Abstract
Accumulation of the antiviral nucleoside analogue fialuridine (FIAU; 1-(2'-deoxy-2'-fluoro-beta-D-arab-inofuranosyl-5-iodouracil) in genomic DNA was examined with a modified version of a recently developed RIA for FIAU. DNA was obtained from tissues of dogs administered FIAU at 0, 1, 2, or 3 mg/kg of body weight per day for 90 days, monkeys administered FIAU at 0 or 25 mg/kg per day for 30 days, and rats administered FIAU at 0, 255, or 510 mg/kg per day for 70 days. FIAU incorporation was observed in all species. In the rat, FIAU was incorporated into DNA of all tissues examined, with highest concentrations in the liver followed by jejunum, spleen, and heart. FIAU was also incorporated into sperm DNA. Incorporation rates were as high as 11,000 pmol of FIAU per mumol of thymidine or 1 FIAU molecule per 90 thymidine molecules. In dogs and rats, the extent of incorporation was dose-dependent. Across species, FIAU concentrations in DNA were not singly dependent on the total dose administered but also may have been dependent on the duration of exposure. These studies show that FIAU accumulates to high concentrations in genomic DNA of liver as well as other tissues during chronic oral administration and suggest that net accumulation of FIAU in DNA may be a critical step in FIAU-induced toxicity.
Full text
PDF

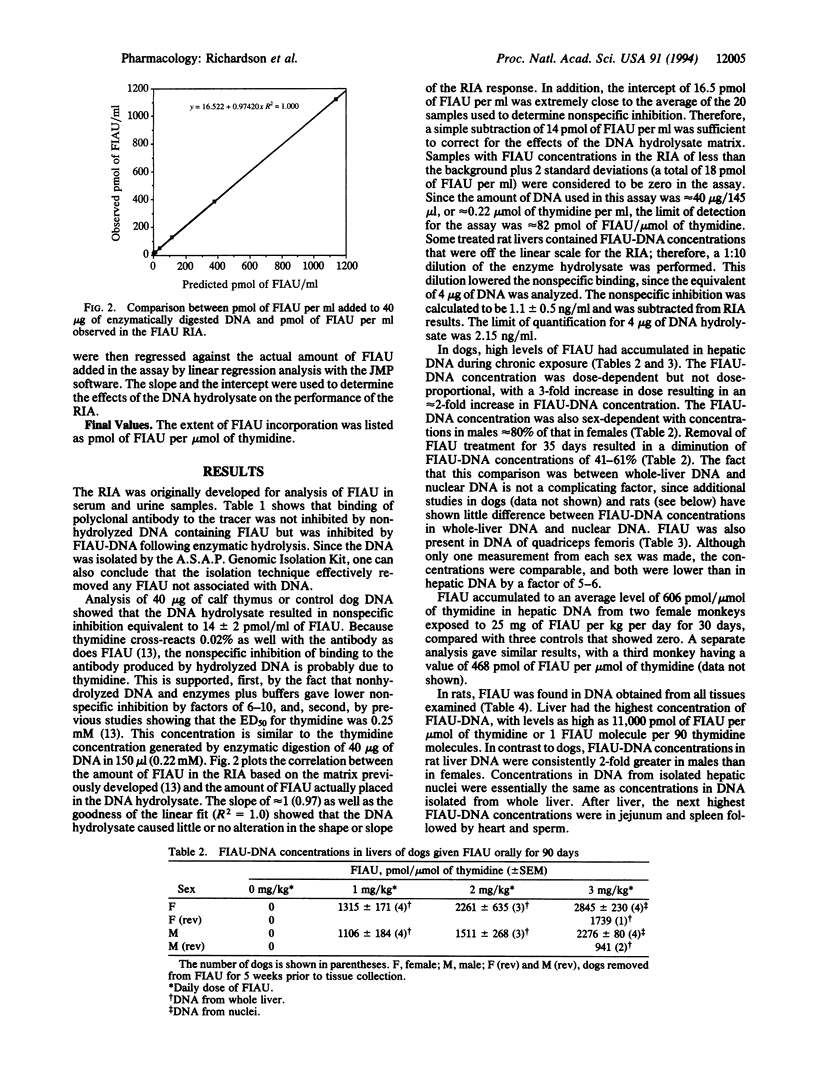

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaudo E., Dalakas M., Shanske S., Moraes C. T., DiMauro S., Schon E. A. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet. 1991 Mar 2;337(8740):508–510. doi: 10.1016/0140-6736(91)91294-5. [DOI] [PubMed] [Google Scholar]
- Bowsher R. R., Compton J. A., Kirkwood J. A., Place G. D., Jones C. D., Mabry T. E., Hyslop D. L., Hatcher B. L., DeSante K. A. Sensitive and specific radioimmunoassay for fialuridine: initial assessment of pharmacokinetics after single oral doses to healthy volunteers. Antimicrob Agents Chemother. 1994 Sep;38(9):2134–2142. doi: 10.1128/aac.38.9.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Cheng Y. C. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2',3'-dideoxycytidine. J Biol Chem. 1989 Jul 15;264(20):11934–11937. [PubMed] [Google Scholar]
- Chen M. S., Van Nostrand M., Oshana S. C. Quantitative determination of antiviral nucleoside analog in DNA. Anal Biochem. 1986 Aug 1;156(2):300–304. doi: 10.1016/0003-2697(86)90256-3. [DOI] [PubMed] [Google Scholar]
- Eacho P. I., Lanier T. L., Brodhecker C. A. Hepatocellular DNA synthesis in rats given peroxisome proliferating agents: comparison of WY-14,643 to clofibric acid, nafenopin and LY171883. Carcinogenesis. 1991 Sep;12(9):1557–1561. doi: 10.1093/carcin/12.9.1557. [DOI] [PubMed] [Google Scholar]
- Eldridge S. R., Goldsworthy T. L., Popp J. A., Butterworth B. E. Mitogenic stimulation of hepatocellular proliferation in rodents following 1,4-dichlorobenzene administration. Carcinogenesis. 1992 Mar;13(3):409–415. doi: 10.1093/carcin/13.3.409. [DOI] [PubMed] [Google Scholar]
- Fourel I., Hantz O., Cova L., Allaudeen H. S., Trepo C. Main properties of duck hepatitis B virus DNA polymerase: comparison with the human and woodchuck hepatitis B virus DNA polymerases. Antiviral Res. 1987 Nov;8(4):189–199. doi: 10.1016/0166-3542(87)90073-8. [DOI] [PubMed] [Google Scholar]
- Grant A. J., Feinberg A., Chou T. C., Watanabe K. A., Fox J. J., Philips F. S. Incorporation of metabolites of 2'-fluoro-5-iodo-1-beta-D-arabinofuranosylcytosine into deoxyribonucleic acid of neoplastic and normal mammalian tissues. Biochem Pharmacol. 1982 Mar 15;31(6):1103–1108. doi: 10.1016/0006-2952(82)90349-5. [DOI] [PubMed] [Google Scholar]
- Hantz O., Allaudeen H. S., Ooka T., De Clercq E., Trepo C. Inhibition of human and woodchuck hepatitis virus DNA polymerase by the triphosphates of acyclovir, 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-5-iodocytosine and E-5-(2-bromovinyl)-2'-deoxyuridine. Antiviral Res. 1984 Aug;4(4):187–199. doi: 10.1016/0166-3542(84)90017-2. [DOI] [PubMed] [Google Scholar]
- Huang P., Chubb S., Hertel L. W., Grindey G. B., Plunkett W. Action of 2',2'-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991 Nov 15;51(22):6110–6117. [PubMed] [Google Scholar]
- Huang P., Chubb S., Plunkett W. Termination of DNA synthesis by 9-beta-D-arabinofuranosyl-2-fluoroadenine. A mechanism for cytotoxicity. J Biol Chem. 1990 Sep 25;265(27):16617–16625. [PubMed] [Google Scholar]
- Korba B. E., Gerin J. L. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antiviral Res. 1992 Jul 1;19(1):55–70. doi: 10.1016/0166-3542(92)90056-b. [DOI] [PubMed] [Google Scholar]
- Kufe D. W., Major P. P., Egan E. M., Beardsley G. P. Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem. 1980 Oct 10;255(19):8997–8900. [PubMed] [Google Scholar]
- Lewis L. D., Hamzeh F. M., Lietman P. S. Ultrastructural changes associated with reduced mitochondrial DNA and impaired mitochondrial function in the presence of 2'3'-dideoxycytidine. Antimicrob Agents Chemother. 1992 Sep;36(9):2061–2065. doi: 10.1128/aac.36.9.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W., Gonzalez B., Chomyn A., Papoian T. Zidovudine induces molecular, biochemical, and ultrastructural changes in rat skeletal muscle mitochondria. J Clin Invest. 1992 Apr;89(4):1354–1360. doi: 10.1172/JCI115722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macilwain C. NIH, FDA seek lessons from hepatitis B drug trial deaths. Nature. 1993 Jul 22;364(6435):275–275. doi: 10.1038/364275d0. [DOI] [PubMed] [Google Scholar]
- Oberg B., Johansson N. G. The relative merits and drawbacks of new nucleoside analogues with clinical potential. J Antimicrob Chemother. 1984 Aug;14 (Suppl A):5–26. doi: 10.1093/jac/14.suppl_a.5. [DOI] [PubMed] [Google Scholar]
- Phillips M. D., Nascimbeni B., Tice R. R., Shelby M. D. Induction of micronuclei in mouse bone marrow cells: an evaluation of nucleoside analogues used in the treatment of AIDS. Environ Mol Mutagen. 1991;18(3):168–183. doi: 10.1002/em.2850180305. [DOI] [PubMed] [Google Scholar]
- Richardson F. C., Copple D. M., Eacho P. I. Effects of methapyrilene on DNA synthesis in mice and rats following continuous dietary exposure. Carcinogenesis. 1992 Dec;13(12):2453–2457. doi: 10.1093/carcin/13.12.2453. [DOI] [PubMed] [Google Scholar]
- Ruiz van Haperen V. W., Veerman G., Vermorken J. B., Peters G. J. 2',2'-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochem Pharmacol. 1993 Aug 17;46(4):762–766. doi: 10.1016/0006-2952(93)90566-f. [DOI] [PubMed] [Google Scholar]
- Schy W. E., Hertel L. W., Kroin J. S., Bloom L. B., Goodman M. F., Richardson F. C. Effect of a template-located 2',2'-difluorodeoxycytidine on the kinetics and fidelity of base insertion by Klenow (3'-->5'exonuclease-) fragment. Cancer Res. 1993 Oct 1;53(19):4582–4587. [PubMed] [Google Scholar]
- Staschke K. A., Colacino J. M., Mabry T. E., Jones C. D. The in vitro anti-hepatitis B virus activity of FIAU [1-(2'-deoxy-2'-fluoro-1-beta-D-arabinofuranosyl-5-iodo)uracil] is selective, reversible, and determined, at least in part, by the host cell. Antiviral Res. 1994 Jan;23(1):45–61. doi: 10.1016/0166-3542(94)90032-9. [DOI] [PubMed] [Google Scholar]



