VOLUME 289 (2014) PAGES 5083–5096
There were several errors in this article. There was a typographical error in Equation 8 in the supplemental data. Equation 8 should be as follows.
ΔG0‡ was calculated using the wrong equation. Equation 18 in the supplemental data should be as follows:
with k‡ = kh/kBT.
The parameter k used for the calculations in the original paper is the kinetic rate constant when the parameter k‡, the kinetic rate constant (k) multiplied by the Planck constant (h), and divided by the Boltzmann (kB) constant and the temperature (T), should have been used. The recalculated ΔG0‡ affects the following text.
PAGE 5093:
In the right column, line 2, ΔH0‡ should be changed to ΔH0‡ (ka1).
In the right column, line 3, “(around −10 to −20 kcal/mol)” should be changed to “(around 20 to 30 kcal/mol).”
In the right column, line 5, “(around −4 to −5 kcal/mol)” should be changed to “(around 4 to 5 kcal/mol).”
PAGE 5094:
The following sentence was incorrect: “In addition, the Ly49*/m157 species has a stability very similar to the transition state (Fig. 11A), making dissociation a more frequent event.” This should be changed to “In addition, the Ly49*/m157 species has a stability very similar to the respective free molecules (Fig. 11A), making dissociation a more frequent event.” Table S3 and Fig 11 were also corrected using the correct Eq. 18. These errors do not affect the results or conclusions of this work.
FIGURE 11.
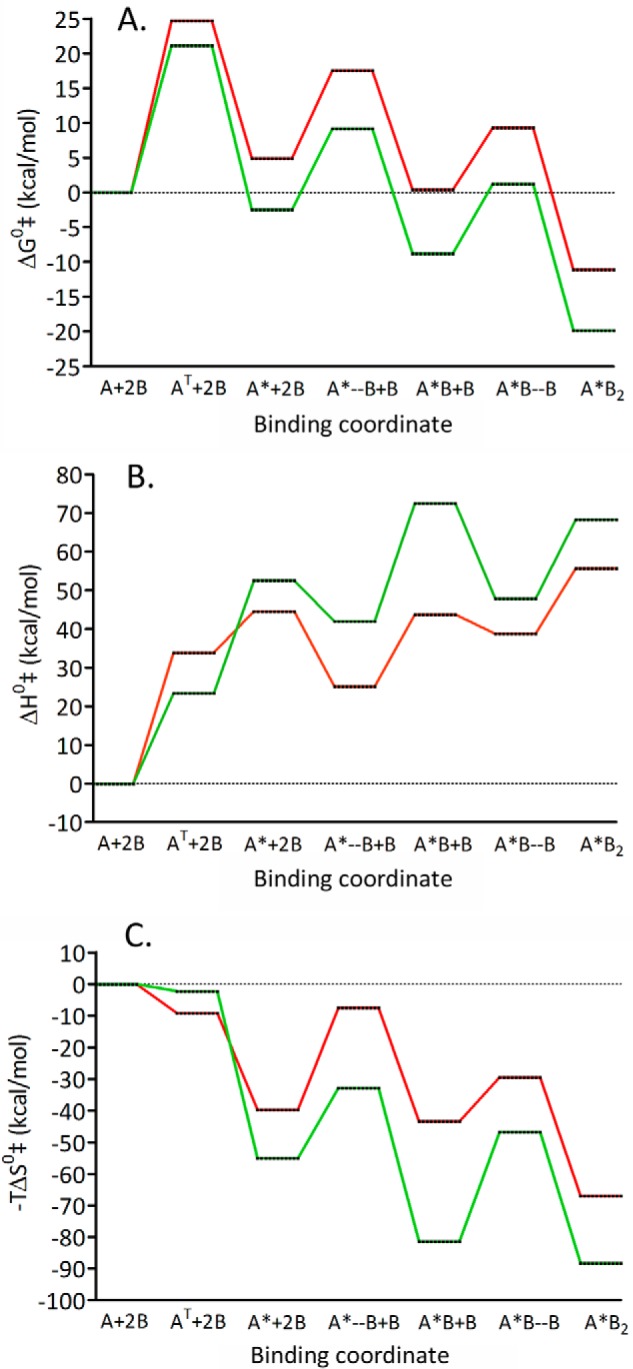
TABLE S3.
Ly49H-m157 and Ly49I-m157 activation free energy (ΔG0‡), enthalpy (ΔH0‡), entropy at 25 °C (−TΔS0‡), and heat capacity (ΔCp0‡ for each step of the model D, estimated using the Eyring equation
| Ly49H/m157 | Ly49I/m157 | |
|---|---|---|
| ka1 | ||
| ΔG0‡ (kcal/mol) | 25 ± 7 | 21 ± 7 |
| ΔH0‡ (kcal/mol) | 30 ± 20 | 23 ± 8 |
| −TΔS0‡ (kcal/mol) | −10 ± 10 | −2 ± 1 |
| −ΔCp0‡ (cal/mol) | 4000 ± 3000 | 2000 ± 1000 |
| kd1 | ||
| − ΔG0‡ (kcal/mol) | 19 ± 1 | 23.6 ± 0.3 |
| −ΔH0‡ (kcal/mol) | −11 ± 9 | −29 ± 9 |
| −TΔS0‡ (kcal/mol) | 30 ± 20 | 50 ± 10 |
| −ΔCp0‡ (cal/mol) | −2000 ± 1000 | −2000 ± 1000 |
| ka2 | ||
| − ΔG0‡ (kcal/mol) | 12.58 ± 0.09 | 11.66 ± 0.07 |
| −ΔH0‡ (kcal/mol) | −19 ± 5 | −11 ± 1 |
| −TΔS0‡ (kcal/mol) | 32 ± 9 | 22 ± 3 |
| −ΔCp0‡ (cal/mol) | −2100 ± 500 | −1100 ± 200 |
| kd2 | ||
| − ΔG0‡ (kcal/mol) | 17.1 ± 0.2 | 18.0 ± 0.1 |
| −ΔH0‡ (kcal/mol) | −19 ± 8 | −31 ± 7 |
| −TΔS0‡ (kcal/mol) | 40 ± 20 | 50 ± 10 |
| −ΔCp0‡ (cal/mol) | −2000 ± 1000 | −3000 ± 900 |
| ka3 | ||
| − ΔG0‡ (kcal/mol) | 9.0 ± 0.1 | 10.03 ± 0.07 |
| −ΔH0‡ (kcal/mol) | −5 ± 4 | −25 ± 9 |
| −TΔS0‡ (kcal/mol) | 14 ± 9 | 30 ± 10 |
| −ΔCp0‡ (cal/mol) | −1300 ± 500 | −3000 ± 1000 |
| kd3 | ||
| − ΔG0‡ (kcal/mol) | 20.5 ± 0.2 | 21.1 ± 0.2 |
| −ΔH0‡ (kcal/mol) | −20 ± 10 | −21 ± 4 |
| −TΔS0‡ (kcal/mol) | 40 ± 30 | 42 ± 9 |
| −ΔCp0‡ (cal/mol) | −2000 ± 1000 | −2400 ± 500 |


