Abstract
Meeting the complex physiological demands of mammalian life requires strict control of the metabolism of long-chain fatty acyl-CoAs because of the multiplicity of their cellular functions. Acyl-CoAs are substrates for energy production; stored within lipid droplets as triacylglycerol, cholesterol esters, and retinol esters; esterified to form membrane phospholipids; or used to activate transcriptional and signaling pathways. Indirect evidence suggests that acyl-CoAs do not wander freely within cells, but instead, are channeled into specific pathways. In this review, we will discuss the evidence for acyl-CoA compartmentalization, highlight the key modes of acyl-CoA regulation, and diagram potential mechanisms for controlling acyl-CoA partitioning.
Keywords: acyltransferase, fatty acid metabolism, fatty acid oxidation, phospholipid, triacylglycerol, acyl-CoA, thermogenesis, thioesterase
Introduction
Two prevailing views of metabolic pathways within the cytosol are a) as sequential steps within a largely empty space with substrates and products traveling from one enzyme to the next, and b), as embedded within a dense network of proteins and metabolites, all jockeying for position. In contrast to these two views, it is likely that synthetic and degradative pathways are composed of enzymes and their regulators in highly organized multi-enzyme assemblies designed to enhance efficiency and regulate steady-state flux (1). Proteins within these assemblies might interact via their transmembrane and extra-membrane domains or be tethered to scaffolds, in a manner that is regulated by allosteric effectors and post-translational modifications (2, 3). Such interactions may be transient, as occurs with the enzymes that comprise purinosomes (4), or they may be semi-permanent as occurs within glycogen granules that contain enzymes that synthesize and degrade glycogen, and their regulatory kinases and phosphatases (5, 6). Assemblies of proteins are advantageous because even without a physical tunnel, they can increase reaction rates by enhancing local substrate concentrations, restricting intermediates from entering competing reactions, and stabilizing chemically unstable intermediates.
Because the dysregulation of metabolic homeostasis has been implicated in a multitude of human diseases, acyl-CoA metabolism represents a critical node for understanding whole-body pathophysiology. Apart from eicosanoid synthesis, the first step in the metabolism of long-chain fatty acids (FAs)2 is their thioesterification. The resulting acyl-CoA is then metabolized by one of six major enzyme families: elongases and desaturases (7), dehydrogenases (8, 9), acyl-CoA thioesterases (10, 11), carnitine palmitoyltransferases (CPT) (12), and lipid and protein acyltransferases (13) (Fig. 1). These competing pathways, often present within a single subcellular compartment and coupled with the highly specialized metabolism of individual cells and tissues, suggest a level of organization extending beyond enzymatic function. In this review, we will use a physiological lens to focus on long-chain mammalian fatty acyl-CoAs and the evidence for compartmentalized acyl-CoA metabolism.
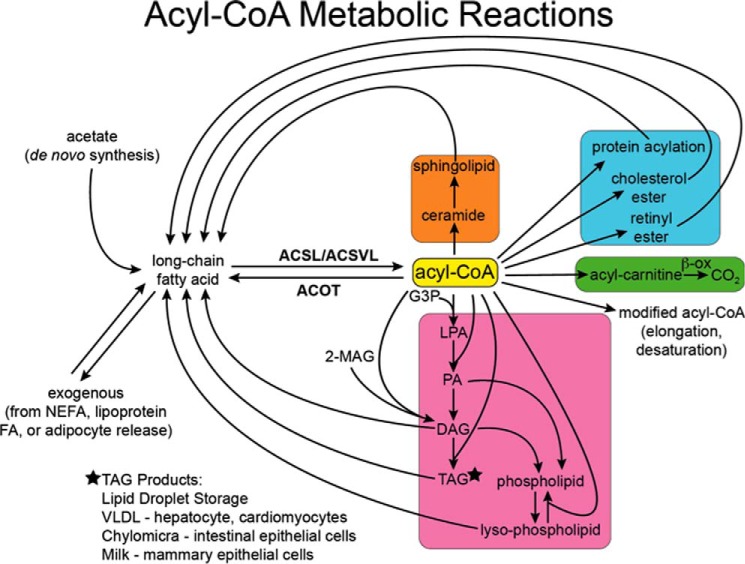
FIGURE 1.
Metabolic reactions of acyl-CoAs. Long-chain FAs are synthesized de novo from acetate or enter cells from the plasma. They are converted to acyl-CoAs by ACSL and ACSVL. The reaction is reversed by acyl-CoA thioesterases (ACOT). Acyl-CoAs can be elongated and desaturated, converted to acylcarnitines, and metabolized to CO2 via mitochondrial and peroxisomal enzymes, esterified to glycerol-3-phosphate to form lysophosphatidic acid (LPA), phosphatidic acid (PA), and TAG, and esterified to monoacylglycerol (MAG) to form diacylglycerol (DAG). Both phosphatidic acid and diacylglycerol are precursors for all the glycerophospholipids. Acyl-CoAs are also esterified to retinol and cholesterol, acylated to proteins, and incorporated into ceramide to form sphingolipids. Lipolysis of these products releases fatty acids back into cellular pools. Triacylglycerol, cholesterol esters, and retinol esters are stored in lipid droplets within cells or secreted from specialized cells as lipoproteins or milk constituents. NEFA, non-esterified fatty acid.
Metabolism of Fatty Acids and Acyl-CoAs
Many pathways rely on enzymes that contain membrane-spanning domains or are tightly associated with membranes. Plasma membrane, endoplasmic reticulum (ER), and Golgi are sites of protein acylation (14), and the ER provides the site for FA ω-oxidation, for the elongation and desaturation of FAs, for the synthesis of retinol esters, cholesterol esters, and most glycerol- and sphingophospholipids, and for the synthesis of triacylglycerol (TAG). The initial substrate for each of these pathways is a long-chain acyl-CoA, the product of one of 13 long-chain or very-long-chain acyl-CoA synthetases (ACSL, ACSVL/FATP, ACS bubblegum (ACSBg)) that activate the FAs of 14–26 carbons (15). The metabolism of acyl-CoAs within cells appears to be highly controlled, and the movement of the acyl-CoAs themselves seems to be compartmentalized. How this occurs remains obscure.
Acyl-CoAs are amphipathic molecules with critical micellar concentrations of 5–42 μm, depending on chain length and degree of unsaturation (16, 17). However, acyl-CoAs are unlikely to self-aggregate because, within cells, they are bound to proteins and membranes. Because the effective free concentration of acyl-CoAs within the cell is estimated to be substantially lower than 200 nm, and because most proteins that have been found to interact with acyl-CoAs in in vitro experiments have specific binding affinities in the μm range, it has been suggested that acyl-CoAs may not be effective modulators of these proteins (18). However, the intracellular concentration of acyl-CoAs, which is measured as between 5 and 160 μm (18), is probably an inaccurate representation of the concentrations within specific membrane domains or near the active sites of metabolic enzymes.
Is Acyl-CoA Metabolism Compartmentalized?
Several independent lines of data support the hypothesis that FAs and acyl-CoAs are compartmentalized within cells and trafficked into specific pathways. Although each is indirect, taken as a whole, the concept is compelling.
Vectorial Acylation
Exogenous FA uptake into cells is enhanced by vectorial acylation, a process that traps entering FAs by converting them into acyl-CoAs, which cannot exit the cell (19, 20), and by downstream metabolic pathways that release the CoA and allow it to be recycled for additional acyl-CoA synthesis (21). The cellular locations of the acyl-CoA synthetases are not critical for FA uptake. Thus, ACSLs and ACSs designated as FA transport proteins (FATPs) enhance FA uptake even when they are located on intracellular membranes (22). An example is provided by similar uptake of arachidonate by each of two differentially spliced variants of ACSL4, one located on the plasma membrane and in the cytosol and the other located on the ER and lipid droplets (23). Overexpressing ACSL4 at the ER generated 50% more phosphatidylinositol than when it was targeted to the mitochondria, compatible with a specific interaction with ER enzymes that synthesize or remodel phosphatidylinositol. These data provide compelling evidence for compartmentalization, as subcellular location of a specific ACSL isoform may be important for the interaction of FA substrates with a specific metabolic pathway.
Many downstream proteins that metabolize acyl-CoAs have a well defined cellular distribution. CPT1, for example, is expressed on the outer mitochondrial membrane as part of the machinery to transport activated long-chain acyl-CoAs into the mitochondrial matrix for β-oxidation (24), and the major esterification pathways that use acyl-CoAs are predominantly located on the ER. However, the location of several putative intrinsic membrane enzymes may not be static. For example, incubating Drosophila S2 cells with oleate causes the ER glycerol-3-phosphate acyltransferase (GPAT) to translocate to newly formed lipid droplets (25), suggesting that GPAT domains formerly believed to constitute transmembrane sequences, can instead integrate within the phospholipid monolayer that surrounds the lipid droplet. With few exceptions, FA channeling has not been rigorously evaluated. ACSL1 and CPT1 interact at the mitochondrial outer membrane, an association that ensures compartmentalized FA channeling into the mitochondrial matrix (26); the interaction of diacylglycerol acyltransferase (DGAT)-2 and monoacylglycerol acyltransferase-2 within a large protein complex via the transmembrane domains of DGAT2 should enable cells to efficiently process acyl-CoAs within the cytosol (27); and recent co-immunoprecipitation studies have identified proteins in the pathway of TAG synthesis that interact with the ER protein seipin (28).
Evidence from Thermogenesis
FA oxidation in brown adipose is impaired by deficiencies in several proteins and enzymes that provide a view of what a multi-protein metabolic cluster might encompass. In addition to the mitochondrial enzymes of β-oxidation, thermogenesis requires proteins involved in TAG lipolysis and FA transport and activation (Fig. 2). These proteins include adipose triglyceride lipase (ATGL), a lipid droplet-associated lipase that releases FAs (29), palmitoyl-protein thioesterase-1, which diminishes TAG lipolysis (30), FA-binding protein (FABP)-3, a FA carrier (31, 32), and ACSL1 (33) and FATP1/ACSVL4 (34), which are FA activators. The block in thermogenesis caused by the absence of any one of these proteins strongly suggests that they form an assembly in brown adipose that metabolizes a pool of FAs that originate in lipid droplets and are specifically targeted to CPT1 for β-oxidation. In addition, the thioesterase, Them2, and its binding partner phosphatidylcholine transfer protein, enhance thermogenesis, suggesting that these, too, are members of the thermogenic cluster (35).
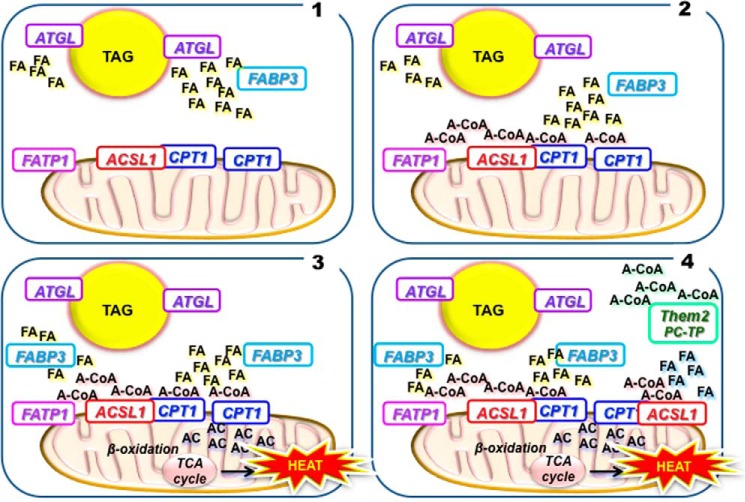
FIGURE 2.
A hypothetical assembly of proteins, substrates, and products that are critical for thermogenesis in brown adipocytes. Mice are unable to maintain a normal body temperature if they lack ATGL, FABP3, FATP1, and ACSL1 or are heterozygotes for CPT1. 1) ATGL hydrolyzes FA from TAG in lipid droplets. 2) FABP3 binds these released FAs and transports them to FATP1 and ACSL1, which convert them to acyl-CoAs (A-CoA). 3) These acyl-CoAs are substrates for CPT1, which converts them to acylcarnitines (AC) that enter the mitochondrial matrix and are metabolized by β-oxidation to produce heat. 4) Thioesterase superfamily member-2 (Them2) and its partner phosphatidylcholine transfer protein (PC-TP) improve the thermogenic process by converting acyl-CoAs, perhaps those formed in other locations, back to fatty acids that can be reactivated by FATP1 and ACSL1 located at the mitochondrial membrane. TCA, tricarboxylic acid cycle.
Metabolism of de Novo Synthesized FA
Studies of mice deficient in acyl-CoA synthetases suggest that acyl-CoAs may be sequentially synthesized, hydrolyzed, and resynthesized, perhaps as they are targeted to different organelles within cells. For example, we can construct a sequence beginning with the insulin-induced de novo synthesis of 16:0 within the cytosol, followed by conversion to 16:0-CoA by an ACSL and then esterification to form lysophosphatidic acid by GPAT1, which is embedded in the outer mitochondrial membrane and prefers saturated FAs that have been synthesized de novo (36). In GPAT1-deficient mice, FA oxidation increases, suggesting that GPAT1 and CPT1 compete at the mitochondrial membrane for a specific pool of acyl-CoAs (37). Regulation occurs via SREBP1- and carbohydrate-responsive element-binding protein (ChREBP)-mediated up-regulation of enzymes of FA synthesis and GPAT1 (13), so that when acyl-CoAs are the product of de novo biosynthesis, their capture by GPAT1 prevents newly synthesized FAs from subsequent β-oxidation.
In contrast to this evidence for intracellular FA compartmentalization, dietary triolein supplementation restores lipid accumulation in stearoyl-CoA desaturase 1 (SCD1)-deficient liver (38). However, because dietary triolein does not correct the phenotype in global SCD1 deficiency, exogenous and endogenous oleate pools may be metabolized differently in extrahepatic tissues.
ACSL1 Knock-out Mice
Studies of ACSL1 knock-out mice provide the most persuasive argument for compartmentalized acyl-CoA metabolism. In liver, ACSL1 is equally distributed on the ER and the mitochondrial outer membrane. Because it comprises only ∼50% of the total ACS activity in either location, its absence results in minor decreases in TAG synthesis and FA oxidation (39). In contrast, in highly oxidative tissues, including heart, skeletal muscle, and brown adipose, ACSL1 constitutes 90% of total ACS activity and its absence severely diminishes FA oxidation, although the remaining ACSL isoforms are sufficient for normal TAG and membrane phospholipid biosynthesis (33, 40, 41). One might reason that when ACSL1 is absent, FA oxidation would be negligible, merely because less exogenous FA enters the cells. Arguing against this interpretation, however, are data from mice deficient in skeletal muscle ACSL1 (41). Because FAs cannot be used for fuel, these mice can run only half as far as their wild type littermates before becoming hypoglycemic. Surprisingly however, their muscle content of long-chain acyl-CoA increases 10-fold during exercise and at exhaustion is 89% higher than controls. The low muscle content of long-chain acylcarnitines confirms that the large amount of acyl-CoA synthesized by other ACSL isoforms in ACSL1-deficient muscle is metabolically unavailable for β-oxidation.
Similar to skeletal muscle, ACSL1 is the predominant ACS in heart. Genetic deletion of ACSL1 results in a 97% decrease in total long-chain acyl-CoAs (40). This decrease results in a nearly complete inhibition of mitochondrial FA oxidation, although membrane phospholipid and TAG synthesis remain intact. Again, it appears that mitochondrial ACSL1 is essential in the heart to partition FA toward oxidation. ACSL1 functions similarly in adipose tissue (33). When mice are challenged with cold exposure, brown adipose FAs cannot be mobilized from the TAG stored in lipid droplets and are unavailable for mitochondrial oxidation and thermogenesis, and even in white adipose, the reduced β-oxidation results in preferential partitioning of FA into TAG stores (33).
ACSL Subcellular Location
Intracellular FAs originate from three primary sources, exogenous FAs that enter cells from the blood or from the gut lumen, FAs that arise via de novo synthesis from acetate, and FAs that are released within the cell by the hydrolysis of acylated proteins, phospholipids, and TAG. Indirect data suggest that FAs originating from each source comprise distinct intracellular pools and are preferentially activated by specific acyl-CoA synthetases and directed into specific metabolic pathways. For example, FAs derived from de novo synthesis versus exogenous sources are metabolized differently by GPAT isoforms located on the mitochondria or the ER (36) and even by the two DGAT isoforms, located on the ER or on lipid droplets (42–44).
Although subcellular location ought to critically define aspects of acyl-CoA partitioning, studies of the ACSL isoforms have been problematic. Subcellular fractionation frequently results in contamination of specific organelles with other cellular constituents. For example, several ACSL isoforms have been identified by proteomic studies of lipid droplets, but it remains unclear as to whether discrepancies in these reports reflect stable or transient associations of a particular ACSL on the lipid droplet or, instead, contamination with fragments of associated ER (45). Confocal studies are superior in that the normal cell structure is not disrupted, but they depend on highly specific antibodies to native proteins, and may not identify proteins expressed at low amounts in some organelles. Overexpression studies, particularly with tagged proteins, may not reflect the endogenous location or interactions with other proteins. The reported interaction of FATP1 and ACSL1 (46), for example, should be re-examined using a confocal method. Massively overexpressing a protein may result in ER retention and may not mirror the location of the native protein.
In addition to technical limitations in assigning location, the individual ACSL isoforms appear to move in response to changes in physiologic stimuli. Similar to studies of GPAT4 (25), treatment with exogenous FA causes tagged ACSL3 to move from the ER to newly forming lipid droplets (47), and a knockdown of Acsl3 in hepatocytes reduces de novo FA synthesis (48, 49), suggesting that ACSL3 is involved in the flux of acyl-CoAs toward storage. Treatment with insulin stimulates FATP1 translocation from the ER to the plasma membrane and increases the intracellular content of long-chain FA.
In addition to physiologic changes, the location of ACSL isoforms may be tissue-dependent, but few studies have reported on both location and function. Thus, the presence of ACSL5 on liver mitochondria (50) suggested that, like ACSL1, ACSL5 would target FA toward β-oxidation. However, although overexpressed ACSL5 in McArdle-RH7777 cells was located on both mitochondria and ER, oleate incorporation increased only into TAG and not into oxidation products (51). In support of these data, the siRNA knockdown of ACSL5 in rat primary hepatocytes decreased oleate-mediated TAG synthesis and lipid droplet formation (52).
Nutritional Regulation
To maintain energy homeostasis, cells must adapt rapidly to the available nutrients. Switching between glucose and FAs (53) is regulated by the cellular level of malonyl-CoA, which inhibits CPT-I, thereby blocking the mitochondrial entry and oxidation of long-chain fatty acyl-CoAs, which are then available for esterification into glycerolipids. During fasting, rising cellular AMP levels activate AMP-activated protein kinase, which enhances ATP-generating pathways that diminish malonyl-CoA production, thereby relieving the inhibition of CPT-I. In addition, fasting induces TAG lipolysis, and the released FAs activate the deacetylase, SIRT1, and the PPAR co-activator, PGC1α, to increase mitochondrial biogenesis (54). Thus, cytosolic metabolites control the metabolic fate of the acyl-CoAs.
Nutrient status similarly controls the transcription of ACSL isoforms. In rat liver, fasting increases and refeeding decreases the mRNA abundance of Acsl1 and Acsl4, whereas the expression of Acsl5 mRNA responds in the opposite manner (55). However, even these changes can be altered by dietary nutrients, so that fasted rats that are refed with a high-fat (20% soybean oil) diet increase Acsl1 mRNA expression in liver (56).
Transcriptional regulation of ACSL isoform mRNA expression depends on activated PPARs. In heart, liver, and skeletal muscle, PPARα activation increases the transcription of Acsl1 (57, 58), but in adipocytes, the transcription of Acsl1 is enhanced by PPARγ (59), Acsl4 transcription in liver is also increased by PPARβ/δ activation (60). Although synthetic ligands were used to activate the PPAR isoforms, these studies suggest that, as PPAR ligands, endogenous and exogenous FAs or acyl-CoAs may themselves alter ACSL isoform expression and activity.
Although in vitro experiments must be interpreted cautiously, FAs appear to control ACSL expression, perhaps via PPAR activation. Exogenous oleate added to rat cardiomyocytes increases Acsl1 expression (57), and in rat insulinoma cells or mouse hepatocytes, polyunsaturated FAs, but not saturated or monounsaturated FAs, reduce both Acsl4 mRNA and protein expression (61, 62). Polyunsaturated FAs also increase ACSL4 protein degradation via a ubiquitination-dependent, proteasomal degradative pathway (62). In addition to promoting changes in subcellular location, insulin increases Acsl1 and Acsl5 expression in cultured hepatoma cells (63), Acsl1 and Acsl3 expression in rat cardiomyocytes, and Acsl6 expression in cardiomyocytes (57). It is not known whether the inductive effect of insulin on the ACSL isoforms is mediated by SREBP. These insulin exposure studies appear to be at odds with fasting studies in which one would expect that insulin levels would be relatively low.
Polyunsaturated FAs are natural ligands for nuclear transcription factors such as the PPARs, liver X receptors (LXRs), HNF-4α, and SREBPs 1 and 2, but their chain length, degrees of unsaturation, and source (exogenous versus de novo) have differential effects on the gene regulation. For example, hepatic PPARα binds oleate, arachidonate, and docosahexaenoic acid with near equal affinity (64); however, in rat hepatocytes, only docosahexaenoic acid activates PPARα (65). These observations suggest that the metabolism of specific FA species is highly organized, supporting the hypothesis that acyl-CoAs are compartmentalized.
ACSL Substrate Preference
The Yamamoto group (66), which cloned the five rat ACSL isoforms, used a coupled spectrophotometric assay to identify the FA preferences of purified recombinant rat isoforms. Although all isoforms activated saturated and unsaturated FAs of 16–20 carbons, ACSL6 (originally called ACSL2) had higher activities with 20:4ω6, 20:5ω3, and 22:6ω3, suggesting that it might be involved in the metabolism of brain phospholipids, which have a high content of polyunsaturated FAs, or in eicosanoid function (67, 68). In fact, in differentiating PC12 cells, ACSL6 enhances both the uptake of 22:6 and neurite outgrowth (69, 70). ACSL4 has a reported preference for arachidonate, and as might be expected, the incorporation and the secretion of eicosanoids and arachidonate are diminished when ACSL4 is knocked down in INS832/13 (61), 3Y1 rat fibroblast (71), and activated hepatic stellate cells (HSC) (72). However, the published substrate preference for each ACSL family member is not invariably observed. For example, although the purified recombinant ACSL1 had no preference for arachidonate or linoleate, ACSL1 is critical for the synthesis of arachidonoyl-CoA in macrophages (73) and was inferred to prefer linoleate in the heart (74). Studies performed in different cell lines also reveal disparate effects of ACSL4 deficiency on arachidonic acid metabolism for phospholipid synthesis and prostaglandin secretion. In 3Y1 and HSC cells, siRNA-mediated knockdown of ACSL4 reduces the incorporation of arachidonic acid into phosphatidylcholine and dramatically decreases the secretion of prostaglandins, but in INS cells, the deficiency of ACSL4 has no effect on the incorporation of arachidonic acid into PC. Instead, the knockdown of ACSL4 in INS cells results in increased unesterified epoxyeicosatrienoic acids because of diminished activation and esterification into glycerolipids.
Most members of the closely related FATP family (also called ACSVL for ACS very-long-chain) can use FAs of 16–24 carbons (75). Because each tissue expresses several ACSL and FATP/ACSVL isoforms, the location of the isoform might direct a particular chain length FA into a specific pathway. Although not experimentally demonstrated, products synthesized by the same acyltransferase, but that differ in FA composition, suggest that a specific ACSL isoform must provide the esterifying enzyme with larger amounts of specific acyl-CoA substrates. Such products include the overwhelming predominance of cholesteryl oleate in liver and LDL (76) and the predominance of cholesteryl adrenate (22:4ω6) and cholesteryl arachidonate (20:4 ω6) in rat adrenal cortex (77). Because the substrate preference of ACAT1, which is present in rat adrenal cortex (76), is oleate, whereas ACAT2 esterifies 18:1, 20:5, and 22:5 equally (78), the composition of tissue cholesteryl esters seems more aligned with the ACSL isoforms than the ACAT isoforms present in each tissue. Similarly, 77% of cardiolipin species in heart and skeletal muscle, in which ACSL1 contributes more than 90% of total ACSL activity, are tetra-18:2 (79), whereas tetralinoleoyl-cardiolipin is ∼50% of the cardiolipin species in liver (69), a tissue in which ACSL1 is responsible for only about 50% of the total ACSL activity (39). In brain, where ACSL1 is minimally expressed (55), the major cardiolipin acyl chains are oleate and arachidonate (80).
PC biosynthesis has also been linked to ACSL3 activity; in human hepatoma Huh7 cells, siRNA-mediated knockdown of Acsl3 diminishes oleate incorporation into PC and reduces VLDL secretion (81). Thus, ACSL3 may activate FAs destined for the synthesis of the phosphatidylcholine that is essential for VLDL secretion (81). Taken together, these findings demonstrate that, depending upon cell type, different ACSL isoforms activate specific FAs that can be incorporated into a variety of glycerolipids. Furthermore, although each ACSL isoform may have a specific substrate preference, the function of each ACSL isoform seems to vary in different cell types.
ACSL Isoforms and Signaling Acyl-CoAs
Lipolysis-derived FAs and acyl-CoAs have been implicated as essential intracellular signaling metabolites (82). As signaling molecules, acyl-CoAs link the nutritional environment to transcription factors capable of reorganizing cellular metabolism to ensure an adequate response to changes in nutrient availability and to maximize metabolic efficiency (83). For example, ACSL3 appears to promote de novo lipogenesis and storage of neutral lipid because the siRNA knockdown of Acsl3 in primary rat hepatocytes also diminishes the expression of PPARγ, carbohydrate-responsive element-binding protein, SREBP1c, and liver X receptor α as well as the expression of the target genes of these transcription factors (48).
Specific pools of FAs or acyl-CoAs are critical in the heart. In hearts deficient in ATGL, the impaired release of FAs from stored TAG results in low expression of PPARα target genes, inadequate FA oxidation, TAG accumulation, and heart failure (84–87). Because treating mice with fibrates increased FA oxidation, reduced TAG stores, and improved heart function, it appeared that the ATGL deficiency had reduced the availability of FAs and/or acyl-CoA ligands required to activate PPARα; thus, despite high levels of acyl-CoAs that partitioned into neutral lipids, exogenously derived FAs were not PPARα ligands (86). A competing interpretation suggests that the effect of ATGL-derived FAs on PPARα is mediated indirectly by SIRT1 (54), but the data from both studies are consistent with transcriptional signaling by distinct pools of FA and acyl-CoAs originating from a specific endogenous source.
Mechanisms, Possibilities, and Unanswered Questions
The wide range of functions initiated by each ACSL isoform suggests three major problems. First, the different effects observed in cell lines of similar origin suggest that analyzing function in the relevant primary cells would provide the most reliable and conclusive information. Second, in addition to mislocalizing proteins, overexpression studies can overwhelm downstream metabolic pathways, resulting in a misleading interpretation of function (88). For example, when ACSL1 is overexpressed in primary hepatocytes, the protein localizes to the ER and increases oleate re-esterification into diacylglycerol and phospholipids (89). In contrast, the liver-specific knock-out of ACSL1 disrupts TAG synthesis and oxidation only mildly (39, 89). Thirdly, the disparate physiological effects observed in different tissues when ACSL1 is absent suggest that protein-protein interactions between ACSL isoforms and downstream metabolic enzymes vary considerably in tissues that have specialized physiological functions. Thus, differing strongly from the FA storage and degradation pathways affected in ACSL1-deficient liver, adipose, skeletal muscle, or heart, ACSL1 in macrophages predominantly mediates inflammatory effects and channels FAs toward the synthesis of phospholipid (90, 91), whereas ACSL1 in endothelial cells is unrelated to inflammatory changes associated with dietary fat (92). Clearly, a better understanding of the locations of each of the ACSLs and their downstream partners is needed.
Important questions remain concerning the trafficking of FAs and acyl-CoAs. We do not know how exogenous FAs are transported from the cytosolic face of the plasma membrane to ACSL isoforms that are located on intracellular membranes. Interaction with the intracellular acyl-CoA-binding proteins or FABP might direct FAs and acyl-CoAs to specific sites within cells. Although directional transport by these binding proteins has been inferred, it has not been demonstrated (93). For example, the absence of FABP4 diminishes PPAR activation by FA (94), and FABP4 enhances lipase activity by interacting with hormone-sensitive lipase (HSL) to bind released FAs (95). Although the absence of FABP and acyl-CoA-binding proteins alters lipid metabolism, the function of these carrier proteins may be more analogous to that of albumin in allowing FAs and acyl-CoAs to be used by enzymes or to bind to transcription factors in a controlled manner (96).
Although indirect data imply the existence of organized assemblies of enzymes that metabolize acyl-CoAs, definitive proof is absent. Newer methods, both physical and in silico, must be sought to identify protein partners and interactions without the potential hazards of overexpression (97–99). We also lack any understanding of why the up-regulation of β-oxidation not only increases the expression of Acsl1, but, counterproductively, also increases the expression of thioesterases such as Them2 and its partners (35). It is unclear as to how protein assemblies form in response to specific nutrient or energy signals. If proteins associate with each other or with scaffolding proteins through the interactions of specific domains, what then causes them to disperse? Phosphorylation and acetylation of ACSL1 are modified hormonally on numerous sites (100), but the functions of these post-translational modifications are unknown. The answers to these questions demand new tools to identify the movements of lipids within cells and new methods to capture transient protein assemblies and to manipulate them.
Author Contributions
D. E. C., P. A. Y., E. L. K., and R. A. C. designed, drafted, revised, and approved the final version of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK56598 and DK59935 (to R. A. C.) and K08 DK090141 (to E. L. K.). The authors declare that they have no conflicts of interest with the contents of this article.
- FA
- fatty acid
- FATP
- FA transport protein
- FABP
- FA-binding protein
- ACS
- acyl-CoA synthetase
- ACSL
- ACS long-chain
- ACSVL
- ACS very-long-chain
- ATGL
- adipose triglyceride lipase
- CPT
- carnitine palmitoyltransferase
- DGAT
- diacylglycerol acyltransferase
- ER
- endoplasmic reticulum
- GPAT
- glycerol-3-phosphate acyltransferase
- TAG
- triacylglycerol
- SREBP
- sterol regulatory element-binding protein
- PPAR
- peroxisome proliferator-activated receptor
- PC
- phosphatidylcholine
- ACAT
- acyl-CoA acyltransferase.
References
- 1. Castellana M., Wilson M. Z., Xu Y., Joshi P., Cristea I. M., Rabinowitz J. D., Gitai Z., Wingreen N. S. (2014) Enzyme clustering accelerates processing of intermediates through metabolic channeling. Nat. Biotechnol. 32, 1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ovádi J., Saks V. (2004) On the origin of intracellular compartmentation and organized metabolic systems. Mol. Cell Biochem. 256–257, 5–12, 10.1023/B:MCBI.0000009855.14648.2c [DOI] [PubMed] [Google Scholar]
- 3. Ovádi J., Srere P. A. (2000) Macromolecular compartmentation and channeling. Int. Rev. Cytol. 192, 255–280 [DOI] [PubMed] [Google Scholar]
- 4. Zhao H., Chiaro C. R., Zhang L., Smith P. B., Chan C. Y., Pedley A. M., Pugh R. J., French J. B., Patterson A. D., Benkovic S. J. (2015) Quantitative analysis of purine nucleotides indicates that purinosomes increase de novo purine biosynthesis. J. Biol. Chem. 290, 6705–6713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shearer J., Graham T. E. (2004) Novel aspects of skeletal muscle glycogen and its regulation during rest and exercise. Exerc. Sport Sci. Rev. 32, 120–126 [DOI] [PubMed] [Google Scholar]
- 6. Graham T. E., Yuan Z., Hill A. K., Wilson R. J. (2010) The regulation of muscle glycogen: the granule and its proteins. Acta Physiol. (Oxf.) 199, 489–498, 10.1111/j.1748-1716.2010.02131.x [DOI] [PubMed] [Google Scholar]
- 7. Guillou H., Zadravec D., Martin P. G., Jacobsson A. (2010) The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog. Lipid Res. 49, 186–199 [DOI] [PubMed] [Google Scholar]
- 8. Goetzman E. S. (2011) Modeling disorders of fatty acid metabolism in the mouse. Prog. Mol. Biol. Transl. Sci. 100, 389–417 [DOI] [PubMed] [Google Scholar]
- 9. Poirier Y., Antonenkov V. D., Glumoff T., Hiltunen J. K. (2006) Peroxisomal β-oxidation: a metabolic pathway with multiple functions. Biochim. Biophys. Acta 1763, 1413–1426 [DOI] [PubMed] [Google Scholar]
- 10. Cohen D. E. (2013) New players on the metabolic stage: How do you like Them Acots? Adipocyte 2, 3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunt M. C., Siponen M. I., Alexson S. E. (2012) The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim. Biophys. Acta 1822, 1397–1410 [DOI] [PubMed] [Google Scholar]
- 12. McGarry J. D., Brown N. F. (1997) The mitochondrial carnitine palmitoyltransferase system: from concept to molecular analysis. Eur. J. Biochem. 244, 1–14 [DOI] [PubMed] [Google Scholar]
- 13. Coleman R. A., Mashek D. G. (2011) Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem. Rev. 111, 6359–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hornemann T. (2015) Palmitoylation and depalmitoylation defects. J. Inherit. Metab. Dis. 38, 179–186 [DOI] [PubMed] [Google Scholar]
- 15. Grevengoed T. J., Klett E. L., Coleman R. A. (2014) Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 34, 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gimeno R. E., Cao J. (2008) Thematic review series: glycerolipids: Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J. Lipid Res. 49, 2079–2088 [DOI] [PubMed] [Google Scholar]
- 17. Schoiswohl G., Schweiger M., Schreiber R., Gorkiewicz G., Preiss-Landl K., Taschler U., Zierler K. A., Radner F. P., Eichmann T. O., Kienesberger P. C., Eder S., Lass A., Haemmerle G., Alsted T. J., Kiens B., Hoefler G., Zechner R., Zimmermann R. (2010) Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J. Lipid Res. 51, 490–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knudsen J., Jensen M. V., Hansen J. K., Faergeman N. J., Neergaard T. B., Gaigg B. (1999) Role of acylCoA binding protein in acylCoA transport, metabolism and cell signaling. Mol. Cell. Biochem. 192, 95–103 [DOI] [PubMed] [Google Scholar]
- 19. Mashek D. G., Li L. O., Coleman R. A. (2007) Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Black P. N., DiRusso C. C. (2007) Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim. Biophys. Acta 1771, 286–298 [DOI] [PubMed] [Google Scholar]
- 21. Mashek D. G., Coleman R. A. (2006) Cellular fatty acid uptake: the contribution of metabolism. Curr. Opin. Lipidol. 17, 274–278 [DOI] [PubMed] [Google Scholar]
- 22. Zhan T., Poppelreuther M., Ehehalt R., Füllekrug J. (2012) Overexpressed FATP1, ACSVL4/FATP4 and ACSL1 increase the cellular fatty acid uptake of 3T3-L1 adipocytes but are localized on intracellular membranes. PLoS One 7, e45087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Küch E. M., Vellaramkalayil R., Zhang I., Lehnen D., Brügger B., Sreemmel W., Ehehalt R., Poppelreuther M., Füllekrug J. (2014) Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim. Biophys. Acta 1841, 227–239 [DOI] [PubMed] [Google Scholar]
- 24. Rufer A. C., Thoma R., Hennig M. (2009) Structural insight into function and regulation of carnitine palmitoyltransferase. Cell. Mol. Life Sci. 66, 2489–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilfling F., Wang H., Haas J. T., Krahmer N., Gould T. J., Uchida A., Cheng J. X., Graham M., Christiano R., Fröhlich F., Liu X., Buhman K. K., Coleman R. A., Bewersdorf J., Farese R. V., Jr., Walther T. C. (2013) Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 24, 384–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee K., Kerner J., Hoppel C. L. (2011) Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J. Biol. Chem. 286, 25655–25662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin Y., McFie P. J., Banman S. L., Brandt C., Stone S. J. (2014) Diacylglycerol acyltransferase-2 (DGAT2) and monoacylglycerol acyltransferase-2 (MGAT2) interact to promote triacylglycerol synthesis. J. Biol. Chem. 289, 28237–28248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Talukder M. M., Sim M. F., O'Rahilly S., Edwardson J. M., Rochford J. J. (2015) Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis. Mol. Metab. 4, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmadian M., Abbott M. J., Tang T., Hudak C. S., Kim Y., Bruss M., Hellerstein M. K., Lee H. Y., Samuel V. T., Shulman G. I., Wang Y., Duncan R. E., Kang C., Sul H. S. (2011) Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13, 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khaibullina A., Kenyon N., Guptill V., Quezado M. M., Wang L., Koziol D., Wesley R., Moya P. R., Zhang Z., Saha A., Mukherjee A. B., Quezado Z. M. (2012) In a model of Batten disease, palmitoyl protein thioesterase-1 deficiency is associated with brown adipose tissue and thermoregulation abnormalities. PloS one 7, e48733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamashita H., Wang Z., Wang Y., Segawa M., Kusudo T., Kontani Y. (2008) Induction of fatty acid-binding protein 3 in brown adipose tissue correlates with increased demand for adaptive thermogenesis in rodents. Biochem. Biophys. Res. Commun. 377, 632–635 [DOI] [PubMed] [Google Scholar]
- 32. Vergnes L., Chin R., Young S. G., Reue K. (2011) Heart-type fatty acid-binding protein is essential for efficient brown adipose tissue fatty acid oxidation and cold tolerance. J. Biol. Chem. 286, 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ellis J. M., Li L. O., Wu P. C., Koves T. R., Ilkayeva O., Stevens R. D., Watkins S. M., Muoio D. M., Coleman R. A. (2010) Adipose acyl-CoA synthetase-1 directs fatty acids toward β-oxidation and is required for cold thermogenesis. Cell Metab. 12, 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu Q., Kazantzis M., Doege H., Ortegon A. M., Tsang B., Falcon A., Stahl A. (2006) Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 55, 3229–3237 [DOI] [PubMed] [Google Scholar]
- 35. Kawano Y., Ersoy B. A., Li Y., Nishiumi S., Yoshida M., Cohen D. E. (2014) Thioesterase superfamily member 2 (Them2) and phosphatidylcholine transfer protein (PC-TP) interact to promote fatty acid oxidation and control glucose utilization. Mol. Cell Biol. 34, 2396–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wendel A. A., Cooper D. E., Ilkayeva O. R., Muoio D. M., Coleman R. A. (2013) Glycerol-3-phosphate acyltransferase (GPAT)-1, but not GPAT4, incorporates newly synthesized fatty acids into triacylglycerol and diminishes fatty acid oxidation. J. Biol. Chem. 288, 27299–27306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hammond L. E., Neschen S., Romanelli A. J., Cline G. W., Ilkayeva O. R., Shulman G. I., Muoio D. M., Coleman R. A. (2005) Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J. Biol. Chem. 280, 25629–25636 [DOI] [PubMed] [Google Scholar]
- 38. Miyazaki M., Flowers M. T., Sampath H., Chu K., Otzelberger C., Liu X., Ntambi J. M. (2007) Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 6, 484–496 [DOI] [PubMed] [Google Scholar]
- 39. Li L. O., Ellis J. M., Paich H. A., Wang S., Gong N., Altshuller G., Thresher R. J., Koves T. R., Watkins S. M., Muoio D. M., Cline G. W., Shulman G. I., Coleman R. A. (2009) Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and β-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 284, 27816–27826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ellis J. M., Mentock S. M., Depetrillo M. A., Koves T. R., Sen S., Watkins S. M., Muoio D. M., Cline G. W., Taegtmeyer H., Shulman G. I., Willis M. S., Coleman R. A. (2011) Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol. Cell Biol. 31, 1252–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li L. O., Grevengoed T. J., Paul D. S., Ilkayeva O., Koves T. R., Pascual F., Newgard C. B., Muoio D. M., Coleman R. A. (2015) Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes 64, 23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y., Millar J. S., Cromley D. A., Graham M., Crooke R., Billheimer J. T., Rader D. J. (2008) Knockdown of acyl-CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim. Biophys. Acta 1781, 97–104 [DOI] [PubMed] [Google Scholar]
- 43. Qi J., Lang W., Geisler J. G., Wang P., Petrounia I., Mai S., Smith C., Askari H., Struble G. T., Williams R., Bhanot S., Monia B. P., Bayoumy S., Grant E., Caldwell G. W., Todd M. J., Liang Y., Gaul M. D., Demarest K. T., Connelly M. A. (2012) The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J. Lipid Res. 53, 1106–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacome-Sosa M. M., Parks E. J. (2014) Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr. Opin. Lipidol. 25, 213–220 [DOI] [PubMed] [Google Scholar]
- 45. Brasaemle D. L., Dolios G., Shapiro L., Wang R. (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279, 46835–46842 [DOI] [PubMed] [Google Scholar]
- 46. Richards M. R., Harp J. D., Ory D. S., Schaffer J. E. (2006) Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J. Lipid Res. 47, 665–672 [DOI] [PubMed] [Google Scholar]
- 47. Poppelreuther M., Rudolph B., Du C., Großmann R., Becker M., Thiele C., Ehehalt R., Füllekrug J. (2012) The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. J. Lipid Res. 53, 888–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bu S. Y., Mashek M. T., Mashek D. G. (2009) Suppression of long chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity. J. Biol. Chem. 284, 30474–30483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chang Y. S., Tsai C. T., Huangfu C. A., Huang W. Y., Lei H. Y., Lin C. F., Su I. J., Chang W. T., Wu P. H., Chen Y. T., Hung J. H., Young K. C., Lai M. D. (2011) ACSL3 and GSK-3β are essential for lipid upregulation induced by endoplasmic reticulum stress in liver cells. J. Cell. Biochem. 112, 881–893 [DOI] [PubMed] [Google Scholar]
- 50. Lewin T. M., Kim J. H., Granger D. A., Vance J. E., Coleman R. A. (2001) Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J. Biol. Chem. 276, 24674–24679 [DOI] [PubMed] [Google Scholar]
- 51. Mashek D. G., McKenzie M. A., Van Horn C. G., Coleman R. A. (2006) Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle-RH7777 cells. J. Biol. Chem. 281, 945–950 [DOI] [PubMed] [Google Scholar]
- 52. Bu S. Y., Mashek D. G. (2010) Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. J. Lipid Res. 51, 3270–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muoio D. M. (2014) Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell 159, 1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khan S. A., Sathyanarayan A., Mashek M. T., Ong K. T., Wollaston-Hayden E. E., Mashek D. G. (2015) ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1α/PPAR-α signaling. Diabetes 64, 418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mashek D. G., Li L. O., Coleman R. A. (2006) Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J. Lipid Res. 47, 2004–2010 [DOI] [PubMed] [Google Scholar]
- 56. Suzuki H., Kawarabayasi Y., Kondo J., Abe T., Nishikawa K., Kimura S., Hashimoto T., Yamamoto T. (1990) Structure and regulation of rat long-chain acyl-CoA synthetase. J. Biol. Chem. 265, 8681–8685 [PubMed] [Google Scholar]
- 57. Durgan D. J., Smith J. K., Hotze M. A., Egbejimi O., Cuthbert K. D., Zaha V. G., Dyck J. R., Abel E. D., Young M. E. (2006) Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am. J. Physiol. Heart Circ. Physiol. 290, H2480–H2497 [DOI] [PubMed] [Google Scholar]
- 58. Schoonjans K., Staels B., Grimaldi P., Auwerx J. (1993) Acyl-CoA synthetase mRNA expression is controlled by fibric-acid derivatives, feeding and liver proliferation. Eur. J. Biochem. 216, 615–622 [DOI] [PubMed] [Google Scholar]
- 59. Martin G., Schoonjans K., Lefebvre A. M., Staels B., Auwerx J. (1997) Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J. Biol. Chem. 272, 28210–28217 [DOI] [PubMed] [Google Scholar]
- 60. Kan C. F., Singh A. B., Dong B., Shende V. R., Liu J. (2015) PPARδ activation induces hepatic long-chain acyl-CoA synthetase 4 expression in vivo and in vitro. Biochim. Biophys. Acta 1851, 577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klett E. L., Chen S., Edin M. L., Li L. O., Ilkayeva O., Zeldin D. C., Newgard C. B., Coleman R. A. (2013) Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J. Biol. Chem. 288, 21618–21629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kan C. F., Singh A. B., Stafforini D. M., Azhar S., Liu J. (2014) Arachidonic acid downregulates acyl-CoA synthetase 4 expression by promoting its ubiquitination and proteasomal degradation. J. Lipid Res. 55, 1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ning J., Hong T., Yang X., Mei S., Liu Z., Liu H. Y., Cao W. (2011) Insulin and insulin signaling play a critical role in fat induction of insulin resistance in mouse. Am. J. Physiol. Endocrinol. Metab. 301, E391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu H. E., Lambert M. H., Montana V. G., Parks D. J., Blanchard S. G., Brown P. J., Sternbach D. D., Lehmann J. M., Wisely G. B., Willson T. M., Kliewer S. A., Milburn M. V. (1999) Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell 3, 397–403 [DOI] [PubMed] [Google Scholar]
- 65. Ren B., Thelen A. P., Peters J. M., Gonzalez F. J., Jump D. B. (1997) Polyunsaturated fatty acid suppression of hepatic fatty acid synthase and S14 gene expression does not require peroxisome proliferator-activated receptor α. J. Biol. Chem. 272, 26827–26832 [DOI] [PubMed] [Google Scholar]
- 66. Mashek D. G., Bornfeldt K. E., Coleman R. A., Berger J., Bernlohr D. A., Black P., DiRusso C. C., Farber S. A., Guo W., Hashimoto N., Khodiyar V., Kuypers F. A., Maltais L. J., Nebert D. W., Renieri A., Schaffer J. E., Stahl A., Watkins P. A., Vasiliou V., Yamamoto T. T. (2004) Revised nomenclature for the mammalian long chain acyl-CoA synthetase gene family. J. Lipid Res. 45, 1958–1961 [DOI] [PubMed] [Google Scholar]
- 67. Iijima H., Fujino T., Minekura H., Suzuki H., Kang M.-J., Yamamoto T. (1996) Biochemical studies of two rat acyl-CoA synthetases, ACS1 and ACS2. Eur. J. Biochem. 242, 186–190 [DOI] [PubMed] [Google Scholar]
- 68. Kang M. J., Fujino T., Sasano H., Minekura H., Yabuki N., Nagura H., Iijima H., Yamamoto T. T. (1997) A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc. Natl. Acad. Sci. U.S.A. 94, 2880–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marszalek J. R., Kitidis C., Dararutana A., Lodish H. F. (2004) Acyl-CoA synthetase 2 overexpression enhances fatty acid internalization and neurite outgrowth. J. Biol. Chem. 279, 23882–23891 [DOI] [PubMed] [Google Scholar]
- 70. Marszalek J. R., Kitidis C., Dirusso C. C., Lodish H. F. (2005) Long-chain acyl-CoA synthetase 6 preferentially promotes DHA metabolism. J. Biol. Chem. 280, 10817–10826 [DOI] [PubMed] [Google Scholar]
- 71. Kuwata H., Yoshimura M., Sasaki Y., Yoda E., Nakatani Y., Kudo I., Hara S. (2014) Role of long-chain acyl-coenzyme A synthetases in the regulation of arachidonic acid metabolism in interleukin 1β-stimulated rat fibroblasts. Biochim. Biophys. Acta 1841, 44–53 [DOI] [PubMed] [Google Scholar]
- 72. Tuohetahuntila M., Spee B., Kruitwagen H. S., Wubbolts R., Brouwers J. F., van de Lest C. H., Molenaar M. R., Houweling M., Helms J. B., Vaandrager A. B. (2015) Role of long-chain acyl-CoA synthetase 4 in formation of polyunsaturated lipid species in hepatic stellate cells. Biochim. Biophys. Acta 1851, 220–230 [DOI] [PubMed] [Google Scholar]
- 73. Kanter J. E., Bornfeldt K. E. (2013) Inflammation and diabetes-accelerated atherosclerosis: myeloid cell mediators. Trends Endocrinol. Metab. 24, 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grevengoed T. J., Martin S. A., Katunga L., Cooper D. E., Anderson E. J., Murphy R. C., Coleman R. A. (2015) Acyl-CoA synthetase 1 deficiency alters cardiolipin species and impairs mitochondrial function. J. Lipid Res. 10.1194/jlr.M059717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Watkins P. A. (2008) Very-long-chain acyl-CoA synthetases. J. Biol. Chem. 283, 1773–1777 [DOI] [PubMed] [Google Scholar]
- 76. Lee R. G., Willingham M. C., Davis M. A., Skinner K. A., Rudel L. L. (2000) Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J. Lipid Res. 41, 1991–2001 [PubMed] [Google Scholar]
- 77. Cheng B., Kowal J. (1994) Analysis of adrenal cholesteryl esters by reversed phase high performance liquid chromatography. J. Lipid Res. 35, 1115–1121 [PubMed] [Google Scholar]
- 78. Seo T., Oelkers P. M., Giattina M. R., Worgall T. S., Sturley S. L., Deckelbaum R. J. (2001) Differential modulation of ACAT1 and ACAT2 transcription and activity by long chain free fatty acids in cultured cells. Biochemistry 40, 4756–4762 [DOI] [PubMed] [Google Scholar]
- 79. Schlame M., Towbin J. A., Heerdt P. M., Jehle R., DiMauro S., Blanck T. J. (2002) Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann. Neurol. 51, 634–637 [DOI] [PubMed] [Google Scholar]
- 80. Kiebish M. A., Han X., Cheng H., Chuang J. H., Seyfried T. N. (2008) Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 49, 2545–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yao H., Ye J. (2008) Long chain acyl-CoA synthetase 3-mediated phosphatidylcholine synthesis is required for assembly of very low density lipoproteins in human hepatoma Huh7 cells. J. Biol. Chem. 283, 849–854 [DOI] [PubMed] [Google Scholar]
- 82. Zechner R., Zimmermann R., Eichmann T. O., Kohlwein S. D., Haemmerle G., Lass A., Madeo F. (2012) Fat Signals: lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Efeyan A., Comb W. C., Sabatini D. M. (2015) Nutrient-sensing mechanisms and pathways. Nature 517, 302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ong K. T., Mashek M. T., Davidson N. O., Mashek D. G. (2014) Hepatic ATGL mediates PPAR-α signaling and fatty acid channeling through an L-FABP independent mechanism. J. Lipid Res. 55, 808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ong K. T., Mashek M. T., Bu S. Y., Mashek D. G. (2013) Hepatic ATGL knockdown uncouples glucose intolerance from liver TAG accumulation. FASEB J. 27, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., Schreiber R., Eichmann T., Kolb D., Kotzbeck P., Schweiger M., Kumari M., Eder S., Schoiswohl G., Wongsiriroj N., Pollak N. M., Radner F. P., Preiss-Landl K., Kolbe T., Rülicke T., Pieske B., Trauner M., Lass A., Zimmermann R., Hoefler G., Cinti S., Kershaw E. E., Schrauwen P., Madeo F., Mayer B., Zechner R. (2011) ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 17, 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wölkart G., Schrammel A., Dörffel K., Haemmerle G., Zechner R., Mayer B. (2012) Cardiac dysfunction in adipose triglyceride lipase deficiency: treatment with a PPARα agonist. Br. J. Pharmacol. 165, 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gibson T. J., Seiler M., Veitia R. A. (2013) The transience of transient overexpression. Nat. Methods 10, 715–721 [DOI] [PubMed] [Google Scholar]
- 89. Li L. O., Mashek D. G., An J., Doughman S. D., Newgard C. B., Coleman R. A. (2006) Overexpression of rat long chain acyl-CoA synthetase 1 alters fatty acid metabolism in rat primary hepatocytes. J. Biol. Chem. 281, 37246–37255 [DOI] [PubMed] [Google Scholar]
- 90. Kanter J. E., Kramer F., Barnhart S., Averill M. M., Vivekanandan-Giri A., Vickery T., Li L. O., Becker L., Yuan W., Chait A., Braun K. R., Potter-Perigo S., Sanda S., Wight T. N., Pennathur S., Serhan C. N., Heinecke J. W., Coleman R. A., Bornfeldt K. E. (2012) Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc. Natl. Acad. Sci. U.S.A. 109, E715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rubinow K. B., Wall V. Z., Nelson J., Mar D., Bomsztyk K., Askari B., Lai M. A., Smith K. D., Han M. S., Vivekanandan-Giri A., Pennathur S., Albert C. J., Ford D. A., Davis R. J., Bornfeldt K. E. (2013) Acyl-CoA synthetase 1 is induced by Gram-negative bacteria and lipopolysaccharide and is required for phospholipid turnover in stimulated macrophages. J. Biol. Chem. 288, 9957–9970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li X., Gonzalez O., Shen X., Barnhart S., Kramer F., Kanter J. E., Vivekanandan-Giri A., Tsuchiya K., Handa P., Pennathur S., Kim F., Coleman R. A., Schaffer J. E., Bornfeldt K. E. (2013) Endothelial acyl-CoA synthetase 1 is not required for inflammatory and apoptotic effects of a saturated fatty acid-rich environment. Arterioscler. Thromb. Vasc. Biol. 33, 232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Storch J., McDermott L. (2009) Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 50, (suppl) S126–S131, 10.1194/jlr.R800084-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Garin-Shkolnik T., Rudich A., Hotamisligil G. S., Rubinstein M. (2014) FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes 63, 900–911 [DOI] [PubMed] [Google Scholar]
- 95. Shen W. J., Sridhar K., Bernlohr D. A., Kraemer F. B. (1999) Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc. Natl. Acad. Sci. U.S.A. 96, 5528–5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Neess D., Bek S., Bloksgaard M., Marcher A. B., Færgeman N. J., Mandrup S. (2013) Delayed hepatic adaptation to weaning in ACBP−/− mice is caused by disruption of the epidermal barrier. Cell Rep. 5, 1403–1412 [DOI] [PubMed] [Google Scholar]
- 97. Rao V. S., Srinivas K., Sujini G. N., Kumar G. N. (2014) Protein-protein interaction detection: methods and analysis. Int. J. Proteomics 2014, 147648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Johnsson N. (2014) Analyzing protein-protein interactions in the post-interactomic era: Are we ready for the endgame? Biochem. Biophys. Res. Commun. 445, 739–745 [DOI] [PubMed] [Google Scholar]
- 99. Maheshwari S., Brylinski M. (2015) Predicting protein interface residues using easily accessible on-line resources. Brief Bioinform., 10.1093/bib/bbv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Frahm J. L., Li L. O., Grevengoed T. J., Coleman R. A. (2011) Phosphorylation and acetylation of acyl-CoA synthetase-I. J. Proteomics Bioinform. 4, 129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]