Background: Inflammation causes bone loss through enhanced osteoclast formation.
Results: Porphyromonas gingivalis stimulates osteoclast formation through Toll-like receptor 2.
Conclusion: Activation of Toll-like receptors may represent a mechanism for inflammation-induced bone loss in diseases like rheumatoid arthritis and periodontitis.
Significance: A thorough understanding of the mechanisms involved in inflammation-induced bone loss will lead to improved treatment.
Keywords: bone, inflammation, innate immunity, osteoblast, osteoclast, toll-like receptor (TLR)
Abstract
Periodontitis has been associated with rheumatoid arthritis. In experimental arthritis, concomitant periodontitis caused by oral infection with Porphyromonas gingivalis enhances articular bone loss. The aim of this study was to investigate how lipopolysaccharide (LPS) from P. gingivalis stimulates bone resorption. The effects by LPS P. gingivalis and four other TLR2 ligands on bone resorption, osteoclast formation, and gene expression in wild type and Tlr2-deficient mice were assessed in ex vivo cultures of mouse parietal bones and in an in vivo model in which TLR2 agonists were injected subcutaneously over the skull bones. LPS P. gingivalis stimulated mineral release and matrix degradation in the parietal bone organ cultures by increasing differentiation and formation of mature osteoclasts, a response dependent on increased RANKL (receptor activator of NF-κB ligand). LPS P. gingivalis stimulated RANKL in parietal osteoblasts dependent on the presence of TLR2 and through a MyD88 and NF-κB-mediated mechanism. Similarly, the TLR2 agonists HKLM, FSL1, Pam2, and Pam3 stimulated RANKL in osteoblasts and parietal bone resorption. LPS P. gingivalis and Pam2 robustly enhanced osteoclast formation in periosteal/endosteal cell cultures by increasing RANKL. LPS P. gingivalis and Pam2 also up-regulated RANKL and osteoclastic genes in vivo, resulting in an increased number of periosteal osteoclasts and immense bone loss in wild type mice but not in Tlr2-deficient mice. These data demonstrate that LPS P. gingivalis stimulates periosteal osteoclast formation and bone resorption by stimulating RANKL in osteoblasts via TLR2. This effect might be important for periodontal bone loss and for the enhanced bone loss seen in rheumatoid arthritis patients with concomitant periodontal disease.
Introduction
Rheumatoid arthritis (RA),2 psoriasis arthritis, septic arthritis, reactive arthritis, periodontitis, peri-implantitis, joint prosthetic loosening, and osteomyelitis are bone-related inflammatory processes associated with infiltration of a wide variety of cells involved in the innate and acquired immune responses. Breakdown of supporting tissues such as cartilage, juxta-articular bone, jaw bone, and bone retaining prosthesis and tooth implants is the reason for joint destruction and for the loosening of teeth and implants (1–4). Bone loss is mainly due to increased formation and activity of osteoclasts generated by fusion of hematopoietic myeloid mononuclear progenitor cells (5). M-CSF is required for proliferation and survival of the progenitors, and the receptor activator of NF-κB ligand (RANKL) is required for fusion and differentiation to osteoclasts (1–5). The decoy receptor osteoprotegerin (OPG) binds and neutralizes RANKL.
In inflammatory conditions, osteoclastogenesis is believed to be caused by increased expression of cytokines, which increases RANKL/OPG ratio in either osteoblasts or in other resident cells, such as synovial fibroblasts or periodontal ligament cells. To this group of cytokines belong IL-1β, IL-6, IL-11, IL-17, TNF-α, LIF, oncostatin M (OSM), and cardiotrophin-1 (5–7). In recent years the potential role of the innate immune system for inflammation-induced bone resorption has attracted increasing interest. Resident cells and infiltrating leukocytes express pattern recognition receptors, including TLRs that respond to pathogen-associated molecular patterns expressed by bacteria, viruses, and fungi (8). These receptors also respond to host-derived molecules generated during cell death, inflammation, and tissue damage (9, 10). It has been repeatedly shown that LPS from different bacteria can stimulate osteoclast formation and bone resorption in vitro and in vivo and that the effect is due to activation of TLR4 (11–13).
Porphyromonas gingivalis is a Gram-negative bacteria present in the biofilm on teeth and associated with periodontitis (14, 15). LPS preparations from P. gingivalis are different from LPS from other bacteria and can be either an agonist or antagonist of TLR4 or even without affinity to TLR4 depending on modifications of the lipid A moiety caused by environmental conditions. LPS preparations from P. gingivalis often are potent agonists of TLR2 due to contamination with a lipoprotein with affinity to TLR2 (16). Oral infection with P. gingivalis in mice causes inflammation-induced alveolar bone loss through activation of TLR2 (17–19). The mechanism by which P. gingivalis induces bone loss is not fully understood as the role of TLR2 in osteoclastogenesis has been studied less as compared with TLR4. P. gingivalis, the synthetic TLR2 ligand Pam3 (palmitoyl-3-Cys-Ser-(Lys)4), and lipoteichoic acid from Staphylococcus aureus, similar to LPS from Escherichia coli, inhibit RANKL-stimulated osteoclast formation in mouse bone marrow macrophage (BMM) cultures (20, 21). At variance, heat shock protein 60 potentiates RANKL-stimulated osteoclast formation in mouse BMM cultures, an effect not observed using cells from Tlr2-deficient mice (22). It was recently reported that the lipoproteins Pam2 (palmitoyl-2-Cys-Ser-(Lys)4) and Pam3 stimulate local and systemic bone loss, as evidenced by microcomputed tomography when administered subcutaneously or intraperitoneally, respectively (23). This effect was mainly attributed to a direct effect by Pams on osteoclast progenitors as Pam2 and Pam3, similar to LPS E. coli, stimulated osteoclast formation in RANKL-primed BMM cultures.
Clinical and epidemiological data indicate that periodontitis is associated with RA (24). Periodontal disease is more common and severe in RA patients than in healthy controls (25–27), and management of periodontitis seems to decrease the severity of RA (28, 29). Several lines of evidence indicate that the link between periodontitis and RA could be the periodontitis-associated bacteria P. gingivalis. DNA from P. gingivalis detected in serum and synovial fluid from patients with RA (30, 31), and enhanced antibody titers against P. gingivalis have been found in RA patients (32, 33). Moreover, periodontitis and RA have been suggested to involve citrullination of proteins by the peptidylarginine deiminase expressed by P. gingivalis, which then could drive autoimmunity in RA (34). Experimentally it has been shown that preexisting subcutaneous inflammation due to infection with heat-killed P. gingivalis resulted in more severe adjuvant arthritis (35) and that preexisting periodontitis caused by oral infections with P. gingivalis caused more advanced arthritis in a mouse model of collagen antibody-induced arthritis (36). Similar observations have been made in mice with concurrent periodontitis caused by oral P. gingivalis infection and collagen type II-induced arthritis (37), where mice with periodontitis exhibited more severe arthritic bone loss with no effect on cartilage destruction.
Data showing stimulatory or inhibitory effects on osteoclastogenesis by stimulation of TLR4 and TLR2 have been obtained using osteoclast progenitor cells from either bone marrow or peripheral blood. Functional osteoclasts are only formed on bone surfaces. We, therefore, focused our studies on the effect by LPS P. gingivalis on periosteal osteoclast formation and bone resorption using ex vivo cultures of mouse parietal bones and an in vivo model using local injections with P. gingivalis. Our aim was also to evaluate if LPS P. gingivalis could enhance osteoclastogenesis not only directly on primed osteoclast progenitors but also indirectly through increased RANKL production in resident cells. We report here that LPS P. gingivalis stimulates periosteal osteoclast formation ex vivo and in vivo due to induction of RANKL in osteoblasts by activation of TLR2.
Experimental Procedures
Materials
Recombinant mouse cytokines and neutralizing antibodies and Quantikine® ELISA kits for RANKL and OPG were from R&D Systems; BMS-345541 and Celastrol were from Sigma; α-minimum essential medium, fetal calf serum, zoledronic acid, and indomethacin were from Invitrogen; 45CaCl2 was from Amersham Biosciences; oligonucleotide primers and probes were from Invitrogen or Applied Biosystems; LPS P. gingivalis (version 10G20-MT) and other TLR2 and TLR4 agonists and primers were from InvivoGen and R&D Systems; RatLapsTM CTX ELISA kit was from Immunodiagnostic Systems; prostaglandin E2 125I-RIA® kit was from PerkinElmer Life Sciences; RNAqueous-4 PCR® kit was from Ambion; High Capacity cDNA Reverse Transcription kit was from Applied Biosystems; Kapa2GTM Robust HotStart PCR kit and KapaTM Probe Fast qPCR kit were from Kapa Biosystems; TaqMan® Fast Advanced Master Mix was from Life Technologies; RNAlater®, RNeasy®, and Cignal Lenti Reporter Assay® kits were from Qiagen; Luciferase Assay System was from Promega.
Animals
CsA mice from our own inbred colony were used for most experiments. CB57BL/6J and B6.129 Tlr2tm1Kir/J mice were purchased from The Jackson Laboratory. MyD88−/− mice (38) and their wild type C57BL/6 mice were bred at the Laboratory for Experimental Biomedicine, Sahlgrenska Academy at the University of Gothenburg. Animal care and experiments were approved and conducted in accordance with the accepted standards of humane animal care and used as deemed appropriate by the animal care and use committees of Umeå University, Umeå, Sweden and the University of Gothenburg, Gothenburg, Sweden.
Osteoclast Formation and Bone Resorption in Cultured Mouse Bones
Parietal bones from 5–7-day-old mice were microdissected, cut into either parietal halves or quarters, and then cultured as previously described (39, 40).
Mineral mobilization was assessed by analyzing the release of 45Ca from bones prelabeled in vivo with 1.5 μCi of 45Ca. For the time-course experiments, mice were injected with 12.5 μCi of 45Ca, and radioactivity was analyzed at different time points by extraction of small amounts of culture medium.
Bone extracellular matrix degradation was assessed by analyzing the amount of type I collagen degradation fragments (CTX) in culture media released from parietal halves using the RatLapsTM kit. Osteoclast formation was assessed by counting the number of cathepsin K-positive osteoclasts and osteoclast differentiation by analyzing expression of osteoclastic and osteoclastogenic genes.
Osteoblast Isolation and Culture
Bone cells were isolated from 2–3-day-old mouse parietal bones by time sequential digestion with bacterial collagenase (41). Cells from digestions 6–10 were used and plated at a density of 104 cells/cm2. At the end of the cultures, RNA was isolated for gene expression analysis.
Osteoclast Formation in Periosteal Cell Cultures
Periosteal and endosteal cells were isolated from 2–3-day-old mice, and cells from all 10 digestions were pooled (41). These isolations contain both osteoblast and osteoclast progenitors, and stimulation by RANKL results in robust formation of bone-resorbing osteoclasts. The periosteal cells were seeded at a density of 103 cells/cm2 and incubated for 9 days. At the end of the cultures, cells were stained for tartrate-resistant acid phosphatase (TRAP), and TRAP+ cells with more than three nuclei were counted (TRAP+MuOCL). RNA was also isolated for gene expression analysis.
Osteoclast Formation in Bone Marrow Macrophage Cultures
Mouse bone marrow cells were incubated with 30 ng/ml M-CSF for 2 days (42). The adhering macrophages were incubated for 3–4 days with 200 μl of medium containing either M-CSF (30 ng/ml) or M-CSF+ RANKL (4 ng/ml) with or without TLR-2 agonists. At the end of the cultures, the cells were stained for TRAP, and TRAP+MuOCL was counted.
Osteoclast Differentiation and Bone Loss in Vivo
Five-week-old male mice were injected with 100 μl of LPS P. gingivalis (500 μg), Pam2 (50 μg), or NaCl subcutaneously over the skull bones and sacrificed after 6 days. The skull bones were dissected and analyzed for the number of TRAP+ osteoclasts for bone loss and for gene expression.
High Resolution Microcomputed Tomography Analysis
Skull bones were scanned by high resolution microcomputed tomography (Skyscan 1172) at 50 kV, 201 μA, and with 13.5-μm voxel size. Image reconstructions were made by NRecon software.
Immunostaining of Osteoclasts
Parietal bones from newborn mice were immunostained for cathepsin K as previously described (43). The number of cathepsin K-positive multinucleated cells per section was determined. Control stainings without primary antibody did not show any positive reaction.
Enzyme Histochemistry
Skull bones from 5-week-old mice were fixed, decalcified in 10% EDTA, and TRAP+MuOCL-detected using the Naphtol AS-BI phosphate method. Staining was performed by Histocenter AB, Gothenburg, Sweden according to its accredited protocol.
Gene Expression Analyses
RNA was isolated from parietal bones and cell cultures using either the RNAqueous®-4 PCR kit or RNeasy kit. RNA from 5-week-old mice skull bones was prepared in TRIzol after homogenization and purified using the RNeasy® kit. RNA from unstimulated and stimulated groups was isolated at each time point. Single-stranded cDNA was synthesized from 0.1–0.5 μg of total RNA using a High Capacity cDNA Reverse Transcription kit. Semi-quantitative RT-PCR analyses of the mRNA expression were performed using the Kapa2GTM Robust HotStart PCR kit. Sequences of the primers are avaibable upon request.
Quantitative real-time PCR analyses were performed using either the KapaTM Probe Fast qPCR kit or the TaqMan® Fast Advanced Master Mix with primers and probes as described previously (44, 45). Amplifications were performed with the ABI PRISM 7900 HT Sequence Detection System and Software or with the StepOnePlus Real-Time PCR system. β-Actin was used as housekeeping gene in all analyses.
RANKL and OPG Protein Analyses
Half parietal bones were cultured for 48 h. Bones cells were lysed with 0.2% Triton X-100, and the amount of RANKL and OPG protein was assessed by measuring the levels of RANKL and OPG in the bone lysates using Quantikine® ELISA kits.
Analysis of Prostaglandin E2
The formation of prostaglandin E2 (PGE2) was assessed by analyzing the release of PGE2 from cultured parietal bones to culture media using the RIA-kit.
Neutralizing Antibody Experiments
Initial control experiments ensured that the antibodies used specifically abolished mRNA expression of Tnfsf11 (encoding RANKL) in parietal bones stimulated by either IL-1β, IL-6+sIL-6R, IL-11, LIF, OSM, or TNF-α, respectively. The antibodies were then added solely or in different combinations with LPS P. gingivalis or Pam2 to parietal bones or isolated osteoblasts. The effects on mineral release and Tnfsf11 mRNA expression were then assessed.
Reporter Gene Experiments
Cells were transduced by lentiviral vectors expressing the luciferase reporter gene under the control of either the NF-κB response elements or positive or negative control at a multiplicity of infection of 10 for 24 h. Cells were then incubated in vector-free media with the TLR2 agonist. Luciferase was measured after harvesting at different time points by using the Luciferase Assay System and Mithras LB940 luminometer.
Statistics
All statistical analysis was performed using one-way analysis of variance with Shapiro-Wilk's normality test and post hoc Holm-Sidak's test or a paired t test (SigmaPlot, Systat Software Inc.). All experiments were performed at least three-five times with comparable results, and all data are presented as the means ± S.E.
Results
Stimulation of Bone Resorption in Parietal Bones by LPS P. gingivalis
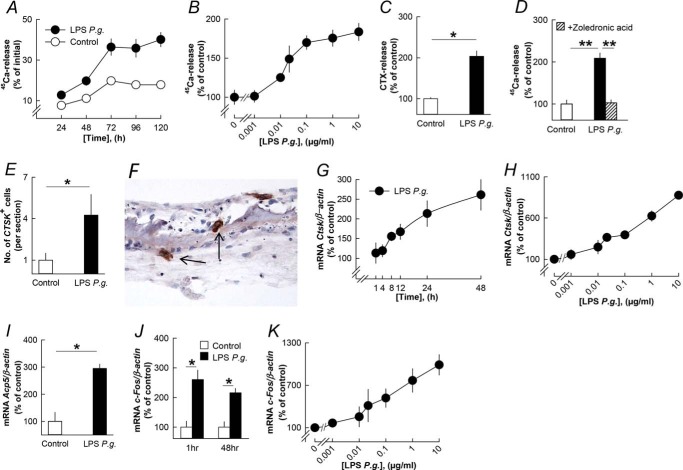
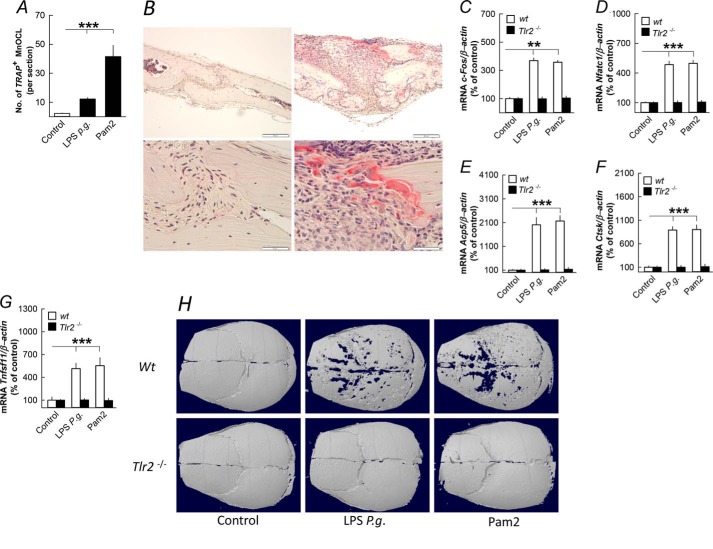
LPS P. gingivalis increased the release of 45Ca from parietal bones in a time- and concentration-dependent manner (Fig. 1, A and B). LPS P. gingivalis also enhanced the release of CTX (Fig. 1C). Stimulation of 45Ca release caused by LPS P. gingivalis was inhibited by the bisphosphonate zoledronic acid (Fig. 1D). LPS P. gingivalis significantly enhanced the number of cathepsin K-positive multinucleated osteoclasts on bone surfaces (Fig. 1, E and F).
FIGURE 1.
LPS from P. gingivalis (P.g.) stimulates bone resorption, osteoclast formation, and expression of osteoclastic and osteoclastogenic genes in organ cultures of neonatal mouse parietal bones. A–C, LPS P. gingivalis time- and concentration-dependently increased 45Ca and CTX release from the parietal bones. D, the stimulatory effect by LPS P. gingivalis on 45Ca release was inhibited by zoledronic acid (0.2 μmol/liter). E, the number of cathepsin K-positive (CTSK+) osteoclasts was enhanced by LPS P. gingivalis. G and H, LPS P. gingivalis time- and concentration-dependently enhanced the mRNA expression of Ctsk. I and J, the mRNA expression of Acp5 (48 h) and c-fos (1 and 48 h) was increased by LPS P. gingivalis. K, LPS P. gingivalis concentration-dependently increased c-fos mRNA. *, p < 0.05; **, p < 0.01 compared with unstimulated controls (C–E, I, and J) or to LPS P. gingivalis-stimulated bones (D). LPS P. gingivalis was used at a concentration of 10 μg/ml in A, C–G, I, and J. Data are the means of 4–5 observations, and S.E. is given as vertical bars when larger than the radius of the symbol.
We next analyzed the effects by LPS P. gingivalis on gene expression by isolating RNA from the parietal bones. The mRNA expression of Ctsk (encoding cathepsin K) was time- and concentration-dependently increased by LPS P. gingivalis (Fig. 1, G and H). LPS P. gingivalis also increased the mRNA expression of Acp5 (encoding TRAP; Fig. 1I). The mRNA expression of the early response gene c-fos was increased by LPS P. gingivalis at 1 h and still at 48 h (Fig. 1J), a response dependent on the concentration of LPS P. gingivalis (Fig. 1K).
Bone Resorption Induced by LPS P. gingivalis Is Due to Increased RANKL
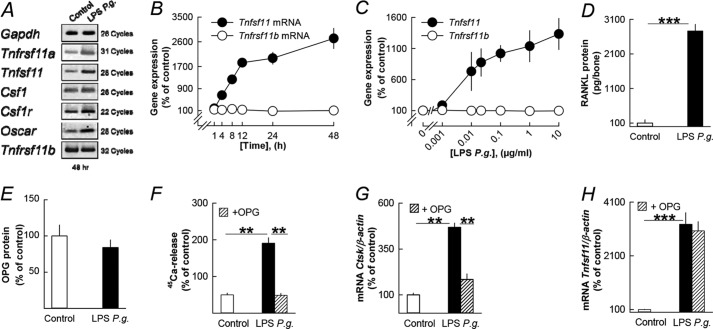
Gene expression analyses using RNA from the parietal bones showed that LPS P. gingivalis enhanced the mRNA expression of Tnfrsf11a (encoding RANK), Tnfsf11 (encoding RANKL), Csf1 (encoding M-CSF), Csf1r (encoding the M-CSF receptor c-Fms), and Oscar, whereas Tnfrsf11b (encoding OPG) mRNA was unaffected (Fig. 2A). Quantitative PCR analyses showed that LPS P. gingivalis caused a time- and concentration-dependent, robust increase of Tnfsf11 mRNA expression (Fig. 2, B and C). In contrast, Tnfrsf11b mRNA was unaffected (Fig. 2, B and C). Quantitative PCR also confirmed that LPS P. gingivalis enhanced Tnfrsf11a, Csf1r, Oscar, and Csf1 mRNA (data not shown). LPS P. gingivalis significantly enhanced RANKL protein in the parietal bones (Fig. 2D), but OPG protein was not significantly changed (Fig. 2E).
FIGURE 2.
The stimulatory effect on bone resorption in neonatal mouse parietal bones by LPS from P. gingivalis (P.g.) is dependent on increased RANKL. A, LPS P. gingivalis enhanced the mRNA expression of Tnfrsf11a, Tnfsf11, Csf1, Csf1r, and Oscar without affecting Tnfrsf11b. B and C, LPS P. gingivalis time- and concentration-dependently enhanced Tnfsf11 mRNA with no effect on Tnfrsf11b mRNA. D and E, LPS P. gingivalis enhanced the cellular level of RANKL protein without affecting OPG protein. F–H, the stimulatory effect by LPS P. gingivalis on 45Ca release, and Ctsk mRNA was inhibited by adding exogenous OPG (300 ng/ml) to the culture medium, whereas Tnfsf11 mRNA was unaffected. **, p < 0.01; ***, p < 0.001 compared with unstimulated controls (D and F–H) or to LPS P. gingivalis-stimulated bones (F and G). LPS P. gingivalis was used at a concentration of 10 μg/ml in A, B, and D–H. Data are the means of 4–5 observations, and S.E. is given as vertical bars when larger than the radius of the symbol.
The increased release of 45Ca induced by LPS P. gingivalis was abolished by the addition of OPG (Fig. 2F). The inhibition of 45Ca release by OPG was associated with decreased mRNA expression of Ctsk (Fig. 2G) but not of Tnfsf11 (Fig. 2H), showing that OPG acted downstream RANKL formation to inhibit osteoclast formation.
The Importance of TLR2 for the Stimulatory Effect of LPS P. gingivalis in Parietal Bones
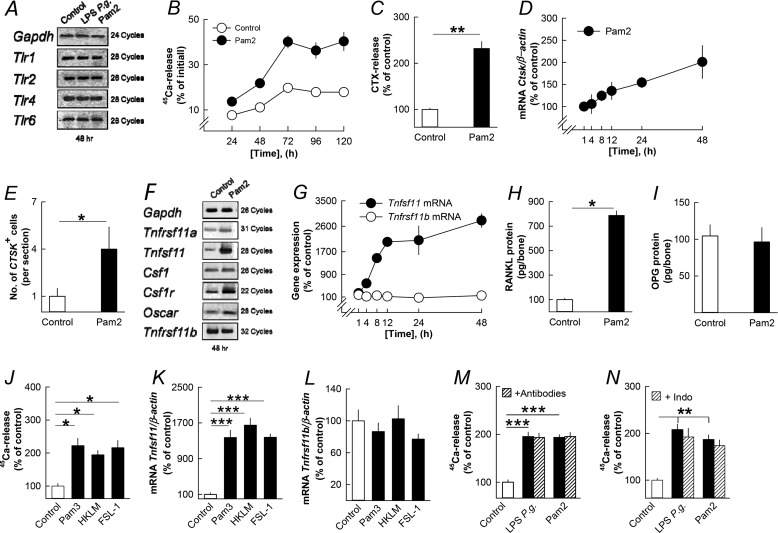
TLR2 forms heterodimers with either TLR1 or TLR6 (46). Mouse parietal bones express Tlr1, Tlr2, Tlr6, and Tlr4 mRNA (Fig. 3A). TLR2-TLR1 and TLR2-TLR6 heterodimers recognize triacylated and diacylated lipopeptides, respectively. Pam2 time-dependently stimulated 45Ca release (Fig. 3B) and increased bone matrix degradation (Fig. 3C) in the organ-cultured parietal bones. RNA was isolated from the parietal bones, and Pam2 was found to increase the mRNA expression of Ctsk (Fig. 3D). In agreement with this finding, Pam2 increased the number of cathepsin K-positive osteoclasts in the parietal bones (Fig. 3E).
FIGURE 3.
The lipopeptide Pam2 and three additional TLR2 agonists stimulate bone resorption, osteoclast formation, and expression of osteoclastic and osteoclastogenic genes in organ cultures of neonatal mouse parietal bones by an effect dependent on RANKL but independent on cytokine and prostaglandin formation. A, LPS P. gingivalis and Pam2 did not affect the mRNA expression of Tlr1, Tlr2, Tlr4, and Tlr6. B–E, Pam2 increased the release of 45Ca (B) and CTX (C), up-regulated Ctsk mRNA (D), and enhanced the number of cathepsin K positive (CTSK+) osteoclasts (E). F, Pam2 increased the mRNA expression of Tnfrsf11a, Tnfsf11, Csf1, Csf1r, and Oscar without affecting Tnfrsf11b. G–I, Pam2 time-dependently increased Tnfsf11 mRNA (G) resulting in increased RANKL protein after 48 h (H) without affecting Tnfrsf11b mRNA (G) or OPG protein (I). J–L, Pam3 (10 ng/ml), HKLM (107 colony-forming units (CFU)) and FSL-1 (0.1 μg/ml)-stimulated 45Ca release (J) and the mRNA expression of Tnfsf11 (K) without affecting Tnfrsf11b (L). M and N, the stimulatory effect by LPS P. gingivalis and Pam2 on 45Ca release was unaffected by adding a mixture of antibodies neutralizing IL-1β, IL-6, IL-11, LIF, OSM, and TNF-α or by adding indomethacin (1 μmol/liter). *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with unstimulated controls (C, E, H, J, K, M, and N). LPS P. gingivalis (P.g.) was used at a concentration of 10 μg/ml in A, M, and N. Pam2 was used at a concentration of 10 ng/ml in A–I, M, and N. Data are the means of four-five observations, and S.E. is given as vertical bars when larger than the radius of the symbol.
Pam2, similar to LPS P. gingivalis, increased the mRNA expression in the parietal bones of Tnfrsf11a, Tnfsf11, Csf1, Csf1r, and Oscar without affecting that of Tnfrsf11b (Fig. 3F). Pam2 caused a robust, time-dependent enhanced Tnfsf11 mRNA expression but did not affect Tnfrsf11b mRNA (Fig. 3G). Quantitative PCR also confirmed that Pam2 increased the mRNA expression of Tnfrsf11a, Csf1r, Oscar, and Csf1 mRNA (data not shown). Pam2 significantly enhanced RANKL protein without affecting OPG in the parietal bones (Fig. 3, H and I).
Three additional TLR2 agonists, Pam3, HKLM (heat-killed preparation of Listeria monocytogenes), and FSL-1 (a synthetic lipoprotein from Mycoplasma salivarium), stimulated 45Ca release from mouse parietal bones (Fig. 3J) and robustly increased Tnfsf11 mRNA in the parietal bones (Fig. 3K) but did not impact Tnfrsf11b mRNA (Fig. 3L).
The Stimulatory Effect by LPS P. gingivalis Is Not Mediated by Osteotropic Cytokines or Prostaglandins
LPS P. gingivalis and Pam2 rapidly (1 h) and concentration-dependently increased the mRNA expression in the parietal bones of Il1b, Il6, Il11, Lif, Osm, and Tnfsf2 (encoding TNF-α) (data now shown), all known to stimulate bone resorption (4, 5). LPS P. gingivalis and Pam2 also enhanced the expression of Ptgs2 (encoding cyclooxygenase-2) and the release of prostaglandin E2 from the parietal bones (data not shown). Neutralizing IL-1β, IL-6, IL-11, LIF, OSM, and TNF-α by specific antibodies, either one-by-one (data not shown) or by adding all together (Fig. 3M), showed that the effects of LPS P. gingivalis and Pam2 on mineral release (Fig. 3M) and on Tnfsf11 mRNA expression (data now shown) were independent of these proinflammatory mediators. Blocking prostaglandin synthesis with indomethacin did not affect LPS P. gingivalis- and Pam2-stimulated 45Ca release (Fig. 3N), but it did partially reduce Tnfsf11 mRNA (data not shown).
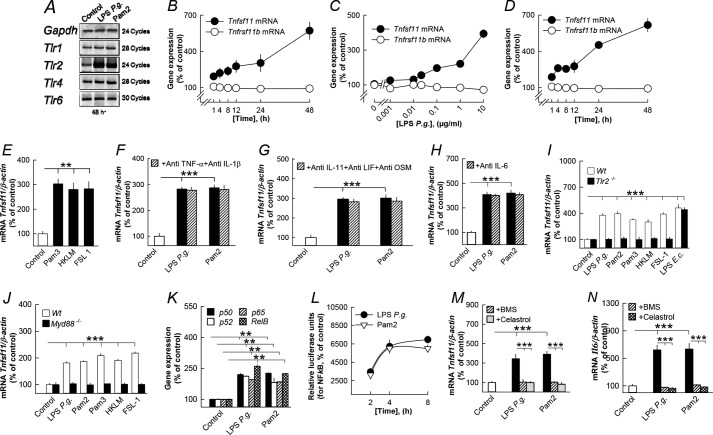
LPS P. gingivalis Stimulates RANKL in Parietal Osteoblasts through TLR2
Because osteoblasts have been shown to produce RANKL in response to a variety of bone resorbing hormones and cytokines (4, 5, 47, 48), we investigated if these cells responded to the different TLR2 agonists with increased RANKL. Mouse parietal osteoblast cultures expressed Tlr1, Tlr2, Tlr4, and Tlr6 mRNA, and Tlr2 mRNA was up-regulated by LPS P. gingivalis and Pam2 (Fig. 4A). LPS P. gingivalis also caused a time- and concentration-dependent increase of Tnfsf11 mRNA expression in the isolated osteoblasts without affecting Tnfrsf11b mRNA (Fig. 4, B and C). Similarly, Pam2 increased Tnfsf11mRNA but not Tnfrsf11b mRNA in these cells (Fig. 4D). Increased Tnfsf11 mRNA in osteoblasts was also observed when cells were stimulated by Pam3, HKLM, and FSL-1 (Fig. 4E).
FIGURE 4.
Five different TLR2 agonists enhanced Tnfsf11 mRNA expression in mouse parietal osteoblasts by a mechanism dependent on TLR2, MyD88, and NF-κB but independent on cytokine formation. A, LPS P. gingivalis (P.g.) and Pam2 up-regulated Tlr2 without affecting Tlr1, Tlr4 or Tlr6. B and C, LPS P. gingivalis time- and concentration-dependently enhanced Tnfsf11 mRNA without affecting Tnfrsf11b. D, Pam2 time-dependently increased Tnfsf11 mRNA without affecting Tnfrsf11b. E, Pam3 (10 ng/ml), HKLM (107 CFU), and FSL-1 (0.1 μg/ml) stimulated Tnfsf11 mRNA. F–H, the stimulatory effect by LPS P. gingivalis and Pam2 on Tnfsf11 mRNA was unaffected by adding antibodies neutralizing IL-1β, IL-6, IL-11, LIF, OSM, and TNF-α. I, LPS P. gingivalis, Pam2, Pam3 (10 ng/ml), HKLM (107 CFU), and FSL-1 (0.1 μg/ml), but not LPS from E. coli (10 μg/ml), increased Tnfsf11 mRNA in osteoblasts from wild type (Wt) but not from Tlr2-deficient mice. J, LPS P. gingivalis, Pam2, Pam3 (10 ng/ml), HKLM (107 CFU), and FSL-1 (0.1 μg/ml) enhanced Tnfsf11 mRNA in osteoblasts from wild-type mice but not from MyD88-deficient mice. K, LPS P. gingivalis and Pam2 increased the mRNA expression of the four NF-κB subunits p50, p52, p65, and RelB. L, LPS P. gingivalis and Pam2 increased NF-κB-driven luciferase in transfected osteoblasts. M and N, the stimulatory effect by LPS P. gingivalis and Pam2 on Tnfsf11 (M) and Il6 mRNA (N) was inhibited by the two NF-κB inhibitors BMS (10 μmol/liter) and Celastrol (0.2 μmol/liter). **, p < 0.01; ***, p < 0.001 compared with unstimulated controls (E–K) or to LPS P. gingivalis-stimulated osteoblasts (M and N). LPS P. gingivalis was used at a concentration of 10 μg/ml in A, B, and F–K. Pam2 was used at a concentration of 10 ng/ml in A, D, and F–N. Data are the means of four-five observations, and S.E. is given as vertical bars when larger than the radius of the symbol.
LPS P. gingivalis and Pam2 increased the mRNA expression of Il1b, Il6, Il11, Lif, Osm, and Tnfsf2 in the parietal osteoblasts (data not shown). Also Pam3, HKLM, and FSL-1 increased the mRNA expression of Il1b, Il6, and Tnfsf2 in the parietal osteoblasts (data not shown). Neutralization of IL-1β, TNF-α, IL-11, LIF, OSM, and IL-6 did not affect LPS P. gingivalis- or Pam2-induced Tnfsf11 mRNA in the osteoblasts (Fig. 4, F–H).
Using osteoblasts isolated from Tlr2-deficient mice we found that Tnfsf11 mRNA induced by LPS P. gingivalis, Pam2, Pam3, HKLM, and FSL-1, but not by LPS E. coli, was dependent on Tlr2 expression (Fig. 4I).
LPS P. gingivalis Stimulates RANKL in Parietal Periosteal Osteoblasts through MyD88 and NF-κB
We next sought to determine by which mechanism stimulation of TLR2 in osteoblasts results in increased Tnfsf11 expression. First, we found that stimulation of Tnfsf11 mRNA in osteoblasts by LPS P. gingivalis, Pam2, Pam3, HKLM, and FSL-1 was critically dependent on the presence of MyD88 (Fig. 4J). Next, we showed that LPS P. gingivalis and Pam2 activated NF-κB as assessed both by increased mRNA expression of p50, p52, p65, and RelB (Fig. 4K) and by activation of a luciferase reporter gene driven by NF-κB (Fig. 4L). Stimulation of Tnfsf11 mRNA as well as of the well known NF-κB target Il6 by LPS P. gingivalis and Pam2 was abolished by BMS and Celastrol, two inhibitors of NF-κB activation acting either on IKKα/IKKβ (BMS) or on TAK1 upstream of IKKβ involved in canonical activation of NF-κB (Celastrol) (Fig. 4, M and N).
P. gingivalis and Pam2 Increase Osteoclast Formation in Vivo by a TLR2-dependent Mechanism
To investigate the in vivo relevance of our in vitro findings, we injected LPS P. gingivalis and Pam2 subcutaneously over skull bones in 5-week-old mice. Six days after the injections, the number of TRAP+ osteoclasts on the periosteal surface of the skull bones was enhanced by LPS P. gingivalis and Pam2 (Fig. 5, A and B). LPS P. gingivalis and Pam2 increased the mRNA expression of c-fos, Nfatc1, Acp5, Ctsk, and Tnfsf11 in the skull bones, effects absent in Tlr2 knock-out mice (Fig. 5, C–G). The enhanced number of osteoclasts resulted in extensive loss of bone in wild type compared with Tlr2-deficient mice as assessed by microcomputed tomography analyses in LPS P. gingivalis- and Pam2-treated mice (Fig. 5H).
FIGURE 5.
Injection of LPS from P. gingivalis (P.g.) and the TLR2 agonist Pam2 above skull bones stimulates osteoclast formation, expression of osteoclastic and osteoclastogenic genes, and bone loss in skull bones from 5-week-old mice. A and B, LPS P. gingivalis and Pam2 enhanced the number of tartrate-resistant acid phosphatase-positive, multinucleated osteoclasts (TRAP+MuOCL); the left panel of B shows a parietal bone 6 days after injection of vehicle, and the right panel of B shows osteoclasts in parietal bones 6 days after injection of LPS P. gingivalis. C–G, injection of LPS P. gingivalis or Pam2 increased the mRNA expression of c-fos, Nfatc1, Acp5, Ctsk, and Tnfsf11 after 3 days in skull bones from wild type (wt) but not from Tlr2-deficient mice. H, injection of LPS P. gingivalis or Pam2 resulted in bone loss after 6 days in skull bones from wild type but not in Tlr2-deficient mice. Images shown are representative of seven images per group. **, p < 0.01; ***, p < 0.001 compared with unstimulated controls (A and C–G). Data are the means of six-seven observations, and S.E. is given as vertical bars.
P. gingivalis Stimulates Osteoclast Formation in Periosteal/Endosteal Cell Cultures through RANKL
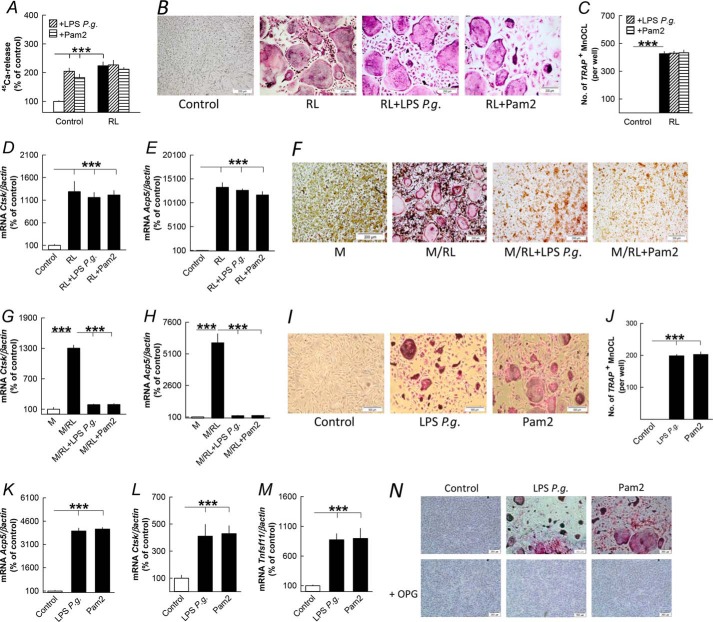
It has been reported that P. gingivalis bacteria and Pam3 inhibit RANKL-induced osteoclast differentiation in BMM cultures (20) in contrast both to the RANKL-dependent stimulation of periosteal osteoclast formation observed in ex vivo cultures of mouse parietal bones and to the induction of osteoclastic genes observed in vivo in the present study. We, therefore, compared the effects by LPS P. gingivalis and Pam2 on osteoclast formation using cells from either periosteum/endosteum or bone marrow.
Co-stimulation with RANKL and either LPS P. gingivalis or Pam2 did not affect RANKL-induced 45Ca release from parietal bones (Fig. 6A). Nor was RANKL-induced osteoclast formation (Fig. 6, B and C) or the expression of osteoclastic genes Ctsk and Acp5 (Fig. 6, D and E) in isolated periosteal/endosteal cell cultures affected by co-treatment with LPS P. gingivalis or Pam2. In agreement with previous findings (20), LPS P. gingivalis and Pam2 abolished osteoclast formation and mRNA expression of Ctsk and Acp5 in BMM cultures stimulated by M-CSF/RANKL (Fig. 6, F–H).
FIGURE 6.
LPS from P. gingivalis and the TLR2 agonist Pam2 regulates bone resorption and osteoclast formation differently in parietal bones, periosteal/endosteal bone cell, and bone marrow cell cultures primed by RANKL (RL). A, RANKL (10 ng/ml)-stimulated 45Ca release from neonatal mouse parietal bones in organ culture was not affected by LPS P. gingivalis (10 μg/ml) or Pam2 (10 ng/ml). B–E, RL (10 ng/ml) stimulation of tartrate-resistant acid phosphatase-positive, multinucleated osteoclasts (TRAP+MuOCL), and mRNA expression of Ctsk and Acp5 in periosteal/endosteal cell cultures from mouse parietal bone were not affected by co-treatment with LPS P. gingivalis (P.g., 10 μg/ml) or Pam2 (10 ng/ml). F–H, increased formation of TRAP+ multinucleated osteoclasts and mRNA expression of Ctsk and Acp5 in M-CSF (30 ng/ml)- and RL (4 ng/ml)-stimulated mouse bone marrow cell cultures were abolished by co-treatment with LPS P. gingivalis and Pam2. I–M, LPS P. gingivalis (10 μg/ml) and Pam2 (10 ng/ml) increased formation of TRAP+ multinucleated osteoclasts and mRNA expression of Acp5, Ctsk and Tnfsf11 in periosteal/endosteal cell cultures from mouse parietal bone. N, the stimulatory effect by LPS P. gingivalis and Pam2 on osteoclast formation in periosteal/endosteal cell cultures was abolished by adding OPG (300 ng/ml) to the culture medium. ***, p < 0.001 compared with unstimulated controls. Data are the means of six-seven observations, and S.E. is given as vertical bars.
In the absence of exogenous RANKL, LPS P. gingivalis and Pam2 stimulated formation of TRAP+ MuOCLs (Fig. 6, I and J) and the expression of Acp5 and Ctsk (Fig. 6, K and L) in periosteal/endosteal cell cultures, an effect associated with increased mRNA expression of Tnfsf11 (Fig. 6M). Osteoclast formation in these cultures by LPS P. gingivalis and Pam2 was abolished by adding OPG (Fig. 6N).
Discussion
Previous studies have shown that oral infection with P. gingivalis not only causes local alveolar bone loss (17–19) but also enhances articular bone loss in arthritic mice (35–37). These studies do not reveal by which mechanisms P. gingivalis infection causes decreased bone mass. In the present study we show that locally injected LPS P. gingivalis subcutaneously above mice skull bones induces bone loss and excessive osteoclast formation due to enhanced osteoclastogenesis as assessed by increased expression of osteoclastogenic transcription factors and osteoclastic genes. Increased osteoclastogenesis might be due either to a direct effect by P. gingivalis on osteoclast progenitors or by an indirect effect due to increased RANKL/OPG ratio. In favor of the latter view, we show here for the first time that LPS P. gingivalis robustly enhances the mRNA expression of Tnfsf11 in vivo. To investigate if P. gingivalis can affect RANKL/OPG in osteoblasts, we studied the effect by LPS P. gingivalis in ex vivo cultures of mouse parietal bones and in isolated mouse parietal osteoblasts.
In the parietal bones, LPS P. gingivalis stimulated the release of mineral and the degradation of bone matrix. Similar to the observations in vivo, LPS P. gingivalis enhanced the number of mature osteoclasts and the expression of osteoclastic genes in addition to the osteoclastogenic transcription factor c-fos. In the organ-cultured bones, LPS P. gingivalis enhanced the RANKL/OPG ratio by a mechanism due exclusively to increased RANKL.
Because osteoblasts/osteocytes are important for RANKL production in physiological bone remodeling (47, 48), we assessed if osteoblasts also could produce RANKL in pathological bone resorption induced by P. gingivalis. Challenge of the osteoblasts with LPS P. gingivalis increased Tnfsf11 mRNA expression with no effect on Tnfrsf11b mRNA, which demonstrates that osteoblasts are target cells for P. gingivalis-induced RANKL production. Moreover, exogenous OPG abolished both LPS P. gingivalis-induced mineral release and the up-regulation of Ctsk mRNA expression without affecting the enhanced Tnfsf11 mRNA, showing that the bone-resorptive response by LPS P. gingivalis was totally dependent on increased RANKL.
We further demonstrated the important role of RANKL for P. gingivalis-induced osteoclast formation by using a cell culture system based upon isolation of periosteal/endosteal cells from mouse parietal bones containing both osteoblasts and osteoclast progenitor cells. LPS P. gingivalis robustly increased the formation of osteoclasts and the expression of osteoclastic genes and Tnfsf11 mRNA, similar to the observations in the intact bones in vivo and ex vivo. Also in this system, the LPS P. gingivalis-induced osteoclast formation was totally dependent on RANKL as osteoclast formation was abolished by adding OPG. Although we demonstrate here the potent stimulatory effect by LPS P. gingivalis on RANKL formation in osteoblasts, we cannot, however, exclude that other cells present in vivo in the inflammatory reaction also contribute to the RANKL response.
Because previous studies have shown that co-stimulation of osteoclast progenitors from bone marrow with RANKL and P. gingivalis inhibits osteoclast differentiation (20), we wondered why differentiation of osteoclast progenitors present on the surfaces of parietal bones was not inhibited but, on the contrary, was stimulated. The fact that RANKL-stimulated mineral release in the parietal bones and that RANKL-stimulated osteoclast formation in the periosteal/endosteal cell cultures was unaffected by co-stimulation with LPS P. gingivalis whereas co-stimulation in the BMM cultures abolished osteoclast formation, shows that the osteoclastic P. gingivalis response in osteoclast progenitors on the bone surfaces are different from that in bone marrow progenitors. We do not know if the difference is because periosteal/endosteal osteoclast progenitors lack TLR2 or if the surrounding cells make them insensitive to P. gingivalis-induced inhibition. The fact that co-cultures of mouse BMM and mouse parietal osteoblasts respond to the TLR2 agonists Pam2 and Pam3 with enhanced RANKL production and increased osteoclast formation argues for the latter explanation (23). It seems that observations made in BMM cultures might not be fully relevant to osteoclastogenesis at the bone surface. Because mature osteoclasts are formed only at bone surfaces, our findings suggest that studies on osteoclastogenesis also should include studies with osteoclast progenitors present at bone surfaces.
Similar to LPS P. gingivalis, four other TLR2 agonists (HKLM, FSL-1, Pam2, and Pam3) stimulated mineral release and Tnfsf11 mRNA in the parietal bones and in parietal osteoblasts, an effect lost in osteoblasts from Tlr2-deficient mice. These data show that TLR2 activation in osteoblasts is linked to RANKL formation, osteoclast formation, and bone resorption. In agreement with these findings, bone loss and enhanced mRNA expression of Acp5 and Ctsk as well as increased Tnfsf11 mRNA were not observed in skull bones in Tlr2-deficient mice when LPS P. gingivalis or Pam2 were injected subcutaneously. Similarly, decreased alveolar bone volume observed in mice with oral infection of P. gingivalis was not seen in Tlr2-deficient mice (17, 19). All together, these findings show that P. gingivalis can stimulate osteoclast formation, bone loss, and RANKL production by activating TLR2, although we cannot exclude that P. gingivalis bacteria can affect bone cells also through other pattern recognition receptors than TLR2.
We next evaluated by which mechanism LPS P. gingivalis and Pam2 stimulate Tnsf11 mRNA in osteoblasts and found the presence of the adapter protein MyD88 to be crucial, similar to the inhibitory effect in RANKL-stimulated BMM and the stimulatory effect in RANKL-primed BMM (20, 23). We then evaluated the importance of NF-κB and found that LPS P. gingivalis and Pam2 activated NF-κB as demonstrated by increased mRNA expression of the four NF-κB subunits p50, p65, p52, and RelB and by activation of a NF-κB reporter gene transfected in the osteoblasts. The crucial role of NF-κB was shown by the finding that two NF-κB inhibitors, BMS and Celastrol, abolished LPS P. gingivalis- and Pam2-induced Tnfsf11 mRNA. BMS inhibits both canonical and non-canonical NF-κB pathways by inhibiting IKKα and IKKβ, whereas Celastrol inhibits TAK1, which is upstream IKKβ activation in the canonical pathway (49).
Recently it was shown in an elegant study that Tlr2−/− mice becomes sensitive to P. gingivalis-induced alveolar bone loss after adoptive transfer of wild type bone marrow-derived macrophages (18). This finding suggests an important role of macrophages in P. gingivalis-induced bone loss in mice with global deletion of Tlr2, including in osteoblasts. One possibility might be that P. gingivalis stimulates macrophages to differentiate to mature osteoclasts. Another reason might be that P. gingivalis stimulates macrophages to release cytokines, thus enhancing RANKL production in osteoblasts. The knowledge about the relative role of osteoblasts and macrophages as primary targets in P. gingivalis-induced bone loss has to await studies using mice with cell-specific deletion of Tlr2.
Here we report that activation of TLR2 in osteoblasts by P. gingivalis increases RANKL production, osteoclast formation, and bone loss both ex vivo and in vivo. Our findings provide an explanation of why P. gingivalis can stimulate alveolar bone loss but might also contribute to our understanding of why oral infection with P. gingivalis seems to cause a more severe loss of juxta-articular bone in RA. TLR2, which is highly expressed in RA synovium (50–52), is not only activated by pathogen-associated molecular patterns such as P. gingivalis but also by endogenous ligands present in RA synovium such as gp96 (53) and Snapin (54). Our data may also help to explain the role of endogenous ligands in the pathogenesis of RA bone erosions.
Author Contributions
A. K. performed most of the experiments and analyses, P. H. performed the gene reporter and MyD88 osteoblast experiments, P. L. and P. S. contributed to study conception and design, C. L. performed immunohistochemical analysis and contributed to study conception and design, and U. H. L. designed and supervised the project. All authors were involved in drafting the article or revising it critically for important intellectual content.
Acknowledgments
We are grateful for the skillful technical assistance by Anita Lie, Ingrid Boström, Inger Lundgren, and Anette Hansevi. We also thank Ann Conaway for editing the manuscript.
This work was supported by the Swedish Research Council, Swedish National Graduate School in Odontological Science, Swedish Dental Association, Swedish Rheumatism Association, Royal 80-Year Fund of King Gustav V, COMBINE, an ALF/LUA research grant from Sahlgrenska University Hospital in Gothenburg, the Lundberg Foundation, and County Council of Västerbotten. The authors declare that they have no conflicts of interest with the contents of this article.
- RA
- rheumatoid arthritis
- RANKL
- receptor activator of NF-κB ligand
- BMM
- bone marrow macrophages
- CTX
- type I collagen degradation fragments
- FSL-1
- a synthetic lipoprotein from M. salivarium
- HKLM
- heat-killed preparation of L. monocytogenes
- M-CSF
- macrophage colony-stimulating factor
- OSM
- oncostatin M
- OPG
- osteoprotegerin
- Pam2
- palmitoyl-2-Cys-Ser-(Lys)4
- Pam3
- palmitoyl-3-Cys-Ser-(Lys)4
- TRAP
- tartrate-resistant acid phosphatase
- TRAP+MuOCL
- TRAP-positive multinucleated osteoclasts
- BMS
- BMS-345541
- TLR
- toll-like receptor.
References
- 1. Walsh N. C., Gravallese E. M. (2010) Bone remodeling in rheumatic disease: a question of balance. Immunol. Rev. 233, 301–312 [DOI] [PubMed] [Google Scholar]
- 2. Bartold P. M., Cantley M. D., Haynes D. R. (2010) Mechanisms and control of pathologic bone loss in periodontitis. Periodontol. 2000 53, 55–69 [DOI] [PubMed] [Google Scholar]
- 3. Schett G., Gravallese E. (2012) Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 8, 656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Souza P. P., Lerner U. H. (2013) The role of cytokines in inflammatory bone loss. Immunol Invest. 42, 555–622 [DOI] [PubMed] [Google Scholar]
- 5. Lorenzo J., Horowitz M., Choi Y. (2008) Osteoimmunology: interactions of the bone and immune system. Endocr. Rev. 29, 403–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garlet G. P. (2010) Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 89, 1349–1363 [DOI] [PubMed] [Google Scholar]
- 7. Liu Y. C., Lerner U. H., Teng Y. T. (2010) Cytokine responses against periodontal infection: protective and destructive roles. Periodontol. 2000 52, 163–206 [DOI] [PubMed] [Google Scholar]
- 8. Bar-Shavit Z. (2008) Taking a toll on the bones: regulation of bone metabolism by innate immune regulators. Autoimmunity 41, 195–203 [DOI] [PubMed] [Google Scholar]
- 9. Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 10. Erridge C. (2010) Endogenous ligands of TLR2 and TLR4: agonists or assistants? J. Leukoc. Biol. 87, 989–999 [DOI] [PubMed] [Google Scholar]
- 11. Itoh K., Udagawa N., Kobayashi K., Suda K., Li X., Takami M., Okahashi N., Nishihara T., Takahashi N. (2003) Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J. Immunol. 170, 3688–3695 [DOI] [PubMed] [Google Scholar]
- 12. Sato N., Takahashi N., Suda K., Nakamura M., Yamaki M., Ninomiya T., Kobayashi Y., Takada H., Shibata K., Yamamoto M., Takeda K., Akira S., Noguchi T., Udagawa N. (2004) MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1α. J. Exp. Med. 200, 601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakamura H., Fukusaki Y., Yoshimura A., Shiraishi C., Kishimoto M., Kaneko T., Hara Y. (2008) Lack of Toll-like receptor 4 decreases lipopolysaccharide-induced bone resorption in C3H/HeJ mice in vivo. Oral Microbiol. Immunol. 23, 190–195 [DOI] [PubMed] [Google Scholar]
- 14. Holt S. C., Ebersole J. L. (2005) Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex,” a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 38, 72–122 [DOI] [PubMed] [Google Scholar]
- 15. Pihlstrom B. L., Michalowicz B. S., Johnson N. W. (2005) Periodontal diseases. Lancet. 366, 1809–1820 [DOI] [PubMed] [Google Scholar]
- 16. Darveau R. P., Pham T. T., Lemley K., Reife R. A., Bainbridge B. W., Coats S. R., Howald W. N., Way S. S., Hajjar A. M. (2004) Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect. Immun. 72, 5041–5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burns E., Bachrach G., Shapira L., Nussbaum G. (2006) Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 177, 8296–8300 [DOI] [PubMed] [Google Scholar]
- 18. Papadopoulos G., Weinberg E. O., Massari P., Gibson F. C., 3rd, Wetzler L. M., Morgan E. F., Genco C. A. (2013) Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J. Immunol. 190, 1148–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin J., Bi L., Yu X., Kawai T., Taubman M. A., Shen B., Han X. (2014) Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect Immun. 82, 4127–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang P., Liu J., Xu Q., Harber G., Feng X., Michalek S. M., Katz J. (2011) TLR2-dependent modulation of osteoclastogenesis by Porphyromonas gingivalis through differential induction of NFATc1 and NF-κB. J. Biol. Chem. 286, 24159–24169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang J., Ryu Y. H., Yun C. H., Han S. H. (2009) Impaired osteoclastogenesis by staphylococcal lipoteichoic acid through Toll-like receptor 2 with partial involvement of MyD88. J. Leukoc. Biol. 86, 823–831 [DOI] [PubMed] [Google Scholar]
- 22. Koh J. M., Lee Y. S., Kim Y. S., Park S. H., Lee S. H., Kim H. H., Lee M. S., Lee K. U., Kim G. S. (2009) Heat shock protein 60 causes osteoclastic bone resorption via toll-like receptor-2 in estrogen deficiency. Bone 45, 650–660 [DOI] [PubMed] [Google Scholar]
- 23. Kim J., Yang J., Park O. J., Kang S. S., Kim W. S., Kurokawa K., Yun C. H., Kim H. H., Lee B. L., Han S. H. (2013) Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. J. Bone Miner. Res. 28, 2381–2391 [DOI] [PubMed] [Google Scholar]
- 24. de Pablo P., Chapple I. L., Buckley C. D., Dietrich T. (2009) Periodontitis in systemic rheumatic diseases. Nat. Rev. Rheumatol. 5, 218–224 [DOI] [PubMed] [Google Scholar]
- 25. Mercado F., Marshall R. I., Klestov A. C., Bartold P. M. (2000) Is there a relationship between rheumatoid arthritis and periodontal disease? J. Clin. Periodontol. 27, 267–272 [DOI] [PubMed] [Google Scholar]
- 26. Pischon N., Pischon T., Kröger J., Gülmez E., Kleber B. M., Bernimoulin J. P., Landau H., Brinkmann P. G., Schlattmann P., Zernicke J., Buttgereit F., Detert J. (2008) Association among rheumatoid arthritis, oral hygiene, and periodontitis. J. Periodontol. 79, 979–986 [DOI] [PubMed] [Google Scholar]
- 27. Scher J. U., Ubeda C., Equinda M., Khanin R., Buischi Y., Viale A., Lipuma L., Attur M., Pillinger M. H., Weissmann G., Littman D. R., Pamer E. G., Bretz W. A., Abramson S. B. (2012) Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 64, 3083–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Katma M. K., Bissada N. F., Bordeaux J. M., Sue J., Askari A. D. (2007) Control of periodontal infection reduces the severity of active rheumatoid arthritis. J. Clin. Rheumatol. 13, 134–137 [DOI] [PubMed] [Google Scholar]
- 29. Ortiz P., Bissada N. F., Palomo L., Han Y. W., Al-Zahrani M. S., Panneerselvam A., Askari A. (2009) Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J. Periodontol. 80, 535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moen K., Brun J. G., Valen M., Skartveit L., Eribe E. K., Olsen I., Jonsson R. (2006) Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin. Exp. Rheumatol. 24, 656–663 [PubMed] [Google Scholar]
- 31. Martinez-Martinez R. E., Abud-Mendoza C., Patiño-Marin N., Rizo-Rodríguez J. C., Little J. W., Loyola-Rodríguez J. P. (2009) Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J. Clin. Periodontol. 36, 1004–1010 [DOI] [PubMed] [Google Scholar]
- 32. Hitchon C. A., Chandad F., Ferucci E. D., Willemze A., Ioan-Facsinay A., van der Woude D., Markland J., Robinson D., Elias B., Newkirk M., Toes R. M., Huizinga T. W., El-Gabalawy H. S. (2010) Antibodies to Porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J. Rheumatol. 37, 1105–1112 [DOI] [PubMed] [Google Scholar]
- 33. Mikuls T. R., Levan T., Gould K. A., Yu F., Thiele G. M., Bynote K. K., Conn D., Jonas B. L., Callahan L. F., Smith E., Brasington R., Moreland L. W., Reynolds R., Gaffo A., Bridges S. L., Jr. (2012) Impact of interactions of cigarette smoking with NAT2 polymorphisms on rheumatoid arthritis risk in African Americans. Arthritis Rheum. 64, 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lundberg K., Wegner N., Yucel-Lindberg T., Venables P. J. (2010) Periodontitis in RA-the citrullinated enolase connection. Nat. Rev. Rheumatol. 6, 727–730 [DOI] [PubMed] [Google Scholar]
- 35. Bartold P. M., Marino V., Cantley M., Haynes D. R. (2010) Effect of Porphyromonas gingivalis-induced inflammation on the development of rheumatoid arthritis. J. Clin. Periodontol. 37, 405–411 [DOI] [PubMed] [Google Scholar]
- 36. Cantley M. D., Haynes D. R., Marino V., Bartold P. M. (2011) Pre-existing periodontitis exacerbates experimental arthritis in a mouse model. J. Clin. Periodontol. 38, 532–541 [DOI] [PubMed] [Google Scholar]
- 37. de Aquino S. G., Abdollahi-Roodsaz S., Koenders M. I., van de Loo F. A., Pruijn G. J., Marijnissen R. J., Walgreen B., Helsen M. M., van den Bersselaar L. A., de Molon R. S., Avila Campos M. J., Cunha F. Q., Cirelli J. A., van den Berg W. B. (2014) Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J. Immunol. 192, 4103–4111 [DOI] [PubMed] [Google Scholar]
- 38. Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. (1998) Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 [DOI] [PubMed] [Google Scholar]
- 39. Ljunggren O., Ransjö M., Lerner U. H. (1991) In vitro studies on bone resorption in neonatal mouse calvariae using a modified dissection technique giving four samples of bone from each calvaria. In vitro studies on bone resorption in neonatal mouse calvariae using a modified dissection technique giving four samples of bone from each calvaria. J. Bone Miner. Res. 6, 543–550 [DOI] [PubMed] [Google Scholar]
- 40. Lerner U. H. (1987) Modifications of the mouse calvarial technique improve the responsiveness to stimulators of bone resorption. J. Bone Miner. Res. 2, 375–383 [DOI] [PubMed] [Google Scholar]
- 41. Granholm S., Henning P., Lindholm C., Lerner U. H. (2013) Osteoclast progenitor cells present in significant amounts in mouse calvarial osteoblast isolations and osteoclastogenesis increased by BMP-2. Bone 52, 83–92 [DOI] [PubMed] [Google Scholar]
- 42. Takeshita S., Kaji K., Kudo A. (2000) Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 15, 1477–1488 [DOI] [PubMed] [Google Scholar]
- 43. Conaway H. H., Pirhayati A., Persson E., Pettersson U., Svensson O., Lindholm C., Henning P., Tuckermann J., Lerner U. H. (2011) Retinoids stimulate periosteal bone resorption by enhancing the protein RANKL, a response inhibited by monomeric glucocorticoid receptor. J. Biol. Chem. 286, 31425–31436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palmqvist P., Lundberg P., Persson E., Johansson A., Lundgren I., Lie A., Conaway H. H., Lerner U. H. (2006) Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J. Biol. Chem. 281, 2414–2429 [DOI] [PubMed] [Google Scholar]
- 45. Schwab A. M., Granholm S., Persson E., Wilkes B., Lerner U. H., Conaway H. H. (2005) Stimulation of resorption in cultured mouse calvarial bones by thiazolidinediones. Endocrinology 146, 4349–4361 [DOI] [PubMed] [Google Scholar]
- 46. Ozinsky A., Underhill D. M., Fontenot J. D., Hajjar A. M., Smith K. D., Wilson C. B., Schroeder L., Aderem A. (2000) The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. U.S.A. 97, 13766–13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xiong J., Onal M., Jilka R. L., Weinstein R. S., Manolagas S. C., O'Brien C. A. (2011) Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J. Q., Bonewald L. F., Kodama T., Wutz A., Wagner E. F., Penninger J. M., Takayanagi H. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 [DOI] [PubMed] [Google Scholar]
- 49. Idris A. I., Krishnan M., Simic P., Landao-Bassonga E., Mollat P., Vukicevic S., Ralston S. H. (2010) Small molecule inhibitors of IκB kinase signaling inhibit osteoclast formation in vitro and prevent ovariectomy-induced bone loss in vivo. FASEB J. 24, 4545–4555 [DOI] [PubMed] [Google Scholar]
- 50. Radstake T. R., Roelofs M. F., Jenniskens Y. M., Oppers-Walgreen B., van Riel P. L., Barrera P., Joosten L. A., van den Berg W. B. (2004) Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-γ. Arthritis Rheum. 50, 3856–3865 [DOI] [PubMed] [Google Scholar]
- 51. Ospelt C., Brentano F., Rengel Y., Stanczyk J., Kolling C., Tak P. P., Gay R. E., Gay S., Kyburz D. (2008) Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 58, 3684–3692 [DOI] [PubMed] [Google Scholar]
- 52. Iwahashi M., Yamamura M., Aita T., Okamoto A., Ueno A., Ogawa N., Akashi S., Miyake K., Godowski P. J., Makino H. (2004) Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 50, 1457–1467 [DOI] [PubMed] [Google Scholar]
- 53. Huang Q. Q., Sobkoviak R., Jockheck-Clark A. R., Shi B., Mandelin A. M., 2nd, Tak P. P., Haines G. K., 3rd, Nicchitta C. V., Pope R. M. (2009) Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J. Immunol. 182, 4965–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shi B., Huang Q., Tak P. P., Vervoordeldonk M. J., Huang C. C., Dorfleutner A., Stehlik C., Pope R. M. (2012) SNAPIN: an endogenous Toll-like receptor ligand in rheumatoid arthritis. Ann. Rheum. Dis. 71, 1411–1417 [DOI] [PubMed] [Google Scholar]