, gene expression analysis was performed on two NE cell clones treated with conditioned medium from NonNE cells or normal culture medium. We found 46 genes that were up-regulated at least fivefold on average by conditioned medium from NonNE cells (Supplemental Fig. S2C). We did not observe genes that were down-regulated more than fivefold among these differentially expressed genes. The ETS transcription factor Pea3 (polyomavirus enhancer activator 3) was one of the highest up-regulated genes (>20-fold) (Supplemental Fig. S2C).
Figure 2.
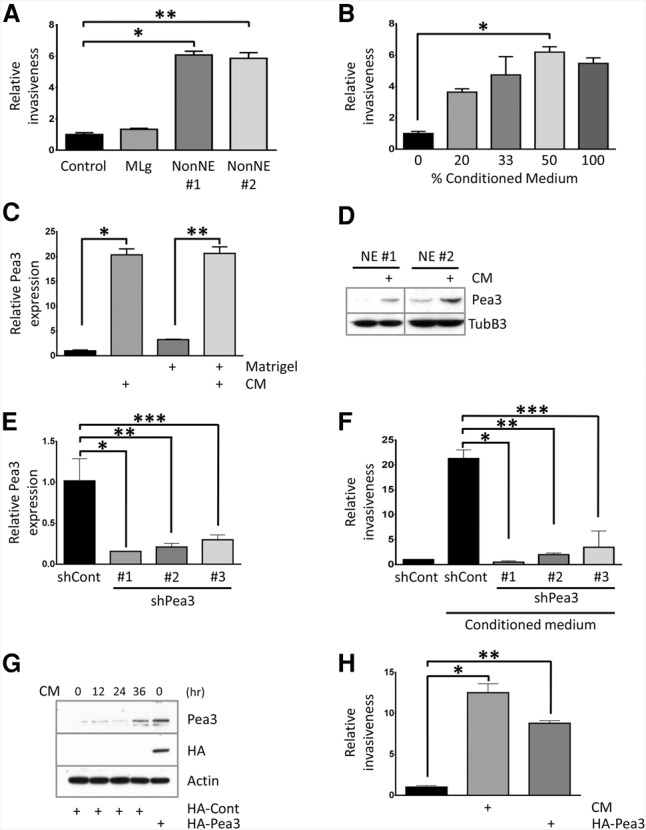
Conditioned medium from NonNE cells enhances NE cell invasion through the induction of Pea3 expression. (A) Quantification of the relative invasiveness of NE cells upon coculture of NonNE cell clones in the lower compartments of Matrigel-coated modified Boyden chambers compared with complete medium-treated control and coculture of MLg. Shown is a representative experiment from five independent experiments. (*) P < 0.0001; (**) P < 0.0001. (B) Quantification of the relative invasion achieved by different dilutions of conditioned medium from NonNE cells with complete culture medium. Error bars represent the SD. (*) P < 0.0001. (C) Quantitative RT–PCR (qRT–PCR) of Pea3 mRNA expression from NE cells either as a floating suspension cells or in Matrigel. NE cells were cultured with conditioned medium from NonNE cells for 12 h. Pea3 levels are presented relative to β-actin mRNA and compared with their expression levels in complete medium-treated NE cells (onefold). Shown is a representative experiment of five independent experiments using both TaqMan and SYBR Green detection methods. (*) P < 0.005; (**) P < 0.005. (D) Western blotting for Pea3 in conditioned medium-treated NE cell clones. Tubulin β-3 was used for the loading control. A similar result was obtained from two independent experiments. (E) Inhibition of Pea3 expression by three individual shRNA lentiviral constructs targeting Pea3 mRNA in conditioned medium-treated NE cells for 12 h. Data are representative of three independent experiments. (*) P < 0.01; (**) P < 0.01; (***) P < 0.02. (F) Matrigel invasion assay of NE cells expressing shRNA targeting Pea3. Data are representative of three independent experiments. (*) P < 0.005; (**) P < 0.005; (***) P < 0.05. (G,H) Constitutive Pea3 overexpression mediates invasiveness of NE cells. (G) Expression of Pea3 was confirmed by Western blot for Pea3 and tagged HA, and the expression level of Pea3 was compared with endogenously induced Pea3. Actin was used as the loading control. Constitutive overexpression of Pea3 increases the invasion activity of NE cells in the absence of conditioned medium. (H) Data are representative of two independent experiments. (*) P < 0.0001; (**) P < 0.0001.
NonNE cells induce the expression of Pea3 in NE cells
PEA3 (also known as ETV4 or E1AF) belongs to the ETS transcription factor family that carries an evolutionarily conserved ETS DNA-binding domain (Oh et al. 2012). PEA3 is known to be expressed in metastatic tumors, and its expression is correlated with metastasis of various human cancers, including breast, non-SCLC, prostate, esophageal, and colorectal cancer (Horiuchi et al. 2003; Sloan et al. 2009; Yuen et al. 2011). Since overexpression of PEA3 can induce the motility and invasiveness of cancer cells through transcriptional activation of metastasis-related genes, we first selected Pea3 as a potential candidate gene for conferring invasiveness to NE cells. Quantitative PCR (qPCR) and Western blot analysis were performed to validate Pea3 expression in NE cells upon treatment of conditioned medium from NonNE cells (Fig. 2C,D). Indeed, conditioned medium from NonNE cells but not from mouse lung fibroblast (MLg) cells or NE cells induced the expression of Pea3 in NE cells, which showed its peak level at 12 h of exposure to conditioned medium and returned to basal level at 72 h (Supplemental Fig. S2D–F). Interestingly, increased expression of PEA3 in NE cells from the human NIH-H446 SCLC cell line was observed upon treatment of conditioned medium from adherent NIH-H446 cells (Supplemental Fig. S2G), indicating that similar signaling is also occurring in human SCLC. Since subcutaneous inoculation of NE and NonNE cells in mice shows intermingled populations of both tumor cell types (Fig. 1B), we further examined the expression of Pea3 in NE cells isolated from cocultures with NonNE cells (Supplemental Fig. S2H). As expected, coculture with NonNE cells also induced Pea3 in NE cells.
Pea3 regulates invasion activity and metastasis of NE cells
To determine whether the acquired invasiveness of NE cells depends on Pea3 expression, three stable knockdown Pea3 NE cell lines were generated as confirmed by qPCR (Fig. 2E; Supplemental Fig. S2I). Knockdown of Pea3 did not show any marked effect on the proliferation rate of NE cells (data not shown) but impaired its invasion activity when exposed to NonNE conditioned medium (Fig. 2F). To determine whether Pea3 by itself could enhance invasion, retroviral-mediated HA-tagged Pea3-overexpressing NE cells were established (Fig. 2G). Pea3 overexpression in NE cells was sufficient to induce the invasion activity in the Matrigel-coated transwell assay in the absence of NonNE conditioned medium (Fig. 2H). These data indicate that, in response to NonNE conditioned medium, Pea3 expression in NE cells is not only required but also largely sufficient for the invasiveness of NE cells in this assay.
To determine whether the effect of Pea3 on invasion activity through Matrigel matches metastatic capacity in vivo, we subcutaneously injected knockdown Pea3 NE cells with NonNE cells into immunodeficient mice and analyzed subsequent tumor growth and metastasis to the liver. Upon knockdown of Pea3, NE cells developed subcutaneous tumors with a growth rate and efficiency similar to those of control shRNA-expressing NE cells (Supplemental Fig. S3A). However, Pea3 knockdown greatly suppressed the ability of NE cells to metastasize to the liver, as shown by the far fewer and smaller liver metastases (Fig. 3A–C). Therefore, expression of Pea3 is critical for effective metastasis of NE cells.
Figure 3.
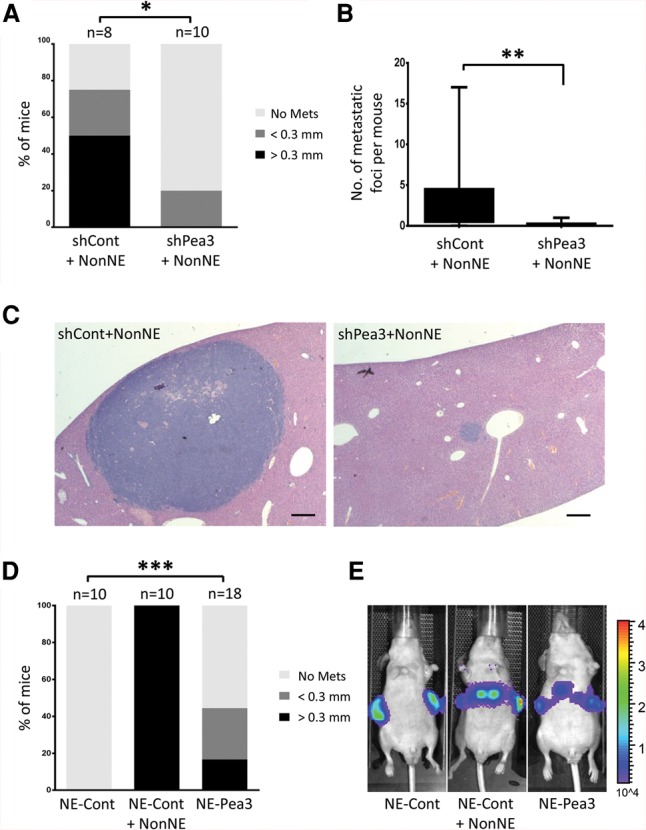
Knockdown of Pea3 in NE cells strongly impairs tumor cell metastasis, and Pea3 overexpression boosts metastasis of NE cells in vivo. (A) The percentage of mice with liver metastases was analyzed by histology. The liver metastases were divided into two sizes: <0.3 mm and >0.3 mm. (*) P < 0.02. (B) The total number of liver metastases was quantified. Statistical significance was determined by Student's t-test. (**) P < 0.01. (C) Representative H&E-stained images of liver sections. Bars, 200 µm. (D,E) A constitutively Pea3- and luciferase-overexpressing NE cell clone was injected subcutaneously into the flanks of Balb/c nude mice. Either control plasmid-overexpressing NE (NE-Cont) cells or mixed NE-Cont and NonNE cells were transplanted as a control group. (D) Statistical significance was determined by Student's t-test. (***) P < 0.01. (E) Representative bioluminescence images for in vivo detection of liver metastasis.
We next examined whether the expression of Pea3 alone is sufficient to endow NE cells with metastatic potential without the support of NonNE cells. Pea3-overexpressing cells alone were subcutaneously injected into immunodeficient mice, and tumor growth and metastasis were assessed. Over 40% of mice engrafted with Pea3-overexpressing NE cells showed liver metastasis, while none of the mice transplanted with control NE cells did (Fig. 3D,E; Supplemental Fig. S3B). However, the number and size of tumor nodules in the liver were smaller than those observed upon cografting of NE and NonNE cells (Fig. 3D; Supplemental Fig. S3C). Therefore, Pea3 is required and partially sufficient to convey the full metastatic capacity to NE cells (for further discussion, see Supplemental Fig. S4D).
Mapk signaling is required upstream of Pea3 for invasion activity of NE cells
Previous studies have demonstrated that PEA3 expression is regulated by RAS/MAPK pathway signaling and is important for the metastatic progression of esophageal, gastric, and prostate adenocarcinoma (Hardy et al. 2011; Keld et al. 2011; Aytes et al. 2013). We therefore examined the activation of this pathway in NE cells. Serum-free conditioned medium from NonNE cells caused strongly elevated phospho-Erk1/2 levels in NE cells (Fig. 4A). To elucidate the significance of this Pea3 induction for the capacity of NE cells to invade, we examined the effect of inhibiting ERK1/2 phosphorylation. The MEK1 inhibitor PD98059 significantly reduced Pea3 expression and blocked the invasion activity of conditioned medium-treated NE cells (Fig. 4B,C). Furthermore, the overexpression of activated Ras (RasV12) alone was sufficient to induce the expression of Pea3 and increase the invasiveness of NE cells without conditioned medium treatment (Fig. 4D,E).
Figure 4.
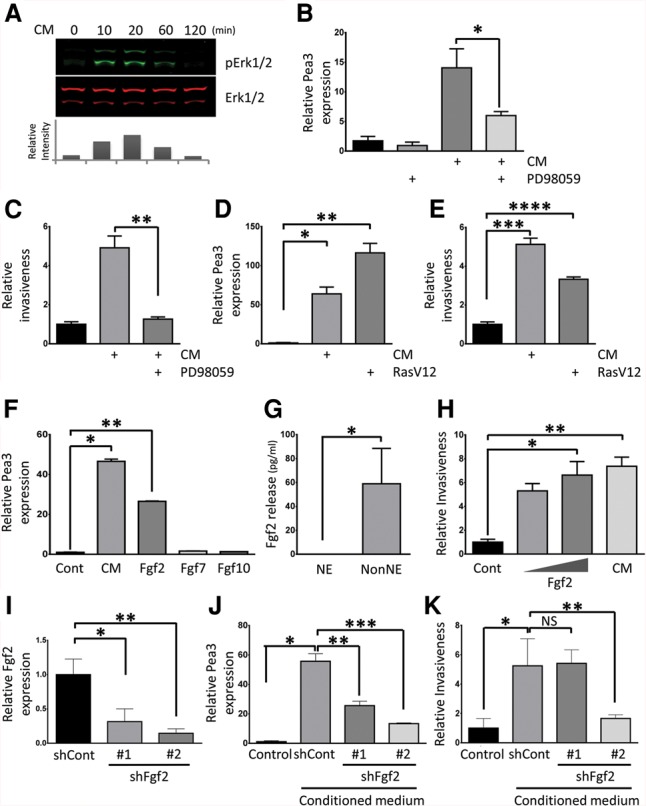
Pea3 expression and invasiveness are induced by fibroblast growth factor (Fgf)/Ras/Mapk pathway activation in NE cells. (A) Erk1/2 activation in NE cells measured after treatment with serum-free conditioned medium from NonNE cells. Samples were immunoblotted with the antibodies against phospho-Erk1/2 (pErk1/2) and total Erk (Erk1/2). The relative intensity of pErk1/2 was normalized to total Erk1/2 using Odyssey software (LI-COR) and is plotted as the fold increase of Erk1/2 phosphorylation as compared with unstimulated NE cells. A similar result was obtained in two independent experiments. (B,C) The effect of MEK1 inhibitor PD98059 on Pea3 expression and invasion of NE cells. (B) NE cells were treated with 50 µM PD98059 and/or conditioned medium from NonNE cells for 12 h, and Pea3 expression was determined by qPCR analysis. (C) NE cells were assayed for their ability to invade Matrigel in the presence of 50 µM PD98059 and conditioned medium from NonNE cells. Data are representative of three independent experiments. (*) P < 0.02; (**) P < 0.02. (D,E) Constitutive Ras activation induces Pea3 expression and invasiveness of NE cells. (D) Lentivirus-mediated overexpression of RasV12 in NE cells induced expression of Pea3 by qPCR in the absence of conditioned medium. (E) Matrigel invasion of constitutive RasV12-expressing NE cells in the absence of conditioned medium treatment. Data are representative of three independent experiments. (*) P < 0.005; (**) P < 0.005; (***) P < 0.01; (****) P < 0.01. (F) Fgf2 induces the expression of Pea3 in NE cells. qRT–PCR was performed to detect the amount of induced Pea3 mRNA in NE cells after treatment with Fgf2, Fgf7, or Fgf10 for 12 h. Data are representative of three independent experiments. (*) P < 0.001; (**) P < 0.0001. (G) Levels of mouse Fgf2 were measured using ELISAs in conditioned medium harvested from NE and NonNE cell clones. Data represent mean ± SEM. Data are representative of three independent experiments. (*) P < 0.01. (H) Effect of Fgf2 (low amount, 1 ng/mL; high amount, 10 ng/mL) on invasion of NE cells in Matrigel. Conditioned medium from NonNE cells was used as a positive control. Data are representative of three independent experiments. (*) P < 0.005; (**) P < 0.001. (I) Inhibition of Fgf2 expression by two distinct shRNA lentiviral constructs in NonNE cells. Data are representative of three independent experiments. (*) P < 0.05; (**) P < 0.005. (J) Conditioned medium from Fgf2 knockdown NonNE cells were used to treat NE cells for 12 h, and Pea3 mRNA expression was measured by qPCR. Data are representative of three independent experiments. (*) P < 0.0001; (**) P < 0.001; (***) P < 0.0001. (K) Quantification of relative invasion of NE cells achieved by conditioned medium from Fgf2 knockdown NonNE cells. Data are representative of two independent experiments. (NS) Not significant. (*) P < 0.005; (**) P < 0.01.
Since MAPK pathway activation is normally driven by external factors, we determined which growth factors secreted by NonNE might be responsible for its induction. Fibroblast growth factors (FGFs) are well known to increase the expression of PEA3 in many mammalian contexts (D'Orazio et al. 1997; Raible and Brand 2001; Firnberg and Neubuser 2002; Brent and Tabin 2004; Mao et al. 2009; Zhang et al. 2009; Hardy et al. 2011). To examine whether members of the FGF subfamily might play a role in the induction of Pea3 expression in NE cells, the expression levels of different Fgfs in NE cells and NonNE cells were determined. NonNE cells expressed Fgf2, Fgf7, and Fgf10 (Supplemental Fig. S4A–C). We therefore tested Pea3 expression upon treatment of NE cells with these Fgfs. Fgf2 augmented expression of Pea3, whereas no effects were observed with Fgf7 and Fgf10 (Fig. 4F). ELISAs showed that Fgf2 could also be detected in conditioned medium from NonNE cells (Fig. 4G). Subsequently, we tested whether Fgf2 treatment is sufficient to confer invasion activity of NE cells. Fgf2 alone increased the invasiveness of NE cells, comparable with the effect seen with conditioned medium-treated NE cells (Fig. 4H). Furthermore, we asked whether Fgf2 is essential for the invasion activity of NE cells. To answer this question, we generated stably Fgf2 knocked down NonNE cells and harvested conditioned medium from these NonNE cells (Fig. 4I). Interestingly, conditioned medium from Fgf2 knockdown NonNE cells showed decreased Pea3-inducing activity, and conditioned medium from NonNE cells with more efficient knocked-down Fgf2 expression showed substantially impaired invasion ability of NE cells (Fig. 4J,K). Therefore, we conclude that Fgf2 secreted by NonNE cells is largely responsible for the enhanced Pea3 expression and invasiveness of NE cells.
Aberrant activation of FGF signaling is frequently observed in the pathogenesis of multiple cancer types, and FGF signaling can promote tumor progression by regulating cancer cell proliferation, survival, migration, invasion, and angiogenesis. Dysregulation of FGF signaling can be achieved by genetic alteration of FGF receptors for ligand-independent activation and excessive production of FGFs for ligand-dependent stimulation (Turner and Grose 2010; Corn et al. 2013). We found that Fgf2 secreted by NonNE cells is responsible and required for inducing the expression of Pea3 and increasing the invasion activity of NE cells. In line with this, increased plasma levels of FGF2 are associated with a poor outcome of SCLC (Ruotsalainen et al. 2002). Moreover, recent NGS of human SCLC identified focal amplifications of the FGFR1 gene (6% of all cases), resulting in high FGF signaling (Peifer et al. 2012). Since overexpression of both FGF2 and FGFR1 is known to regulate human melanoma and non-SCLC (Wang and Becker 1997), it will be interesting to examine whether NonNE cells will also be found as components of SCLC tumors carrying amplification of the FGFR1 gene.
Materials and methods
Mouse SCLC cell lines
Clonal cell lines derived from Trp53F/F;Rb1F/F SCLC tumors have been previously described (Calbo et al. 2011) and were cultured at 37°C in a humidified atmosphere of 5% CO2.
Transplantation of SCLC cell lines
All experiments involving animals comply with local and international regulations and ethical guidelines and have been authorized by the local experimental animal committee at The Netherlands Cancer Institute (DEC-NKI). Balb/c nude immunosuppressed mice were used for subcutaneous and intravenous transplantation of tumor cell lines.
Supplementary Material
Acknowledgments
We thank the personnel of the animal facility for their excellent animal husbandry, P. Krimpenfort and E. Semenova for critically reading the manuscript, and A. Fish and R. Bhaskaran for technical supports. M.-C.K was a recipient of the National Research Foundation of Korea grant funded by the Korean Government (NRF-2009-352-C00133), and K.S. was a recipient of a National Health and Medical Research Council of Australia Overseas-based Biomedical Training Fellowship (no. 516781). This work was also supported by a grant of the Dutch Cancer Society and a European Research Council Synergy grant to A.B.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.262998.115.
References
- Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM, Kempski H, Moorman AV, Titley I, Swansbury J, et al. 2011. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 469: 356–361. [DOI] [PubMed] [Google Scholar]
- Aytes A, Mitrofanova A, Kinkade CW, Lefebvre C, Lei M, Phelan V, LeKaye HC, Koutcher JA, Cardiff RD, Califano A, et al. 2013. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc Natl Acad Sci 110: E3506–E3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. 2004. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131: 3885–3896. [DOI] [PubMed] [Google Scholar]
- Burrell RA, Swanton C. 2014. Tumour heterogeneity and the evolution of polyclonal drug resistance. Mol Oncol 8: 1095–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R, Berns A. 2011. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 19: 244–256. [DOI] [PubMed] [Google Scholar]
- Corn PG, Wang F, McKeehan WL, Navone N. 2013. Targeting fibroblast growth factor pathways in prostate cancer. Clin Cancer Res 19: 5856–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orazio D, Besser D, Marksitzer R, Kunz C, Hume DA, Kiefer B, Nagamine Y. 1997. Cooperation of two PEA3/AP1 sites in uPA gene induction by TPA and FGF-2. Gene 201: 179–187. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. 2003. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3: 453–458. [DOI] [PubMed] [Google Scholar]
- Firnberg N, Neubuser A. 2002. FGF signaling regulates expression of Tbx2, Erm, Pea3, and Pax3 in the early nasal region. Dev Biol 247: 237–250. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg Robert A. 2011. Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hardy KM, Yatskievych TA, Konieczka J, Bobbs AS, Antin PB. 2011. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev Biol 11: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S, Yamamoto H, Min Y, Adachi Y, Itoh F, Imai K. 2003. Association of ets-related transcriptional factor E1AF expression with tumour progression and overexpression of MMP-1 and matrilysin in human colorectal cancer. J Pathol 200: 568–576. [DOI] [PubMed] [Google Scholar]
- Jackman DM, Johnson BE. 2005. Small-cell lung cancer. Lancet 366: 1385–1396. [DOI] [PubMed] [Google Scholar]
- Junttila MR, de Sauvage FJ. 2013. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501: 346–354. [DOI] [PubMed] [Google Scholar]
- Keld R, Guo B, Downey P, Cummins R, Gulmann C, Ang YS, Sharrocks AD. 2011. PEA3/ETV4-related transcription factors coupled with active ERK signalling are associated with poor prognosis in gastric adenocarcinoma. Br J Cancer 105: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MC, Berns A. 2013. Mouse models for lung cancer. Mol Oncol 7: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, Chen L, Ewing CM, Eisenberger MA, Carducci MA, et al. 2009. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 15: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, McGlinn E, Huang P, Tabin CJ, McMahon AP. 2009. Fgf-dependent Etv4/5 activity is required for posterior restriction of Sonic Hedgehog and promoting outgrowth of the vertebrate limb. Dev Cell 16: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, Cibulskis K, Bhutkar A, McKenna A, Dooley A, Vernon A, et al. 2014. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell 156: 1298–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. 2003. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4: 181–189. [DOI] [PubMed] [Google Scholar]
- Notta F, Mullighan CG, Wang JC, Poeppl A, Doulatov S, Phillips LA, Ma J, Minden MD, Downing JR, Dick JE. 2011. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature 469: 362–367. [DOI] [PubMed] [Google Scholar]
- Oh S, Shin S, Janknecht R. 2012. ETV1, 4 and 5: an oncogenic subfamily of ETS transcription factors. Biochim Biophys Acta 1826: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander T, et al. 2012. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 44: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible F, Brand M. 2001. Tight transcriptional control of the ETS domain factors Erm and Pea3 by Fgf signaling during early zebrafish development. Mech Dev 107: 105–117. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen T, Joensuu H, Mattson K, Salven P. 2002. High pretreatment serum concentration of basic fibroblast growth factor is a predictor of poor prognosis in small cell lung cancer. Cancer Epidemiol Biomarkers Prev 11: 1492–1495. [PubMed] [Google Scholar]
- Sloan KA, Marquez HA, Li J, Cao Y, Hinds A, O'Hara CJ, Kathuria S, Ramirez MI, Williams MC, Kathuria H. 2009. Increased PEA3/E1AF and decreased Net/Elk-3, both ETS proteins, characterize human NSCLC progression and regulate caveolin-1 transcription in Calu-1 and NCI-H23 NSCLC cell lines. Carcinogenesis 30: 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. 2012. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res 72: 2473–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R. 2010. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10: 116–129. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg Robert A. 2011. Tumor metastasis: molecular insights and evolving paradigms. Cell 147: 275–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. 2011. Small-cell lung cancer. Lancet 378: 1741–1755. [DOI] [PubMed] [Google Scholar]
- Wang Y, Becker D. 1997. Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med 3: 887–893. [DOI] [PubMed] [Google Scholar]
- Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJ, Witt H, Croul S, Bouffet E, Fults DW, Eberhart CG, et al. 2012. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature 482: 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. 2010. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467: 1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen HF, Chan YK, Grills C, McCrudden CM, Gunasekharan V, Shi Z, Wong AS, Lappin TR, Chan KW, Fennell DA, et al. 2011. Polyomavirus enhancer activator 3 protein promotes breast cancer metastatic progression through Snail-induced epithelial-mesenchymal transition. J Pathol 224: 78–89. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Verheyden JM, Hassell JA, Sun X. 2009. FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev Cell 16: 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


