Koltowska et al. used a forward genetic screen in zebrafish to identify the transcription factor mafba as essential for lymphatic vessel development. Vegfc signaling increases mafba expression to control downstream transcription, and this relationship is SoxF transcription factor-dependent.
Keywords: lymphatic, vascular, Mafb, Vegfc, Sox18, Sox7, zebrafish
Abstract
The lymphatic vasculature plays roles in tissue fluid balance, immune cell trafficking, fatty acid absorption, cancer metastasis, and cardiovascular disease. Lymphatic vessels form by lymphangiogenesis, the sprouting of new lymphatics from pre-existing vessels, in both development and disease contexts. The apical signaling pathway in lymphangiogenesis is the VEGFC/VEGFR3 pathway, yet how signaling controls cellular transcriptional output remains unknown. We used a forward genetic screen in zebrafish to identify the transcription factor mafba as essential for lymphatic vessel development. We found that mafba is required for the migration of lymphatic precursors after their initial sprouting from the posterior cardinal vein. mafba expression is enriched in sprouts emerging from veins, and we show that mafba functions cell-autonomously during lymphatic vessel development. Mechanistically, Vegfc signaling increases mafba expression to control downstream transcription, and this regulatory relationship is dependent on the activity of SoxF transcription factors, which are essential for mafba expression in venous endothelium. Here we identify an indispensable Vegfc–SoxF–Mafba pathway in lymphatic development.
Lymphangiogenesis is the formation of new lymphatic vessels from pre-existing vessels. Lymphangiogenesis plays integral roles in cancer metastasis, lymphedema, and cardiovascular disease (Lim et al. 2013; Martel et al. 2013; Stacker et al. 2014). Common molecular pathways control lymphangiogenesis in development and disease, and much of our current understanding has come from the study of embryogenesis. In the embryo, the lymphatic vasculature derives chiefly from pre-existing veins through a process involving cellular transdifferentiation, migration, and proliferation and vessel morphogenesis (Oliver and Srinivasan 2010; Koltowska et al. 2013).
Lymphangiogenesis is dependent on VEGFR3 signaling in all known contexts, including during development. Knockout mice for Vegfr3 are embryonic-lethal due to cardiovascular failure (Dumont et al. 1998), and heterozygous mutation of Vegfr3 in the Chy mouse model leads to lymphedema and lymphatic vascular defects (Karkkainen et al. 2001). Furthermore, the transgenic overexpression of a soluble inhibitory (ligand trap) form of VEGFR3 disrupts tissue lymphangiogenesis (Makinen et al. 2001). Mouse mutants for Vegfc fail to form the earliest lymphatic sprouts from embryonic veins, and, indicative of the instructive role for VEGFC, overexpression of this ligand promotes ectopic tissue lymphangiogenesis (Jeltsch et al. 1997; Karkkainen et al. 2004; Hagerling et al. 2013). In humans, the VEGFC/VEGFR3 pathway also controls lymphatic vessel development, and patients with mutations in either VEGFR3 or VEGFC develop primary lymphedema in familial Milroy's disease or Milroy's-like lymphedema, respectively (Irrthum et al. 2000; Karkkainen et al. 2000; Gordon et al. 2013). Finally, several coreceptors and modulators of VEGFC/VEGFR3 signaling play crucial roles in lymphangiogenesis (for review, see Schulte-Merker et al. 2011; Koltowska et al. 2013; Zheng et al. 2014), underscoring how central the pathway is in lymphatic development.
Characterization of the zebrafish lymphatic vasculature has demonstrated the highly conserved function of the VEGFC/VEGFR3 signaling pathway in vertebrates (Kuchler et al. 2006; Yaniv et al. 2006). Forward genetic screens for mutants lacking lymphatics in zebrafish discovered a function for Ccbe1 (Hogan et al. 2009a), which was subsequently shown to play a conserved role in mice and humans (Alders et al. 2009; Connell et al. 2010; Bos et al. 2011). CCBE1 regulates the processing and activation of immature VEGFC to its mature, functional form necessary for lymphangiogenesis (Jeltsch et al. 2014; Le Guen et al. 2014). In addition, zebrafish mutants have been described in vegfc and vegfr3 themselves, and we now know that zebrafish Vegfc signaling controls all secondary angiogenesis, including the formation of lymphatic precursors (PLs) from the posterior cardinal vein (PCV) (Hogan et al. 2009a,b; Villefranc et al. 2013; Le Guen et al. 2014). The coordinated activity of both Vegfc and Vegfd further controls lymphangiogenesis of the facial lymphatic network (Okuda et al. 2012; Astin et al. 2014).
Although the apical signaling pathway that governs lymphatic sprouting is well established, we still have a very limited understanding of how signaling controls downstream pathways and regulates endothelial cell (EC) transcription. Several transcription factors are known to control the initial specification and maintenance of lymphatic EC (LEC) fate in mice, including PROX1, COUPTFII, and SOX18 (Wigle and Oliver 1999; Francois et al. 2008; Srinivasan et al. 2010; Srinivasan and Oliver 2011). Other transcription factors controlling later differentiation and specialization of lymphatic vessels include FOXC2 and GATA2 (Petrova et al. 2004; Norrmen et al. 2009; Kazenwadel et al. 2012; Lim et al. 2012). Interestingly, emerging data have suggested that both the maintenance of PROX1 and the induction of SOX18 activity can be driven by VEGF signaling mechanisms (Deng et al. 2013; Duong et al. 2014; Srinivasan et al. 2014). While this integration of cell-extrinsic signaling and cell-intrinsic transcriptional information appears to play an important role in lymphatic development, the spatiotemporal control of signaling and the complexity of EC transcriptional mechanisms remain far from fully understood.
The initial genetic screens performed for zebrafish lymphatic development used a pan-endothelial marker [Tg(fli1a:EGFP)y1] and scored the presence of the thoracic duct as a proxy for systemic lymphangiogenesis. We took advantage of the recently described Tg(-5.2lyve1b:dsRed) strain, which labels the comprehensive network of developing lymphatic vessels and embryonic veins (Okuda et al. 2012), to perform a phenotypically sensitized forward genetic screen and integrated whole-genome sequence mapping for rapid gene discovery. Here, we report the first mutant characterized from this screen, a mafba mutant. We found that mafba is an essential Vegfc-regulated transcription factor controlling lymphangiogenesis and identified a role for SoxF transcription factors in inducing mafba expression.
Results
uq4bh mutants fail to form lymphatic vasculature
We designed and performed an ENU mutagenesis screen in zebrafish. Briefly, we mutagenized the Tg(-5.2lyve1b:dsRed) strain and performed a classical F3 embryonic screen in this background. We sequenced the genomes of the transgenic mutagenized founders and our in-house WIK strain, which we used to generate mapping crosses with a predefined genomic variation. In total, we used 838 mutagenized F1 genomes (419 F2 families) with, on average, four F2 in-crosses scored per family (or 573 genomes screened). We identified 34 mutants that gave lymphatic deficiency/venous sprouting defects at either of two time points: 3 d post-fertilization (dpf) and 5 dpf.
The uq4bh mutant showed a highly selective reduction, up to a complete loss, of lymphatic vessels in the embryonic trunk and face but formed a grossly normal blood vasculature (Fig. 1A; Supplemental Fig. 1B). Overall, the body plan was normal except for an otic vesicle defect and edema by 7 dpf (Figs. 1B, 2D; Supplemental Fig. 1A). Quantification of the number of LECs in the trunk and face using a nuclear marker of ECs coupled with Tg(-5.2lyve1b:dsRed) demonstrated a significant reduction in mutants (Fig. 1C–E). In the developing facial lymphatics, reductions were observed in medial facial lymphatics and branchial arch lymphatics (Fig. 1D,E; Supplemental Fig. 1C). The number of LECs in the otolithic lymphatic vessel was increased but was presumed to reflect altered, local tissue patterning. Earlier in development, examination of parachordal PL cell number showed no difference in mutants (Fig. 1F,G). We analyzed the expression of prox1a in the Tg(prox1a:Kalt4-4xUAS:uncTagRFP) transgenic line that has been previously reported (Dunworth et al. 2014; van Impel et al. 2014) crossed onto a Tg(10xUAS:Venus) reporter strain. Interestingly, we saw no change in prox1a expression in sprouts leaving the PCV, suggesting normal establishment of cell identity in mutants (Fig. 1H,I). Overall, these observations indicate that the uq4bhmutants initiate lymphatic fate and sprouting secondary angiogenesis, but ongoing formation of a comprehensive lymphatic network fails.
Figure 1.
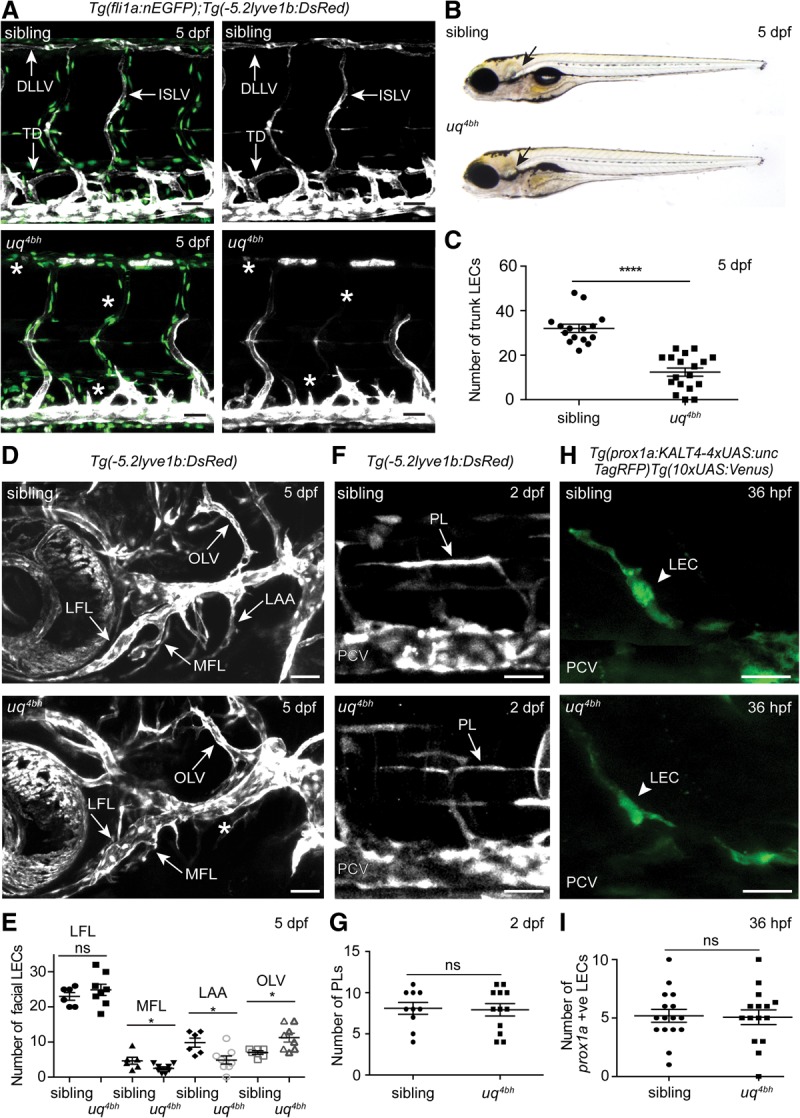
The uq4bh mutant fails to form a lymphatic vasculature. (A) Vascular nuclei [Tg(fli1a:nlsEGFP); green] and veins and lymphatic vessels [Tg(lyve1:DsRed); white] in the trunk of sibling (top panels) and mutant (bottom panels) embryos at 5 dpf. (TD) Thoracic duct; (DLLV) dorsal longitudinal lymphatic vessel; (ISLV) intersegmental lymphatic vessel. Arrows indicate lymphatics, and asterisks indicate their absence. Bars, 50 μm. (B) Gross morphology of sibling (top) and mutant (bottom) embryos at 5 dpf. (C) Quantification of total LEC number using the overlay of transgenic marker expression from A. Mean ± SEM; scored siblings, n = 15; uq4bh, n = 18; t-test. (****) P < 0.0001. (D) The facial lymphatic network in sibling (top) and mutant (bottom) embryos at 5 dpf. (LFL) Lateral facial lymphatic; (OLV) otolithic lymphatic; (LAA) branchial arch lymphatics; (MFL) medial facial lymphatics. Bars, 50 μm. (E) Quantification of facial lymphatic cell numbers in individual embryos using the overlay of transgenic marker expression from A. Mean ± SEM; scored siblings, n = 6; uq4bh, n = 8; t-test. (*) P < 0.05. (F) PLs in the horizontal myoseptum in sibling (top) and mutant (bottom) embryos. Bars, 30 μm. (G) Quantification of PL numbers in individual embryos using the overlay of transgenic marker expression from A. Mean ± SEM; scored siblings, n = 10; uq4bh, n = 12; t-test. (ns) No significant difference. (H) prox1a-expressing LECs as they emerge from the PCV at 36 h post-fertilization (hpf) in sibling (top) and mutant (bottom) embryos labeled by Tg(prox1a:Kalt4-4xUAS:uncTagRFP);Tg(10xUAS:Venus). (Green) α-GFP. Bars, 30 μm. (I) Quantification of prox1a-expressing LECs in individual embryos at 36 hpf. Mean ± SEM; scored siblings, n = 16; uq4bh, n = 15; t-test. (ns) No significant difference.
Figure 2.
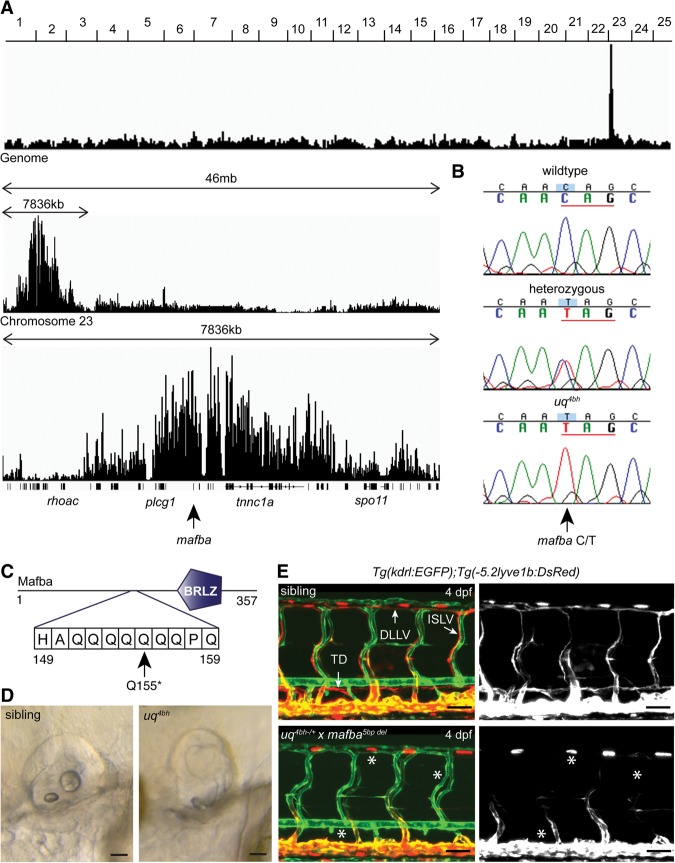
Positional cloning of mafbauq4bh using whole-genome sequence-based homozygosity mapping and mutation detection. (A) Schematic plot of genomic homozygosity across all 25 chromosomes (top), chromosome 23 (middle), and the region of linkage (bottom) (see the Materials and Methods for details). The mafba gene is located centrally within the region of highest homozygosity. (B) Sequence chromatograms confirming the nonsense allele in the mafba gene. (Top) Wild type (n = 6 reads). (Middle) Heterozygous (n = 18 reads). (Bottom) Mutant (n = 19 reads). (C) Schematic of the location of the Q155* allele predicted to truncate the critical BRLZ domain of Mafba. (D) Otic vesicle morphology of sibling (left) and mutant (right) embryos at 3 dpf. Bars, 80 μm. (E) Confirmation of causative mafbauq4bh allele by complementation test with a mafba5 bp del transmitting founder. Vasculature visualized by Tg(kdrl:EGFP) (green) and Tg(-5.2lyve1b:DsRed) (red) in the sibling (top) and transheterozygous mafbauq4bh;mafba5 bp del (bottom). Siblings, n = 10; mafbauq4bh; mafba5 bp del, n = 10. (Right) Red channel. Bars, 30 μm. See also Supplemental Figure 2.
uq4bh is a mafba mutant
We used a whole-genome sequence-based approach to map the genomic location of the affected gene (Supplemental Material; data not shown). We identified a region of homozygosity on chromosome 23, and, within this linked region, a candidate mutation was identified in the mafba gene (Fig. 2A; Supplemental Fig. 2A). A C/T nonsense mutation was identified (encoding Q155*) and predicted to truncate Mafba prior to the critical basic region leucine zipper (BRLZ) domain and thus is a predicted loss-of-function allele (Fig. 2B,C). The zebrafish neural segmentation mutant valentino (Moens et al. 1996) is a mafba mutant and displays the same otic vesicle phenotype as uq4bh (Fig. 2D), suggesting a causative mafba mutation. MAFB is a bZIP transcription factor that can act as an activator or repressor. MAFB homologs regulate cellular differentiation in various developmental contexts, controlling posterior hindbrain/otic fate decisions (Moens et al. 1996; Moens and Prince 2002), podocyte development (Sadl et al. 2002; Moriguchi et al. 2006), pancreatic β-cell differentiation (Artner et al. 2007), and hematopoietic lineage decisions (Sieweke et al. 1996, 1997; Kelly et al. 2000; Bakri et al. 2005). However, MafB has no previously described role in either lymphangiogenesis or vascular development.
To confirm that the mutation in mafba causes the lymphatic vascular developmental phenotype, we used CRISPR genome editing to generate germline mosaic zebrafish transmitting mutations in mafba (Supplemental Fig. 2B). Mosaic founders were then crossed to carriers for the uq4bh mutation, and the progeny were analyzed for phenotypes. Compound heterozygous embryos (confirmed by genotyping) displayed the described valentino otic phenotype, and this was coincident with a loss of lymphatic vascular development (Fig. 2D,E). This complementation test confirmed the mutant as mafbauq4bh (Fig. 2E; Supplemental Fig. 2C,D).
mafba is expressed in the PCV and enriched in secondary sprouts
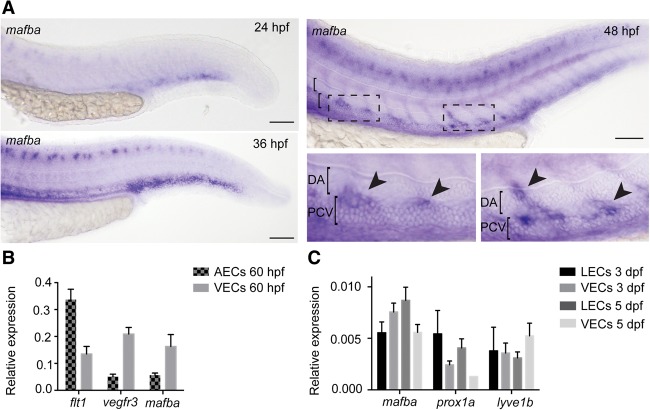
To determine the cell type in which mafba is active, we examined gene expression by in situ hybridization (ISH). mafba expression was observed in neurons, rhombomeres, and the pancreas (data not shown) as well as the PCV. We observed weak PCV expression at 24 h post-fertilization (hpf), which was increased by 30 and 36 hpf, preceding and concomitant with secondary angiogenesis (Fig. 3A; Supplemental Fig. 3A). At 48 hpf, when secondary sprouts have formed, we observed enriched expression of mafba dorsally in ECs sprouting or sprouted from the PCV (Fig. 3A). As ISH is insensitive at later developmental stages, we examined mafba expression in FACS (fluorescent-activated cell sorting)-sorted populations of arterial ECs (AECs), venous ECs (VECs), and LECs that we isolated from 60 hpf, 3 dpf, and 5 dpf embryos (Fig. 3B,C; Coxam et al. 2014, 2015). Indicative of lineage-restricted expression in the vasculature, we found that mafba was highly enriched in VECs compared with AECs at early time points (comparable with known VEC markers) and was enriched in LECs compared with VECs by 5 dpf (comparable with known venous and lymphatic markers lyve1b and prox1a). We examined expression of other Maf family genes and the duplicate Mafb homolog mafbb, which showed detectable EC expression, suggesting that it could play a compensatory role (Supplemental Fig. 4B,C); however, given the phenotype of mafba mutants, any compensation must be partial.
Figure 3.
mafba is expressed in endothelium during lymphangiogenesis. (A) Expression of mafba in the zebrafish trunk at 24 hpf and 36 hpf (left) and 48 hpf (right). (Bottom right panels) Higher-magnification images of secondary sprouts (PCV; arrowheads). (Boxed area) High-magnification region; (bracket) dorsal aorta (DA). Bars, 100 μm. (B) Quantitative PCR (qPCR) on FACS-sorted AEC versus VEC populations at 60 hpf; mafba expression is VEC-enriched, comparable with vegfr3 and in contrast to AEC marker flt1. Mean ± SEM. Expression is relative to kdrl. (C) qPCR on FACS-sorted LEC and VEC populations from 3 dpf and 5 dpf; the mafba expression profile is similar to LEC markers prox1a and lyve1b. Mean ± SEM. Expression is relative to rpl13.
mafba acts cell-autonomously during zebrafish lymphangiogenesis
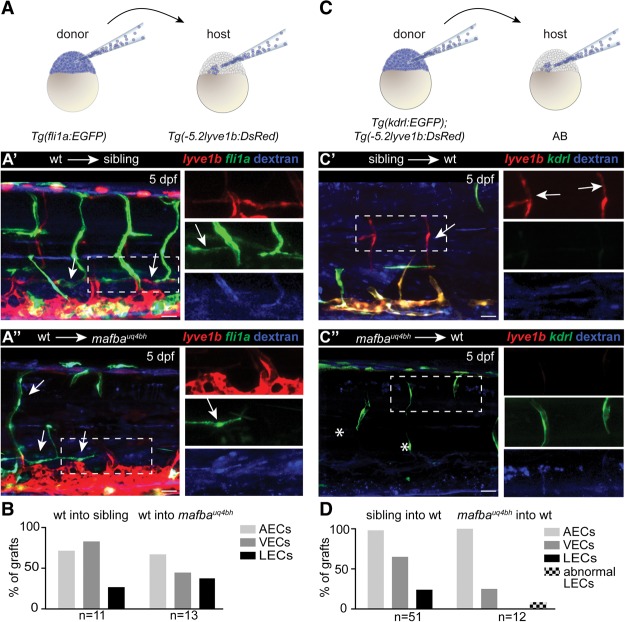
mafba expression is suggestive of a cell-autonomous function; hence, we performed cellular transplantation experiments to formally test autonomy. We transplanted wild-type Tg(fli1a:EGFP) cells into Tg(-5.2lyve1b:DsRed) recipients from a mafba heterozygous in-cross and scored for the presence of grafted (EGFP-positive) cells in arteries (AECs), veins (VECs), and lymphatics (LECs) at 5 dpf. We found that wild-type cells could as readily contribute AECs, VECs, and LECs in mutant embryos (n = 13 vascular grafts) as they could in sibling controls (n = 11 vascular grafts) (Fig. 4A,B). We traced all grafted cells with dextran and noted the positions of non-ECs, which were in variable locations and not consistent in rescued mutant embryos. Together, these analyses suggest that mafba is sufficient in ECs to direct lymphangiogenesis in mutant embryos.
Figure 4.
A cell-autonomous function for mafba in lymphatic vascular development. (A) Transplantation of Tg(fli1a:EGFP)-labeled (green) and dextran blue-labeled (blue) donor cells into a Tg(lyve1b:DsRed) (red) host derived from a mafbauq4bh in-cross. (A′,A′′) Wild-type ECs were observed to contribute to arteries (AECs), veins (VECs), and lymphatics (LECs) (white arrows) in sibling and mutant recipients. The boxed areas indicate high-power regions (shown at right). (B) Quantification of endothelial contributions in A′ and A′′. Percentage contributions of a cell to AECs, VECs, and LECs for all vascular grafted embryos (n = 11 wild type into sibling embryos with vascular grafts; n = 13 wild type into mutant embryos with vascular grafts). (C) Transplantation of Tg(lyve1b:DsRed)-labeled (red), Tg(kdrl:EGFP)-labeled (green), and dextran blue-labeled (blue) donor cells derived from a mafbauq4bh in-cross into an unlabeled wild-type host. Genotyping confirmed donor identity. (C′) Sibling ECs contributed to AECs, VECs, and LECs (arrows). (C′′) Mutant ECs contributed AECs (n = 12/12 grafts) and VECs (n = 3/12 grafts) but not LECs. The boxed areas indicate high-power images (shown at right). (D) Quantification of endothelial contributions in C′ and C′′. Percentage contributions to AECs, VECs, and LECs for all vascular grafts. One mutant graft was observed with lymphatic vascular morphology but expressed Tg(lyve1b:DsRed) and Tg(kdrl:EGFP), indicative of a differentiation defect (see Supplemental Fig. 3B). Bars, 30 μm.
Reciprocally, we transplanted mutant cells from Tg(kdrl:EGFP);Tg(-5.2lyve1b:DsRed) double-transgenic embryos into unlabeled wild-type hosts. In this experimental setting, AECs will express only EGFP, VECs will express EGFP plus dsRED, and LECs will express only dsRED in successful vascular grafts. Transplanted mutant cells contributed to AECs (n = 12/12 vascular grafts) and VECs (n = 3/12 vascular grafts) but not Tg(lyve1:DsRed)-expressing LECs (Fig. 4C,D). Interestingly, in one transplanted embryo (n = 1/12), mutant cells formed a section of vasculature that appeared to be lymphatic based on morphology, but this vessel expressed Tg(kdrl:EGFP), which is normally restricted to blood vessels. Hence, this grafted vessel was considered to have a differentiation defect (Supplemental Fig. 3B). Taken together, reciprocal cellular mosaic experiments demonstrate that mafba acts autonomously in ECs during lymphatic vessel development.
A set of mafba-dependent endothelial genes are responsive to Vegfc signaling
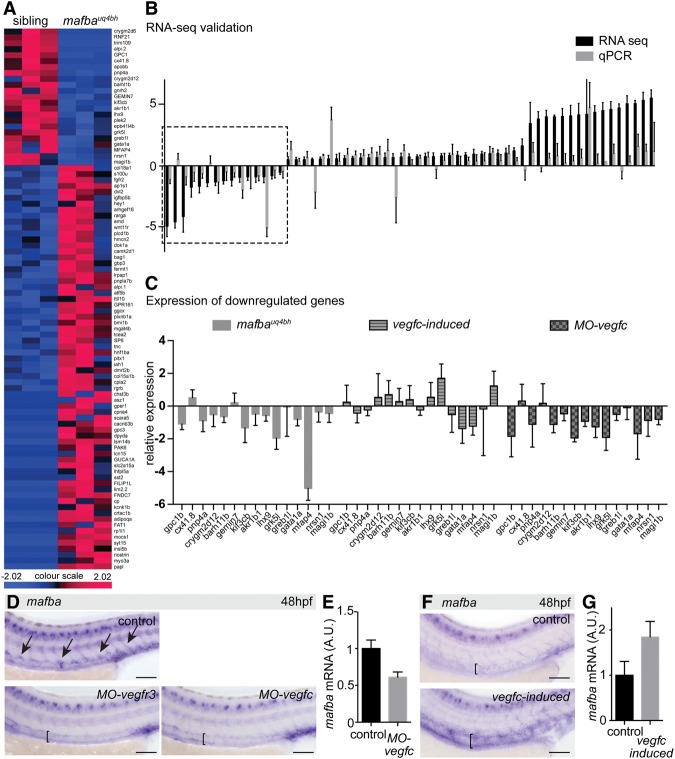
We next isolated Tg(kdrl:EGFP)-expressing ECs from sibling and mafbauq4bh mutant embryos at 48 hpf by embryo dissociation and FACS for EGFP (Supplemental Fig. 4A). We performed RNA sequencing (RNA-seq) in triplicate and generated a concordant data set identifying 23 down-regulated and 68 up-regulated genes (Fig. 5A; Supplemental Table 1). These dysregulated genes were generally lowly expressed, probably indicative of low numbers of cells derived from secondary sprouts (which express mafba) in the larger pool of Tg(kdrl:EGFP)-expressing ECs. To confirm that these genes were dysregulated, we used quantitative PCR (qPCR) for 15 down-regulated and 47 up-regulated genes and confirmed that the majority was misexpressed, as indicated in the RNA-seq data (Fig. 5B).
Figure 5.
mafba-dependent endothelial genes are Vegfc-dependent, and mafba is up-regulated by Vegfc signaling. (A) Schematic summary of RNA-seq from FACS-sorted sibling and mafbauq4bh mutants at 48 hpf. n = 23 down-regulated genes; n = 68 up-regulated genes. P < 0.01 using EdgeR analysis. (B) qPCR validation of RNA-seq. RNA-seq relative changes and qPCR fold changes are displayed side by side for a subset of up-regulated and down-regulated genes. Mean ± SEM. The boxed area contains down-regulated genes. (C) Fifteen down-regulated genes analyzed by qPCR in FACS-sorted ECs from 48-hpf vegfc-induced and 30-hpf MO-vegfc embryos. Reductions in expression were observed in both mafbauq4bh and MO-vegfc embryonic endothelium. Mean ± SEM. (D) Expression of mafba in control uninjected (n = 30/33), MO-vegfc-injected (n = 18/18), and MO-vegfr3-injected (n = 33/33) embryos at 48 hpf. Enriched dorsal PCV expression (arrows) is absent in MO-vegfc and MO-vegfr3 embryos (brackets). Bars, 100 μm. (E) qPCR validation of mafba reduction in FACS-sorted ECs from MO-Vegfc embryos. Expression is given relative to the geometric average of kdrl, cdh5, and lyve1b expression. Mean ± SEM. (F) Expression of mafba in control and vegfc-induced [Tg(prox1a:Kalt4);Tg(10xUAS:Vegfc)] embryos. mafba expression is induced throughout the PCV in vegfc-induced embryos. n = 16/16. ISH staining was for shorter periods than in E to highlight increased expression. Bars, 100 μm. (G) qPCR validation of mafba induction in FACS-sorted ECs from Tg(prox1a:Kalt4);Tg(10xUAS:Vegfc) embryos. Expression is given relative to the geometric average of kdrl, cdh5, and lyve1b expression. Mean ± SEM.
To understand how selective to secondary angiogenesis these genes are, we sorted ECs using FACS from 30-hpf MO-vegfc knockdown embryos (prior to venous sprouting) and 48-hpf Tg(prox1a:Kalt4-4xUAS:uncTagRFP);Tg(10xUAS:Vegfc) “vegfc-induced” embryos, which overexpress Vegfc in all prox1a-expressing tissues and hence show ectopic venous angiogenesis (Helker et al. 2013; Le Guen et al. 2014; K Koltowska, AK Lagendijk, C Pichol-Thievend, JC Fischer, M Francois, EA Ober, AS Yap, and BM Hogan, in prep.). We then performed qPCR for 15 genes that were down-regulated in mafba mutants. Of these 15 genes, 13 were down-regulated in MO-vegfc ECs, and eight were up-regulated in Vegfc-induced ECs (Fig. 5C). This observation suggested that, in ECs, mafba-dependent genes are also dependent on normal Vegfc signaling.
mafba expression is up-regulated by Vegfc
We investigated mafba expression in MO-vegfc and MO-vegfr3 embryos at 30 hpf and saw no change in mafba expression (Supplemental Fig. 4D). At 48 hpf, when mafba normally becomes enriched in the dorsal PCV, this enrichment was lost in MO-vegfc and MO-vegfr3 embryos, and overall EC mafba levels (normalized) were reduced by qPCR (Fig. 5D,E). Venous sprouts fail to form in MO-vegfc and MO-vegfr3 embryos, and so the loss of expression may be a consequence of failed morphogenesis. However, the observation is consistent with selective enrichment in cells responding to Vegfc signaling, so we next examined mafba expression in vegfc-induced embryos. We observed vastly increased mafba expression in the vasculature of vegfc-induced embryos by ISH and confirmed this increase by qPCR (normalized) using FACS-sorted ECs from vegfc-induced embryos (Fig. 5F,G). We did not find any evidence for regulation of vegfc, vegfr3, or other Vegf family downstream from mafba (Supplemental Fig. 4E–G).
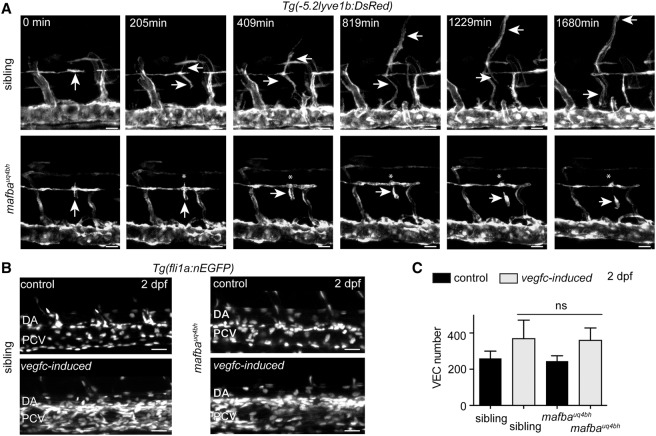
mafba regulates LEC migration from the horizontal myoseptum but is dispensable for vegfc-induced proliferation
To determine the earliest cellular defect in trunk lymphangiogenesis in mafba mutants, we time-lapse-imaged PLs in the horizontal myoseptum from 48 hpf onward in Tg(-5.2lyve1:DsRed);Tg(kdrl:EGFP) double-transgenic embryos. While wild-type PLs actively migrated out of the myoseptum and elongated along their arterial substrates (Bussmann et al. 2010; Cha et al. 2012), mutant PLs commonly failed to migrate from the myoseptum (Fig. 6A; Supplemental Movies 1, 2). Some PLs displayed distinctly broad and rounded morphology during migration, which was not observed in wild-type cells (Fig. 6A). Interestingly, we did not find any evidence for dysregulation of chemokine signaling components that control this migratory event (Cha et al. 2012) or ephrinb2a/ephB4, which have been implicated in EC migration (Supplemental Fig. 5A–C). We next examined mafba mutants in the vegfc-induced transgenic overexpression background and found that vegfc overexpression induced the proliferation of VECs at the same level in mafba mutants as in wild-type transgenic embryos (Fig. 6B,C). Together, these data show that mafba controls the ongoing migration of PLs after initial secondary angiogenesis but is not required for vegfc transgene-induced venous proliferation.
Figure 6.
mafba controls LEC migration and is dispensable for Vegfc transgene-induced proliferation. (A) Representative images from time lapse of LEC migration from sibling (n = 3) and mafbauq4bh mutant (n = 3) embryos. Visualized in Tg(lyve1:DsRed) (white) embryos from 48 hpf. See Supplemental Movies 1 and 2. (Arrows) LECs. Bars, 20 μm. (B) vegfc-induced embryos display increased VEC numbers (bottom left panel) compared with control embryos (top left panel). (Right panels) Increased VEC number is still observed in vegfc-induced mafbauq4bh mutant embryos. Tg(fli1a:nlsEGFP) (white) labels endothelial nuclei. Bars, 30 μm. (C) Quantification of VEC number in sibling (n = 4), sibling vegfc-induced (n = 5), mafbauq4bh (n = 6), and mafbauq4bh vegfc-induced (n = 6). Mean ± SEM; t-test. (ns) No significant difference.
Vegfc up-regulates mafba expression in a SoxF transcription factor-dependent manner
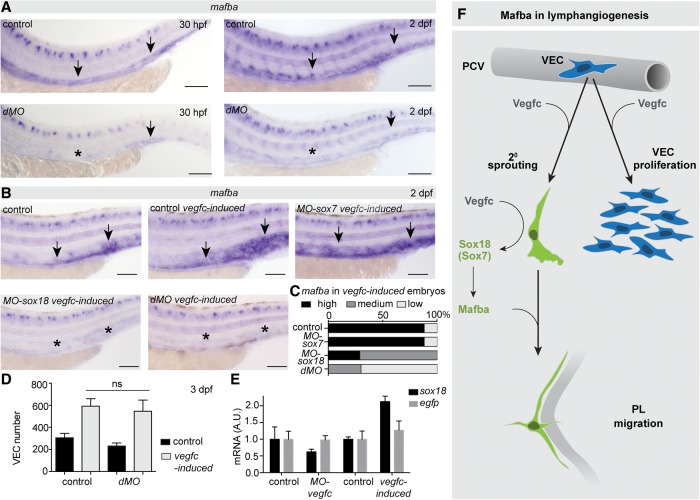
We and others have previously shown that VEGF signaling can control the activity of SOX18 in human ECs, mice, and zebrafish (Deng et al. 2013; Duong et al. 2014). To determine whether SoxF family transcription factors play a role in mafba induction, we examined mafba expression in sox7/sox18 double morpholino (dMO)-injected embryos. We found a strong reduction to complete absence of mafba expression in the PCV at 30 and 48 hpf (Fig. 7A), an unexpected observation because sox7/sox18 dMO embryos display expanded expression of classical VEC markers (Herpers et al. 2008; Pendeville et al. 2008). Given that SOXF transcription factors can respond to VEGFs and that we observed that mafba expression is also Vegfc-responsive, we next examined whether the up-regulation of mafba by Vegfc is dependent on SoxF transcription factors. We found that knockdown of sox7 alone did not have an impact on the induction of mafba by Vegfc (Fig. 7B,C). sox18 knockdown strongly reduced the intensity of induction, and the dMO knockdown further reduced the induction of mafba expression (Fig. 7B,C). Supporting this regulatory relationship in vitro, transfection of human umbilical vein ECs with SOX18 but not SOX7 induced MAFB expression (Supplemental Fig. 6A,B). Furthermore, expression of sox18 and sox7 was normal in mafbauq4bh mutants, suggesting that these genes are upstream of but not downstream from mafba (Supplemental Fig. 6D,E).
Figure 7.
mafba is downstream from Vegfc and SoxF transcription factors. (A) Expression of mafba at 30 hpf in control uninjected (n=38/41) and MO-sox18/sox7 dMO-injected (n = 24/25) embryos (left) and at 48 hpf in control uninjected (n = 30/33) and MO-sox18/sox7 dMO-injected (n = 18/18) embryos (right). Arrows indicate PCV, and an asterisk indicates reduced expression. Bars, 100 μm. (B) mafba expression at 48 hpf in control and vegfc-induced embryos as well as vegfc-induced embryos injected with MO-sox7, MO-sox18, and MO-sox18/sox7 dMO. Arrows indicate PCV expression, and asterisks indicate reduced/absent expression. Bars, 100 μm. (C) Quantification of mafba expression in embryos from E, with embryos scored as high, medium, and low mafba expressors. Control, 88%, n = 37/42, high; MO-sox7, 89%, n = 39/44, high; MO-sox18, 71%, n = 25/35, medium; dMO, 71%, n = 34/48, low. (D) Quantification of VEC number in vegfc-induced control and MO-sox18/sox7 dMO-injected embryos. Mean ± SEM. Control, n = 12; control vegfc-induced, n = 34; dMO, n = 11; dMO vegfc-induced, n = 25; t-test. (ns) No significant difference. (E) qPCR for sox18 expression in FACS-sorted zebrafish ECs from MO-vegfc, vegfc-induced, and control embryos. egfp served as a control for EC expression levels. Expression is relative to the geometric average of kdrl, cdh5, and lyve1b expression. Mean ± SEM. (F) Working model of Mafba function in lymphangiogenesis: Vegfc up-regulates mafba expression in secondary sprouts and in a Sox18-dependent (redundancy with Sox7) manner. Mafba is essential for PL migration. Vegfc controls venous proliferation independently of Mafba or Sox18/7.
Given that the transgenic overexpression of Vegfc induces proliferation of VECs, we asked whether the reduction of mafba VEC expression in vegfc-induced/MO-soxF-injected embryos was concurrent with a reduction in VEC proliferation. Importantly, Vegfc overexpression in sox7/sox18 double-knockdown embryos still induced robust VEC proliferation (Fig. 7D). This observation is strikingly in line with the fact that mafba also played no role in vegfc-induced VEC proliferation in this transgenic background. Finally, we examined sox7 and sox18 expression by qPCR on embryonic ECs FACS-sorted [using Tg(kdrl:EGFP)] from MO-vegfc and vegfc-induced embryos at 48 hpf. We found that both were mildly reduced in loss-of-function scenarios and increased in gain-of-function scenarios, where normalized expression of endothelial egfp was not changed (Fig. 7E; Supplemental Fig. 6C).
Discussion
Taken together, the observations above demonstrate that the transcription factor Mafba is essential for embryonic lymphangiogenesis during zebrafish development. In mice, MAFB has established roles in directing hindbrain segmentation and podocyte, pancreatic β-cell, and hematopoietic lineage differentiation but no known function in vascular lineages (Sieweke et al. 1996, 1997; Moens et al. 1998; Kelly et al. 2000; Sadl et al. 2002; Bakri et al. 2005; Moriguchi et al. 2006; Artner et al. 2007). Our findings of a cell-autonomous role in lymphatic vessel development, given the previously described functions in cell fate and differentiation, suggest a likely role in endothelial lineage decisions or differentiation. A function in ongoing LEC differentiation could explain the migration defect observed if critical machinery was not switched on in PLs after they initially sprout from the PCV. To fully understand how Mafba elicits such a specific phenotype in LECs, it is clear that the characterization of downstream genes and pathways is now needed.
Vegfc is the major driver of developmental lymphangiogenesis in vertebrates (Karkkainen et al. 2004). In zebrafish, Vegfc induces VEC proliferation in the PCV (Helker et al. 2013; Le Guen et al. 2014) and the sprouting of venous precursors and PLs from the PCV during secondary angiogenesis (Hogan et al. 2009a; Villefranc et al. 2013). Our observations of mafba expression and the expression of a subset of mafba-dependent genes in Vegfc loss-of-function and gain-of-function embryos indicate that mafba is responsive to Vegfc signaling during development. We showed previously that mutations in genes within the Vegfc pathway completely block the phenotypes caused by overexpression of Vegfc (Le Guen et al. 2014), yet here we observed that loss of mafba has no impact on transgene-induced proliferation while being crucial for lymphatic development. This suggests that Vegfc signaling has multiple downstream outcomes in ECs and that different cellular responses are controlled by different effectors. In line with this observation, the cellular defect in mafbauq4bh mutants occurs later than in vegfc, vegfr3, or ccbe1 mutants (Hogan et al. 2009a; Villefranc et al. 2013; Le Guen et al. 2014), with mafba controlling LEC migration from the horizontal myoseptum rather than sprouting of LEC precursors from the PCV. It will be intriguing to discover whether MAFB proteins play specialized roles regulating a discrete subset of functional genes rather than broad roles in LEC identity/transcription and determine how this compares with other transcription factors, such as PROX1, which is maintained by VEGFC signaling in mice (Srinivasan et al. 2014).
We further investigated potential mechanisms by which Vegfc might regulate the levels of mafba in zebrafish. In mice, MafB can act together with Ets1, and there is evidence that Vegfc can modulate Ets-mediated transcription (Sieweke et al. 1996; Yoshimatsu et al. 2011); however, we examined morpholino knockdown models and gene expression levels and found no evidence that Mafba acts coordinately with Ets factors in lymphatic development (Supplemental Fig. 6F–J). In mice, the transcription factor Sox18 controls lymphangiogenesis in a partially redundant manner with other SOXF transcription factors in different mouse strains (Francois et al. 2008; Hosking et al. 2009). The transcription factors Sox18 and Sox7 function redundantly during zebrafish blood vascular development, with dMO-injected embryos displaying early arterial–venous defects that are recapitulated in genetic mutants and do not allow for controlled analysis of later lymphangiogenesis (Cermenati et al. 2008; Herpers et al. 2008; Pendeville et al. 2008; Hermkens et al. 2015). Interestingly, recent work has shown that SOXF transcription factors can be up-regulated and nuclear-localized in response to VEGF/VEGFC–ERK signaling to control downstream gene expression and vascular lineage decisions (Deng et al. 2013; Duong et al. 2014). We investigated whether SOXF transcription factors could link Vegfc signaling to mafba expression. We found that Sox18 and Sox7 are necessary for normal mafba expression and further showed that they indeed mediate the observed induction of mafba by transgenic overexpression of Vegfc. Taken together, these observations led us to a working model of how this pathway functions to control lymphatic development (Fig. 7F).
The question of how cells acquire LEC identity and differentiate has led to the characterization of a number of transcriptional regulators of LEC fate and differentiation (Wigle and Oliver 1999; Francois et al. 2008; Srinivasan et al. 2010; Srinivasan and Oliver 2011). How these transcription factors combine and integrate as a functional regulatory network and how they are controlled by extrinsic signals to modulate precise spatiotemporally controlled gene expression remain to be elucidated. The addition of Mafb as a crucial regulator of lymphangiogenesis provides a new direction and increases the complexity of LEC gene regulation. Importantly, many transcriptional regulators of LEC development play central roles in lymphatic vascular diseases (Fang et al. 2000; Finegold et al. 2001; Irrthum et al. 2003; Ostergaard et al. 2011). In addition to gaining a deeper mechanistic understanding of LEC fate acquisition and differentiation, it will be interesting to determine whether MAFB transcription factors contribute to vascular pathologies in the future.
Materials and methods
Zebrafish
Animal work followed the guidelines of the animal ethics committee at the University of Queensland. The forward genetic screen was based on previous studies, with mutagenesis as described previously (de Bruijn et al. 2009). The genomic sequencing pipeline was based on previous studies (Leshchiner et al. 2012) and will be described in full elsewhere. Published zebrafish lines were Tg(fli1a:nEGFP)y7 (Lawson and Weinstein 2002), Tg(-5.2lyve1b:DsRed)nz101 (Okuda et al. 2012), TgBAC(prox1a:KalTA4-4xUAS-ADV.E1b:TagRFP)nim5 (Dunworth et al. 2014; van Impel et al. 2014), Tg(flt1:YFP)hu4624 (Hogan et al. 2009a), and Tg(kdrl:EGFP)s84 3 (Jin et al. 2005).
Transgenesis, morpholinos, genotyping, and genome editing
10xUAS:vegfc plasmid DNA was generated using the full-length zebrafish vegfc cDNA cloned into the Gateway pME vector (pDON-221) using Gateway technology (Hartley et al. 2000). To generate the Tg(10xUAS:vegfc)uq2bh strain, 20 ng/μL plasmid DNA and 25 ng/μL tol2 transposase mRNA were injected in one-cell stage embryos, and F1 founders were identified by PCR. mafba full-length cDNA was cloned into the pCS2+ vector and used as a template for riboprobe synthesis. MO-vegfr3, MO-vegfc, MO-sox7, and MO-sox18 were described previously (Herpers et al. 2008; Le Guen et al. 2014). Two morpholino against ets1 (Pham et al. 2007) were used as described previously, and two morpholinos against ets2 were designed and injected (5 ng per embryo). CRISPR genome editing for mafba was performed as described in Gagnon et al. (2014) to generate the mafbauq5bh(5-base-pair [bp] del) allele. All primer and morpholino sequence details are provided in the Supplemental Material.
Whole-mount ISH
Whole-mount ISH was performed as described previously (Kartopawiro et al. 2014) using the mafba probe (see above) generated from plasmid linearized using ClaI. Probes for vegfc (Ober et al. 2004), vegfr3 (Hogan et al. 2009b), cxcr4a and cxcl12b (Coxam et al. 2014), efnb2a (Durbin et al. 1998), ephb4a (Cooke et al. 1997), and sox18 and sox7 (Herpers et al. 2008) were used as previously described.
FACS and gene expression analysis
Isolation of zebrafish embryonic ECs, RNA extraction, cDNA preparation, and qPCR were performed as described previously (Coxam et al. 2014; Kartopawiro et al. 2014). For isolating cells from sibling and mafbauq4bh mutants, embryos were divided into the phenotypic categories based on the otic phenotype at 48 hpf. For isolating cells from vegfc-induced and siblings, embryos were divided into phenotypic categories based on vascular phenotype at 48 hpf. For MO-vegfc and uninjected control, cells were isolated at 30 hpf and 48 hpf. Primer sequences are in the Supplemental Material. RNA-seq was performed using the Illumina NextSeq500 and generated average 76-bp reads, which were then mapped to the reference genome (danRer7/Zv9). Full details on library preparation, sequencing, and analysis are in the Supplemental Material.
Immunohistochemistry
Immunohistochemistry for anti-GFP was performed according to the following protocol. Embryos were fixed in 4% PFA (paraformaldehyde) overnight and washed five times with PBST (0.1% Tween in PBS [phosphate buffered saline]). Embryos were blocked in PBDT (PBS with 1% BSA, 1% DMSO, 0.1% Triton-100) with 10% horse serum for 3 h, anti-GFP (chicken polyclonal to GFP, 1:200; ab13970) was added, and embryos were incubated overnight. Embryos were washed five times for 30 min in PBDT and then incubated in PBDT and 10% horse serum with secondary antibody (goat anti-chicken IgG, 1:400; Alexa 488; Invitrogen, A11039) and DAPI (1:1000; Sigma Aldrich) overnight. Embryos were washed five times for 30 min in PBST and imaged.
Transplantation
Transplantation was performed essentially as described previously (Hogan et al. 2009a) with the following changes. Donor embryos were injected with dextran cascade blue (10,000 MW, Invitrogen) at 5 ng/nL (1 nL per embryo). Cells from wild-type donor embryos [Tg(fli1a:EGFP)] were transplanted into host embryos derived from mafba heterozygous in-crosses [Tg(-5.2lyve1b:DsRed)]. For reciprocal transplants, mutant cells [Tg(kdrl:EGFP);Tg(-5.2lyve1b:DsRed)] were transplanted into unlabeled wild-type hosts, and donors were genotyped. Embryos with successfully transplanted ECs were cultured until 5 dpf.
Imaging and quantification
Live and fixed embryos were mounted laterally and imaged using a Zeiss LSM 710 FCS confocal microscope. All images were processed using either ImarisX64 7.70 and/or ImageJ 1.47 (National Institutes of Health) software. The number of LEC nuclei [expressing Tg(fli1a:nEGFP)] coexpressing Tg(-5.2lyve1b:DsRed) across five somites through a Z-stack was manually counted using ImageJ 1.47 (National Institutes of Health) software (Fig. 1A,C,D–G; Supplemental Fig. 1C) The number of Prox1-positive LECs expressing Tg(prox1aBAC:KalTA4-4xUAS-E1b:uncTagRFP)nim5;Tg(10xUAS:Venus) detected by α-GFP in green and costained with DAPI (to label nuclei) across five somites was manually counted using ImageJ 1.47 (National Institutes of Health) software (Fig. 1H,I). For the quantification of EC numbers shown in Figure 5, the spot tool in ImarisX64 7.70 software was used. For each sample, the Tg(fli1a:nEGFP) nuclei were selected based on fluorescence intensity, and the total number of GFP-positive nuclei (across five somites) in a full Z-stack was calculated.
Supplementary Material
Acknowledgments
Imaging was performed in the Australian Cancer Research Foundation's Dynamic Imaging Facility at the Institute for Molecular Bioscience. Sequencing was performed by the Institute for Molecular Bioscience Sequencing Facility. K.K. was supported by a Lymphatic Education and Research Network Post-doctoral Fellowship, A.K.L. was supported by a University of Queensland Post-doctoral Fellowship, B.M.H. was supported in part by an Australian Research Council Future Fellowship (FT100100165) and in part by a National Health and Medical Research Council/National Heart Foundation Career Development Fellowship (1083811), M.F. was supported by a National Health and Medical Research Council Career Development Fellowship (1011242), and K.A.S. was supported by an Australian Research Council Future Fellowship (FT110100496). J.W.A. and P.S.C. receive funding from The Ministry of Business, Innovation, and Employment; the Health Research Council of New Zealand; and the Auckland Medical Research Council. This research was supported by National Health and Medical Research Council grant 1050138 and the Cariplo Foundation.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.263210.115.
References
- Alders M, Hogan BM, Gjini E, Salehi F, Al-Gazali L, Hennekam EA, Holmberg EE, Mannens MM, Mulder MF, Offerhaus GJ, et al. 2009. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet 41: 1272–1274. [DOI] [PubMed] [Google Scholar]
- Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. 2007. MafB is required for islet β cell maturation. Proc Natl Acad Sci 104: 3853–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astin JW, Haggerty MJ, Okuda KS, Le Guen L, Misa JP, Tromp A, Hogan BM, Crosier KE, Crosier PS. 2014. Vegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sprouting. Development 141: 2680–2690. [DOI] [PubMed] [Google Scholar]
- Bakri Y, Sarrazin S, Mayer UP, Tillmanns S, Nerlov C, Boned A, Sieweke MH. 2005. Balance of MafB and PU.1 specifies alternative macrophage or dendritic cell fate. Blood 105: 2707–2716. [DOI] [PubMed] [Google Scholar]
- Bos FL, Caunt M, Peterson-Maduro J, Planas-Paz L, Kowalski J, Karpanen T, van Impel A, Tong R, Ernst JA, Korving J, et al. 2011. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ Res 109: 486–491. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Bos FL, Urasaki A, Kawakami K, Duckers HJ, Schulte-Merker S. 2010. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137: 2653–2657. [DOI] [PubMed] [Google Scholar]
- Cermenati S, Moleri S, Cimbro S, Corti P, Del Giacco L, Amodeo R, Dejana E, Koopman P, Cotelli F, Beltrame M. 2008. Sox18 and Sox7 play redundant roles in vascular development. Blood 111: 2657–2666. [DOI] [PubMed] [Google Scholar]
- Cha YR, Fujita M, Butler M, Isogai S, Kochhan E, Siekmann AF, Weinstein BM. 2012. Chemokine signaling directs trunk lymphatic network formation along the preexisting blood vasculature. Dev Cell 22: 824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell F, Kalidas K, Ostergaard P, Brice G, Homfray T, Roberts L, Bunyan DJ, Mitton S, Mansour S, Mortimer P, et al. 2010. Linkage and sequence analysis indicate that CCBE1 is mutated in recessively inherited generalised lymphatic dysplasia. Hum Genet 127: 231–241. [DOI] [PubMed] [Google Scholar]
- Cooke JE, Xu QL, Wilson SW, Holder N. 1997. Characterisation of five novel zebrafish Eph-related receptor tyrosine kinases suggests roles in patterning the neural plate. Dev Genes Evol 206: 515–531. [DOI] [PubMed] [Google Scholar]
- Coxam B, Sabine A, Bower NI, Smith KA, Pichol-Thievend C, Skoczylas R, Astin JW, Frampton E, Jaquet M, Crosier PS, et al. 2014. Pkd1 regulates lymphatic vascular morphogenesis during development. Cell Rep 7: 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxam B, Neyt C, Grassini DR, Le Guen L, Smith KA, Schulte-Merker S, Hogan BM. 2015. Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (cad) regulates Notch signaling and vascular development in zebrafish. Dev Dyn 244: 1–9. [DOI] [PubMed] [Google Scholar]
- de Bruijn E, Cuppen E, Feitsma H. 2009. Highly efficient ENU mutagenesis in zebrafish. Methods Mol Biol 546: 3–12. [DOI] [PubMed] [Google Scholar]
- Deng Y, Atri D, Eichmann A, Simons M. 2013. Endothelial ERK signaling controls lymphatic fate specification. J Clin Invest 123: 1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. 1998. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282: 946–949. [DOI] [PubMed] [Google Scholar]
- Dunworth WP, Cardona-Costa J, Bozkulak EC, Kim JD, Meadows S, Fischer JC, Wang Y, Cleaver O, Qyang Y, Ober EA, et al. 2014. Bone morphogenetic protein 2 signaling negatively modulates lymphatic development in vertebrate embryos. Circ Res 114: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong T, Koltowska K, Pichol-Thievend C, Le Guen L, Fontaine F, Smith KA, Truong V, Skoczylas R, Stacker SA, Achen MG, et al. 2014. VEGFD regulates blood vascular development by modulating SOX18 activity. Blood 123: 1102–1112. [DOI] [PubMed] [Google Scholar]
- Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. 1998. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev 12: 3096–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW. 2000. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet 67: 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold DN, Kimak MA, Lawrence EC, Levinson KL, Cherniske EM, Pober BR, Dunlap JW, Ferrell RE. 2001. Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Hum Mol Genet 10: 1185–1189. [DOI] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, et al. 2008. Sox18 induces development of the lymphatic vasculature in mice. Nature 456: 643–647. [DOI] [PubMed] [Google Scholar]
- Gagnon JA, Valen E, Thyme SB, Huang P, Ahkmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, Schier AF. 2014. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS One 9: e98186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K, Schulte D, Brice G, Simpson MA, Roukens MG, van Impel A, Connell F, Kalidas K, Jeffery S, Mortimer PS, et al. 2013. Mutation in vascular endothelial growth factor-C, a ligand for vascular endothelial growth factor receptor-3, is associated with autosomal dominant milroy-like primary lymphedema. Circ Res 112: 956–960. [DOI] [PubMed] [Google Scholar]
- Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F. 2013. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 32: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley JL, Temple GF, Brasch MA. 2000. DNA cloning using in vitro site-specific recombination. Genome Res 10: 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helker CS, Schuermann A, Karpanen T, Zeuschner D, Belting HG, Affolter M, Schulte-Merker S, Herzog W. 2013. The zebrafish common cardinal veins develop by a novel mechanism: lumen ensheathment. Development 140: 2776–2786. [DOI] [PubMed] [Google Scholar]
- Hermkens DM, van Impel A, Urasaki A, Bussmann J, Duckers HJ, Schulte-Merker S. 2015. Sox7 controls arterial specification in conjunction with hey2 and efnb2 function. Development 142: 1695–1704. [DOI] [PubMed] [Google Scholar]
- Herpers R, van de Kamp E, Duckers HJ, Schulte-Merker S. 2008. Redundant roles for sox7 and sox18 in arteriovenous specification in zebrafish. Circ Res 102: 12–15. [DOI] [PubMed] [Google Scholar]
- Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte-Merker S. 2009a. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet 41: 396–398. [DOI] [PubMed] [Google Scholar]
- Hogan BM, Herpers R, Witte M, Helotera H, Alitalo K, Duckers HJ, Schulte-Merker S. 2009b. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 136: 4001–4009. [DOI] [PubMed] [Google Scholar]
- Hosking B, Francois M, Wilhelm D, Orsenigo F, Caprini A, Svingen T, Tutt D, Davidson T, Browne C, Dejana E, et al. 2009. Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development 136: 2385–2391. [DOI] [PubMed] [Google Scholar]
- Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. 2000. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet 67: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M. 2003. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet 72: 1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. 1997. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276: 1423–1425. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppanen VM, Holopainen T, Kivela R, Ortega S, Karpanen T, Alitalo K. 2014. CCBE1 enhances lymphangiogenesis via ADAMTS3-mediated VEGF-C activation. Circulation 129: 1962–1971. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. 2005. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132: 5199–5209. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN. 2000. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet 25: 153–159. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, et al. 2001. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci 98: 12677–12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. 2004. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5: 74–80. [DOI] [PubMed] [Google Scholar]
- Kartopawiro J, Bower NI, Karnezis T, Kazenwadel J, Betterman KL, Lesieur E, Koltowska K, Astin J, Crosier P, Vermeren S, et al. 2014. Arap3 is dysregulated in a mouse model of hypotrichosis-lymphedema-telangiectasia and regulates lymphatic vascular development. Hum Mol Genet 23: 1286–1297. [DOI] [PubMed] [Google Scholar]
- Kazenwadel J, Secker GA, Liu YJ, Rosenfeld JA, Wildin RS, Cuellar-Rodriguez J, Hsu AP, Dyack S, Fernandez CV, Chong CE, et al. 2012. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood 119: 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LM, Englmeier U, Lafon I, Sieweke MH, Graf T. 2000. MafB is an inducer of monocytic differentiation. EMBO J 19: 1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltowska K, Betterman KL, Harvey NL, Hogan BM. 2013. Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development 140: 1857–1870. [DOI] [PubMed] [Google Scholar]
- Kuchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. 2006. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol 16: 1244–1248. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. 2002. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248: 307–318. [DOI] [PubMed] [Google Scholar]
- Le Guen L, Karpanen T, Schulte D, Harris NC, Koltowska K, Roukens G, Bower NI, van Impel A, Stacker SA, Achen MG, et al. 2014. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 141: 1239–1249. [DOI] [PubMed] [Google Scholar]
- Leshchiner I, Alexa K, Kelsey P, Adzhubei I, Austin-Tse CA, Cooney JD, Anderson H, King MJ, Stottmann RW, Garnaas MK, et al. 2012. Mutation mapping and identification by whole-genome sequencing. Genome Res 22: 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KC, Hosoya T, Brandt W, Ku CJ, Hosoya-Ohmura S, Camper SA, Yamamoto M, Engel JD. 2012. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J Clin Invest 122: 3705–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. 2013. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab 17: 671–684. [DOI] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, et al. 2001. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med 7: 199–205. [DOI] [PubMed] [Google Scholar]
- Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, et al. 2013. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest 123: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens CB, Prince VE. 2002. Constructing the hindbrain: insights from the zebrafish. Dev Dyn 224: 1–17. [DOI] [PubMed] [Google Scholar]
- Moens CB, Yan YL, Appel B, Force AG, Kimmel CB. 1996. valentino: a zebrafish gene required for normal hindbrain segmentation. Development 122: 3981–3990. [DOI] [PubMed] [Google Scholar]
- Moens CB, Cordes SP, Giorgianni MW, Barsh GS, Kimmel CB. 1998. Equivalence in the genetic control of hindbrain segmentation in fish and mouse. Development 125: 381–391. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, et al. 2006. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol 26: 5715–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, et al. 2009. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol 185: 439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober EA, Olofsson B, Makinen T, Jin SW, Shoji W, Koh GY, Alitalo K, Stainier DY. 2004. Vegfc is required for vascular development and endoderm morphogenesis in zebrafish. EMBO Rep 5: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda KS, Astin JW, Misa JP, Flores MV, Crosier KE, Crosier PS. 2012. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139: 2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Srinivasan RS. 2010. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development 137: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, Dafou D, Kilo T, Smithson S, Lunt P, et al. 2011. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet 43: 929–931. [DOI] [PubMed] [Google Scholar]
- Pendeville H, Winandy M, Manfroid I, Nivelles O, Motte P, Pasque V, Peers B, Struman I, Martial JA, Voz ML. 2008. Zebrafish Sox7 and Sox18 function together to control arterial-venous identity. Dev Biol 317: 405–416. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, et al. 2004. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 10: 974–981. [DOI] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. 2007. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol 303: 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadl V, Jin F, Yu J, Cui S, Holmyard D, Quaggin S, Barsh G, Cordes S. 2002. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol 249: 16–29. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Sabine A, Petrova TV. 2011. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol 193: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke MH, Tekotte H, Frampton J, Graf T. 1996. MafB is an interaction partner and repressor of Ets-1 that inhibits erythroid differentiation. Cell 85: 49–60. [DOI] [PubMed] [Google Scholar]
- Sieweke MH, Tekotte H, Frampton J, Graf T. 1997. MafB represses erythroid genes and differentiation through direct interaction with c-Ets-1. Leukemia 11(Suppl 3): 486–488. [PubMed] [Google Scholar]
- Srinivasan RS, Oliver G. 2011. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev 25: 2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, Porto MP, Lagutin O, Oliver G. 2010. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev 24: 696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, Mukatira S, Gil HJ, Nurmi H, Alitalo K, et al. 2014. The Prox1–Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev 28: 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. 2014. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14: 159–172. [DOI] [PubMed] [Google Scholar]
- van Impel A, Zhao Z, Dorien MA, Hermkens M, Roukens G, Fischer JC, Peterson-Maduro J, Duckers H, Ober EA, Ingham PW, et al. 2014. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 141: 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villefranc JA, Nicoli S, Bentley K, Jeltsch M, Zarkada G, Moore JC, Gerhardt H, Alitalo K, Lawson ND. 2013. A truncation allele in vascular endothelial growth factor c reveals distinct modes of signaling during lymphatic and vascular development. Development 140: 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778. [DOI] [PubMed] [Google Scholar]
- Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. 2006. Live imaging of lymphatic development in the zebrafish. Nat Med 12: 711–716. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu Y, Yamazaki T, Mihira H, Itoh T, Suehiro J, Yuki K, Harada K, Morikawa M, Iwata C, Minami T, et al. 2011. Ets family members induce lymphangiogenesis through physical and functional interaction with Prox1. J Cell Sci 124: 2753–2762. [DOI] [PubMed] [Google Scholar]
- Zheng W, Aspelund A, Alitalo K. 2014. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 124: 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.