Background: NRG1 and its receptor ErbB4 are schizophrenia susceptibility genes.
Results: NRG1 induced the up-regulation of EAAC1 with an increase in glutamate uptake. Conclusion: NRG1/ErbB4 signaling influences glutamate uptake by increasing the EAAC1 protein level.
Significance: These results contribute to understanding of a possible mechanism of NRG1/ErbB4 signaling that may be linked to the neural circuitry disruption in schizophrenia.
Keywords: γ-Aminobutyric acid (GABA), neuron, neurotransmitter transport, neurotrophic factor, schizophrenia, EAAC1, ErbB4, Glutamatergic hypofunction, Neuregulin 1
Abstract
Neuregulin 1 (NRG1) is a trophic factor that is thought to have important roles in the regulating brain circuitry. Recent studies suggest that NRG1 regulates synaptic transmission, although the precise mechanisms remain unknown. Here we report that NRG1 influences glutamate uptake by increasing the protein level of excitatory amino acid carrier (EAAC1). Our data indicate that NRG1 induced the up-regulation of EAAC1 in primary cortical neurons with an increase in glutamate uptake. These in vitro results were corroborated in the prefrontal cortex (PFC) of mice given NRG1. The stimulatory effect of NRG1 was blocked by inhibition of the NRG1 receptor ErbB4. The suppressed expression of ErbB4 by siRNA led to a decrease in the expression of EAAC1. In addition, the ablation of ErbB4 in parvalbumin (PV)-positive neurons in PV-ErbB4−/− mice suppressed EAAC1 expression. Taken together, our results show that NRG1 signaling through ErbB4 modulates EAAC1. These findings link proposed effectors in schizophrenia: NRG1/ErbB4 signaling perturbation, EAAC1 deficit, and neurotransmission dysfunction.
Introduction
Neuregulin 1 (NRG1)3 signaling proteins contain an epidermal growth factor (EGF)-like motif, which binds to and activates the ErbB receptor tyrosine kinase. NRG1 has multiple actions during synaptogenesis in the developing brain. Recent studies have clearly shown its role in the regulation of synaptic plasticity and neurotransmission (1). NRG1 acts to suppress the induction of long-term potentiation (LTP) (2, 3) in the hippocampus (2–5). In vitro studies have shown that NRG1 suppresses NMDA receptor-mediated currents in prefrontal cortex (PFC) neurons (6). NRG1 has also been shown to induce the internalization of surface AMPA receptors in hippocampal neurons (2). ErbB4 expression in the brain is mainly restricted to γ-amino butyric acid (GABA) ergic interneurons (4, 7). NRG1 has been shown to stimulate GABA release in PFC slices through the ErbB4 receptor (8). NRG1 suppresses LTP by enhancing GABA release. Interestingly, these effects require ErbB4 in parvalbumin (PV)-positive neurons (9). ErbB4 is located predominantly in the PV-positive interneurons (4, 10) that contribute to regulate the neuronal network balance. Which synaptic molecules are linked with the NRG1-mediated regulation of neuronal activity remains unclear.
The concentrations of synaptic glutamate are tightly regulated by Na+-dependent high affinity glutamate transporters that ensure crisp synaptic neurotransmission. This family consists of five members: GLAST, GLT-1, EAAC1, EAAT4, and EAAT5 (also known collectively as EAAT1–5). Excitatory amino acid carrier 1 (EAAC1) is a glutamate transporter present in the presynaptic pool, in opposition to two other main transporters, GLAST and GLT1, that are expressed at the excitatory amino acid synapse by surrounding astrocytes (11). EAAC1 is found throughout the brain on the somas and dendrites of small and large pyramidal neurons (12–14). EAAC1 is also localized to presynaptic GABA containing terminals and may have a metabolic role in providing glutamate for GABA metabolism (13, 15). The loss of brain EAAC1 expression interferes with GABA synthesis and results in epilepsy (12, 16). Moreover, EAAC1 expression is altered in pathological conditions, such as hypoxia/ischemia, multiple sclerosis, schizophrenia, and epilepsy (17). These findings suggest that EAAC1 may be important for the function or development of GABAergic metabolism and neurotransmission in the brain.
Our study demonstrates that NRG1/ErbB4 signaling influences glutamate uptake by regulating EAAC1 and suggests that this pathway can contribute to neurotransmission and to the etiology of schizophrenia.
Experimental Procedures
Materials and Animals
NRG1βEGF-like domain peptide was obtained from Prospec-Tany TechnoGene. Antibodies were supplied by Santa Cruz Biotechnology Inc. (Santa Cruz, CA) (ErbB4, sc-283; ErbB2, sc-284; EAAT1, sc-15316; EAAT2, sc-7760; EAAT3, sc-25658; ERK2, sc-100752; Mouse IgG, sc-2025; Rabbit IgG, sc-66931; β-actin, sc-47778; HRP-conjugated anti-rabbit IgG, sc-2004; HRP-conjugated anti-mouse IgG, sc-2005 and HRP-conjugated anti-goat IgG, sc-2020), Millipore Corp. (Chemicon, MA) (EAAT3(EAAC1), MAB1587), Abcam (Cambridge, MA) (PV, ab11427), and Synaptic System (Gottingen, Germany) (PV, 195011C3). AG1478 and AG879 were from Calbiochem. (3S)-3-[[3-[[4-(trifluoromethyl) benzoyl] amino]phenyl]methoxy]-l-aspartic acid (TFB-TBOA) was from Tocris. DMSO was from Sigma. PV-ErbB4 mice and GAD (glutamic acid decarboxylase)-GFP mice were kindly provided by Prof. Lin Mei at Georgia Regents University (9). Experiments with animals were performed in accordance with institutional and Eulji University guidelines.
Cell Culture
Primary cortical neurons were cultured essentially as described (8). Dissociated cortical neurons from E18 Sprague-Dawley rat embryos were cultured for 14 days after seeding (DIV) in Neurobasal medium supplemented with B27 (Gibco, Carlsbad, CA). Rat C6 glioma cells were obtained from the Korean cell line bank (Seoul, Korea) and grown in DMEM supplemented with 10% fetal bovine serum and a penicillin-streptomycin mixture.
RT-PCR and Quantitative Real-time PCR (qRT-PCR)
Total RNA was isolated from primary cortical neurons and C6 cells with TRIzol (Invitrogen). The following primers were used for PCR: slc1a1 (S), 5′-atgcttctgcctcgtctttggac-3′, (AS), 5′-atgcccagcgattaggaacaaaa-3′. All of the primers were designed with the help of primer 3 programs according to the known or predicted rat sequences reported in GenBankTM. qRT-PCR was performed using Bio-Rad Bio-Plex Systems with the DyNAmo SYBR Green qRT-PCR Kit (Finnzymes). For all of the probands, each cycle consisted of a denaturation step at 95 °C for 10 s, followed by separate annealing (20 s) and extension (30 s) steps at a temperature characteristic for each proband. Fluorescence was monitored at the end of each extension step. The specificity of each PCR product was verified by performing dissociation reaction plots. Data were normalized to GAPDH. Real-time PCR data presented here had a p < 0.05 in Student's t test.
Small Interfering RNA (siRNA) Transfection
siRNAs targeting rat ErbB4, negative control (AllStars Negative Control siRNA) and positive control (Mapk1 Control siRNA) were designed and synthesized by Qiagen with the following sequences: ErbB4–1, 5′-AAGGATAACATCGGATCACAA-3′; ErbB4–2, 5′-ACCGAGTTAGTCGAGCCCTTA-3′; ErbB4–3, 5′-TACGCATTATTCGTGGGACAA-3′; ErbB4–4, 5′-TCGCTATGCCTTAGCAATATT-3′. Cells were transfected with each siRNA using HiPerFect transfection reagent according to the manufacturer's protocol (Qiagen).
Western Blotting
Immunoblotting was performed as previously described (8). Samples were separated on a polyacrylamide gel and transferred to PVDF membranes. The membranes were blocked with TBS containing 0.05% Tween-20 and 5% skim milk. The blots were then reacted with primary antibodies overnight at 4 °C and subsequently incubated using secondary antibodies conjugated with horseradish peroxidase and an enhanced chemiluminescent substrate (Amersham Biosciences Pharmacia).
Immunofluorescence
Immunostaining of ratprimary cortical neurons (E18, DIV14), C6 glioma cells, and brain sections were performed as described (8). Briefly, the cells were reacted in the blocking solution containing antibodies against ErbB4 (1:100), EAAC1 (1:100), and EAAT1 (1:100) at 4 °C overnight. The cells were then incubated with FITC-conjugated goat anti-mouse IgG or Cy3-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., 1:200) in buffer for 2 h at room temperature. Nuclei were counterstained with Hoechst (10 μm in PBS) for 30 min. PFC sections (20 μm) were prepared and incubated in the blocking solution containing rabbit anti-PV and mouse anti-EAAC1. GAD-GFP-positive terminals were examined by excitation at 488 nm. Immunoactivity were visualized by Oyster 550 fluorescence-labeled anti-PV and Alexa 488 or Alexa 595-conjugated secondary antibodies for anti-EAAC1. Stained cells were mounted in Vectorshield (Vector Laboratories) and observed under the LSM 510 laser scanning microscope (Carl Zeiss, Germany).
In Vitro Glutamate Uptake Assay
Glutamate uptake was measured as described previously (18). After the cells were washed twice with uptake buffer (pH 7.4, 10 mm glucose, 5 mm KCl, 127 mm NaCl, 10 mm Hepes, 2.5 mm CaCl2, 1.2 mm MgSO4, and 1.3 mm KH2PO4), glutamate uptake measurements were initiated by adding L-[3H]glutamate (250 μCi/μmol, PerkinElmer Life Sciences, Boston, MA) at 5 μm final concentration (4 μm cold plus 1 μm radioactive glutamate). l-Glutamate uptake was terminated after 2 min incubations at 37 °C. The reaction was stopped by adding cold, sodium-free buffer. To dissolve the cells, 1 N NaOH was added to the culture dishes and the radioactivity was measured by a Liquid Scintillation Analyzer Tri-Carb 2900TR (PerkinElmer). The values of L-[3H]glutamate incorporation were divided by protein content. Na+-dependent uptake was calculated as the difference in radioactivity accumulated in the presence and absence of Na+.
Biotinylation
The biotinylation assay was performed as described previously (18). In brief, cells were rinsed three times with ice-cold PBS containing 0.1 mm calcium and 1.0 mm magnesium (Ca/Mg PBS). The cells were then incubated with 1 mg/ml sulfo-NHS-SS-biotin in Ca/Mg PBS for 20 min at 4 °C with gentle shaking. The unreacted biotin was removed, and the biotin was quenched using Ca/Mg PBS containing 100 mm glycine. The cells were lysed with RIPA buffer containing protease and phosphatase inhibitors. The lysates were centrifuged at 16,500 × g for 30 min to remove cellular debris. Aliquots of the lysate were further incubated with an equal volume of a 50% slurry of avidin beads for 1 h and centrifuged at 16,500 × g for 10 min at 4 °C. SDS sample buffer was added to the cell lysate, biotinylated proteins (cell surface proteins), and non-biotinylated proteins (intracellular proteins). Each of these fractions was diluted and loaded so that the sum of immunoreactivity in the non-biotinylated and biotinylated fractions equaled the immunoreactivity in the lysate (if the yield from avidin extraction was 100%).
Intracerebroventricular (ICV) Infusion of NRG1 using an Osmotic Pump
The ICV infusion experiments were carried out with Alzet Micro-Osmotic Pumps (model 1004, DURECT, with a capacity of 100 μl and at a pump rate of 0.11 μl/h for 4 weeks) as described previously (19). The osmotic pumps were filled with NRG1 peptide (28 ng/kg) or PBS according to the manufacturer's instructions. A group of mice underwent ICV cannulation with the Alzet Brain Infusion kit (DURECT), and subcutaneous osmotic pumps were used to infuse into the right lateral ventricle of the brain. Mice were anesthetized via an intramuscular injection of Rompun (17.5 mg/kg) and Zoletil (12.5 mg/kg) and were sacrificed after infusion of NRG1 for 4-weeks. The mice were immediately intracardially perfused with PBS containing heparin. After perfusion, each brain was removed and stored at −70 °C.
Statistical Analysis
The results are expressed as the mean ± S.E. The differences between several groups were analyzed by one-way ANOVA followed by Bonferroni's post-hoc test. Comparisons between two groups were performed with Student's paired t test. Significance was accepted for a p value of < 0.05.
Results
NRG1 Induces the Up-regulation of EAAC1
Several previous studies have shown that NRG1 signaling regulates neurotransmission (8). However, it is not clear how NRG1 signaling controls the GABA circuit. Because EAAC1 expression has been found on presynaptic GABA terminals (20, 21), where the transporter is thought to supply glutamate as a precursor for GABA synthesis (11, 16), we hypothesized that NRG1 signaling may be involved in the regulation of EAAC1. Based on this hypothesis, we investigated the effect of NRG1 on the regulation of glutamate transporters in C6 cells. EAAC1 represents the predominant glutamate transporter expressed by C6 cells, although the expression of GLT1 and GLAST is also detectable at very low levels in these cells (22).
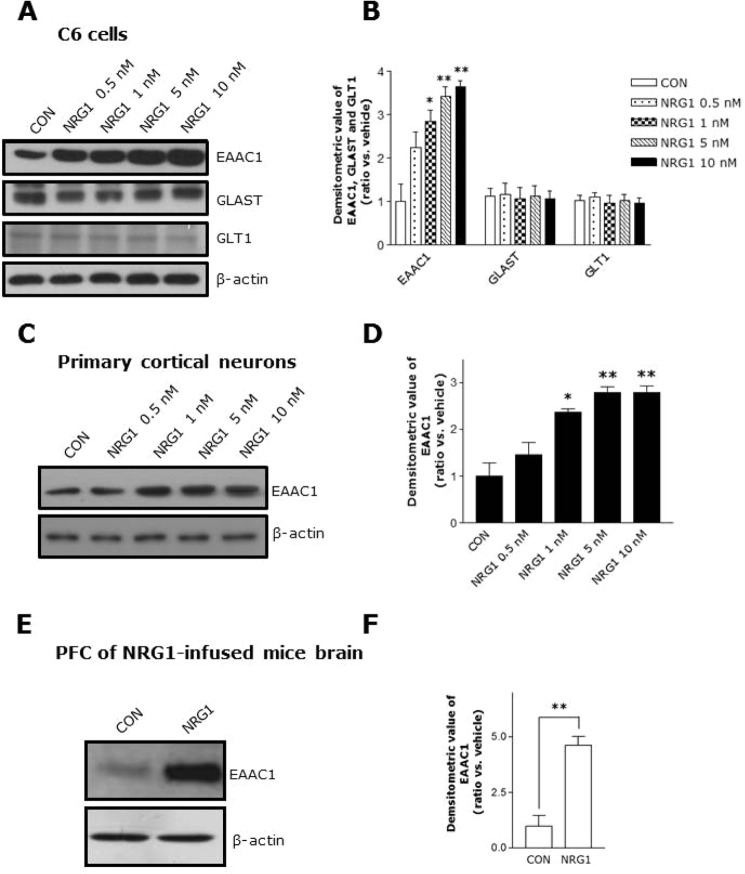
NRG1 showed no effect on the protein levels of the astroglial transporters (GLAST and GLT1); however, NRG1 treatment in C6 cells increased the neuronal transporter EAAC1. Moreover, this effect was shown in a concentration-dependent manner (Fig. 1, A and B). As shown in Fig. 1, C and D, NRG1 also up-regulated EAAC1 in primary cortical neurons.
FIGURE 1.
NRG1 up-regulates EAAC1 expression. A, C6 cells were treated with varying concentrations of NRG1 at 30 min and resulted in dose-dependent increases in EAAC1 expression. B, quantification of the data in A. n = 6. *, p < 0.05; **, p < 0.01. C, dose-dependent increases in EAAC1 expression in primary cortical neuronal cells. D, quantification of the data in C. n = 6. *, p < 0.05; **, p < 0.01. E, increased EAAC1 expression in PFC of NRG1-infused mice. Experimental scheme of the intraventricular infusion of the NRG1 peptide using an osmotic pump. NRG1 (28 ng/kg) or PBS was infused into 6-month-old mice. F, statistical analysis of the expression of EAAC1 in the PFC. n = 4. **, p < 0.01.
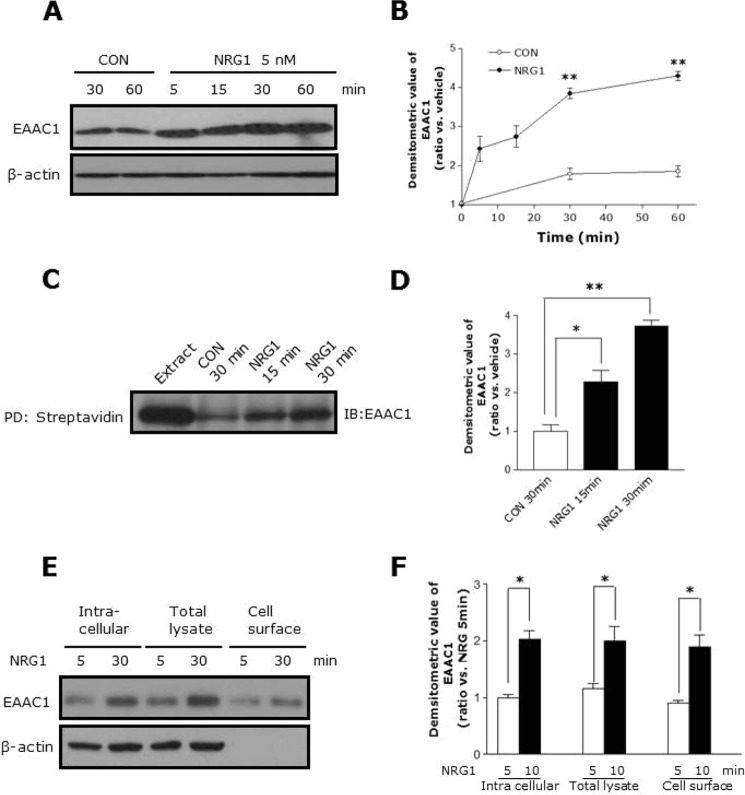
To verify these results in vivo, we infused vehicle or NRG1 into the lateral ventricle of mice brains using an osmotic pump with a stereotaxic apparatus. Consistent with the in vitro result, NRG1 dramatically increased EAAC1 protein levels in PFC of NRG1-infused mice brain (Fig. 1, E and F). Treatment with 5 nm NRG1 showed a maximal response in 30 min; this dose was used for further experiments (Fig. 2, A and B).
FIGURE 2.
Time course of the NRG1-induced increase in total and cell surface EAAC1. A, C6 cells were treated with 5 nm NRG1 for different periods of time. Time-dependent increases in total EAAC1 in C6 cell lysates. B, statistical analysis of the expression of total EAAC1 in a time-dependent manner. n = 8. **, p < 0.01. C, cell surface expression of EAAC1 was measured by biotinylation followed by batch extraction and analysis by Western blot. D, statistical analysis of expression of surface EAAC1 in the time-dependent manner. n = 8. *, p < 0.05; **, p < 0.01. E, representative Western blot probed with EAAC1 and actin antibodies demonstrating the effect of NRG1 on intracellular, total lysate, and cell surface expression of EAAC1 in C6 cells. F, bar graph summarizes the data in E. n = 8. *, p < 0.05.
The surface expression of EAAC1 is important for its function, so we investigated whether NRG1 affects the protein level of EAAC1 in the cell membrane by performing a biotinylation assay. NRG1 increased the surface expression of EAAC1 and increased the EAAC1 expression in total cell lysates (Fig. 2, C and D). However, NRG1 increased not only the expression of surface EAAC1 but also the intracellular expression of EAAC1 (Fig. 2, E and F). These results indicate that NRG1 regulates EAAC1 expression in the brain without affecting the astroglial transporters, GLAST and GLT1.
ErbB4 Is Necessary for EAAC1 Up-regulation by NRG1
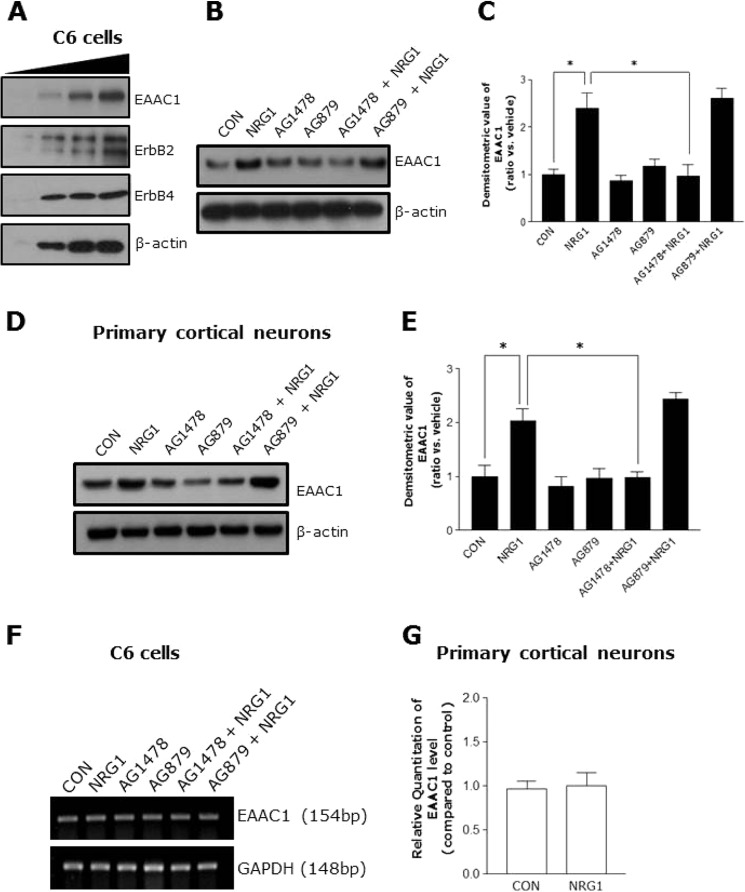
We investigated the possible role of ErbB receptors in the NRG1-mediated regulation of EAAC1 expression. The cells were treated with AG1478 (an inhibitor of ErbB4) and AG879 (an inhibitor of ErbB2) (8). First, we confirmed that EAAC1, ErbB2, and ErbB4 were expressed in C6 cells (Fig. 3A). Treatment with AG1478 prevented NRG1 from up-regulating EAAC1 in C6 cells, but treatment with AG879 did not (Fig. 3, B and C).
FIGURE 3.
NRG1 induced an increase of EAAC1 expression through activation of the ErbB4 receptor. A, Western blot analysis of EAAC1 (upper panel), ErbB2 (middle panel), and ErbB4 expression (lower panel) in C6 cells. The cell lysates were loaded with a different amount of protein (10, 20, 30, and 40 μg). B, inhibition of NRG1 by AG1478 induced the increase of EAAC1 expression. C6 cells were pretreated without or with 5 μm AG879 (ErbB2 inhibitor) and 5 μm AG1478 (ErbB4 inhibitor) for 15 min prior to the addition of NRG1 (5 nm, final concentration) for 30 min. C, quantification analysis of data in B. n = 6. *, p < 0.05. D, inhibition of ErbB4 blocks NRG1-induced increases of EAAC1 in primary cortical neurons. E, bar graph summarizes the data in D. n = 6. *, p < 0.05. F, no effect of NRG1 was observed on mRNA levels of EAAC1 in C6 cells (n = 6). G, qRT-PCR was performed to examine EAAC1 expression in cultured primary hippocampal neurons after treatment with 5 nm NRG1.
In addition, we investigated whether ErbB4 was involved in the EAAC1 up-regulating effect of NRG1in primary cortical neurons. Similar to C6 cells, treatment with AG1478, prevented NRG1 from up-regulating EAAC1 in the cells (Fig. 3, D and E). We checked the mRNA level of EAAC1 by performing RT-PCR and qRT- PCR after treating C6 cells and primary cortical neurons with 5 nm NRG1. NRG1 did not affect the mRNA level of EAAC1 in the cells (Fig. 3, F and G). These data suggest that ErbB4 activation is required for NRG1-mediated regulation of EAAC1, whereas ErbB2 is not involved.
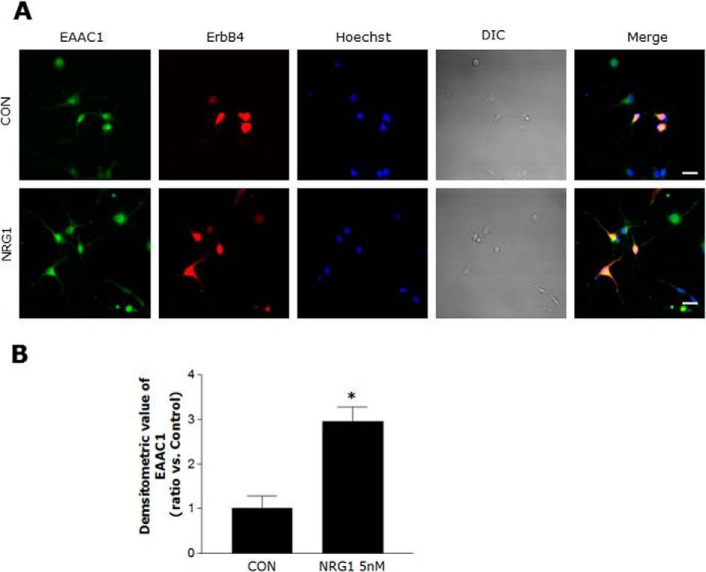
We performed immunocytochemistry to investigate EAAC1 immunoreactivity and localization in primary cortical neurons and found that NRG1 significantly increased EAAC1 immunoreactivity in comparison to control and that EAAC1 co-localized with ErbB4 in primary cortical neurons (Fig. 4, A and B).
FIGURE 4.
Colocalization of EAAC1 and ErbB4 in primary cortical neurons. A, double immuno-labeling studies on ErbB4 and EAAC1 in primary cortical neurons. Primary cortical neurons were treated with 5 nm NRG1 for 30 min, fixed, and stained with anti-ErbB4 and anti-EAAC1 antibodies that were visualized with FITC and Cy3-coupled secondary antibodies, respectively. Scale bar, 20 μm. B, bar graph summarizing the data from neurons with EAAC1 fluorescence. n = 5. *, p < 0.05.
NRG1 Increases Glutamate Uptake in Primary Cortical Neurons
To confirm that EAAC1 up-regulated by NRG1 is functional, we measured glutamate uptake in primary cortical neurons by employing a [3H]glutamate in vitro uptake assay system. NRG1 treatment increased glutamate uptake in primary cortical neurons in a dose-dependent manner (Fig. 5A). To further investigate the involvement of EAAC1, we characterized the glutamate uptake assay by treatment with TFB-TBOA (a selective non-transportable EAAT inhibitor) at a concentration of 50 nm, which is known to block GLAST and GLT1 without affecting EAAC1 (23). In the presence of TFB-TBOA, the effect of NRG1 was not affected (Fig. 5B). Moreover, treatment with AG1478 prevented NRG1 from increasing glutamate uptake. In contrast, AG879 had no effect (Fig. 5C).
FIGURE 5.
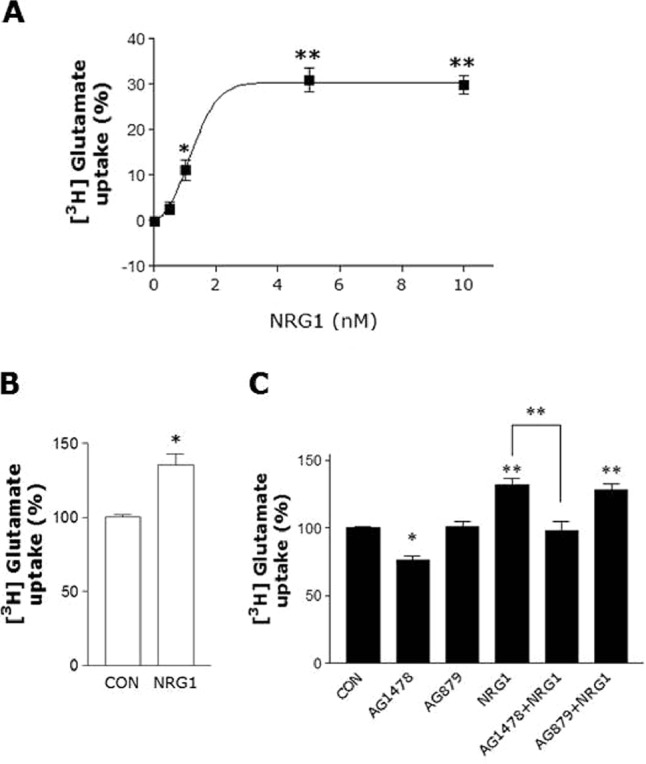
NRG1 Increases glutamate uptake in primary cortical neurons. A, glutamate transport activity in primary cortical neurons. 1, 5, or 10 nm NRG1 was added for 30 min. Glutamate uptake activity was then assessed by the incorporation of [3H]glutamate during 2 min at 37 °C. Dose-dependent potentiation of glutamate uptake. B, pretreatment with 50 nm TAB-TBOA did not influence the NRG1 (5 nm) effect on glutamate uptake activity. n = 6. *, p < 0.05. C, inhibition of NRG1 (5 nm, final concentration) induced increase of glutamate uptake by 5 μm AG1478, but not AG879. A one-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test was performed. n = 6. *, p < 0.05; **, p < 0.01.
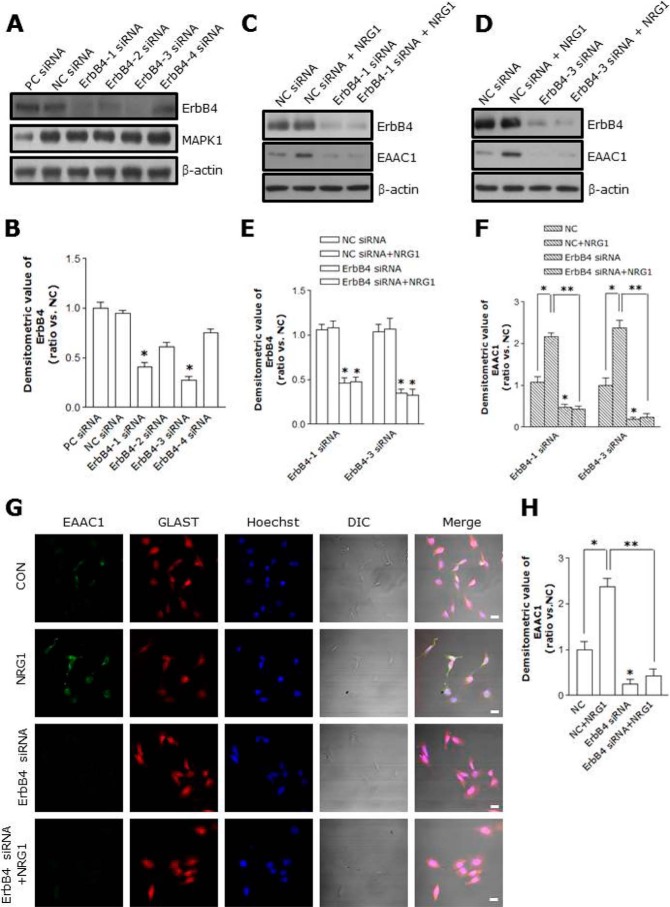
Knock-down of ErbB4 by siRNA Inhibits NRG1 from Up-regulating EAAC1
To investigate the involvement of endogenous ErbB4, we employed siRNA to knock down ErbB4 (24, 25). Four different siRNA reduced the ErbB4 protein expression to different degrees (Fig. 6, A and B), and ErbB4–1 and ErbB4–3 siRNA were effective (Fig. 6, C, D, and E). ErbB4–3 specific siRNA (33 nm) down-regulated ErbB4 in C6 cells by ∼82% (Fig. 6, D and E). Knock-down of ErbB4 significantly reduced the total amount of EAAC1 in C6 cells (control, 1 ± 0.17; ErbB4–3 siRNA, 0.19 ± 0.1, n = 8; p < 0.05; Fig. 6, D and F). Furthermore, transfection with ErbB4–3 siRNA prevented NRG1 from increasing EAAC1 expression (NC + NRG1, 2.38 ± 0.18; ErbB4–3 siRNA+ NRG1, 0.24 ± 0.1, n = 8; p < 0.01; Fig. 6, D and F).
FIGURE 6.
Suppression of NRG1-induced EAAC1 expression by ErbB4 siRNA. A, ErbB4 and MAPK1 expression levels after transfection with each ErbB4 siRNA (ErbB4–1, ErbB4–2, ErbB4–3, or ErbB4–4), negative control (NCsiRNA) and positive control (PCsiRNA). B, quantification of ErbB4 siRNA (ErbB4–1, ErbB4–2, ErbB4–3, or ErbB4–4) in C6 cells. ErbB4–1 and ErbB4–3 efficiently suppressed ErbB4 expression. n = 8. *, p < 0.05. C, ErbB4 and EAAC1 protein levels after transfection with ErbB4–1 siRNA and NCsiRNA. C6 cells were treated with NRG1 (5 nm, 30 min). After 36 h, an immunoblot analysis was performed on the ErbB4–1 siRNA-transfected C6 cells. D, ErbB4 and EAAC1 expression levels after transfection with ErbB4–3 siRNA and NCsiRNA. E, quantification of ErbB4 expression data in C and D, respectively. n = 8. *, p < 0.05. F, quantification of EAAC1 expression data in C and D, respectively. n = 8. *, p < 0.05; **, p < 0.01. G, C6 cells were transfected with ErbB4–3 siRNA for 36 h. C6 cells were treated with 5 nm NRG1 for 30 min after ErbB4–3 siRNA transfection, and then stained with anti-EAAC1 and anti-GLAST antibodies. Scale bar, 20 μm. H, bar graph summarizing data from C6 cells with EAAC1 fluorescence. ErbB4 siRNA transfection inhibits NRG1-induced EAAC1 expression. n = 8. *, p < 0.05; **, p < 0.01.
We performed immunocytochemistry to investigate EAAC1 immunoreactivity in C6 cells and found that ErbB4 siRNA significantly decreased EAAC1 immunoreactivity in comparison to control (Fig. 6, G and H). These observations confirm a role of endogenous ErbB4 in the NRG1-mediated up-regulation of EAAC1.
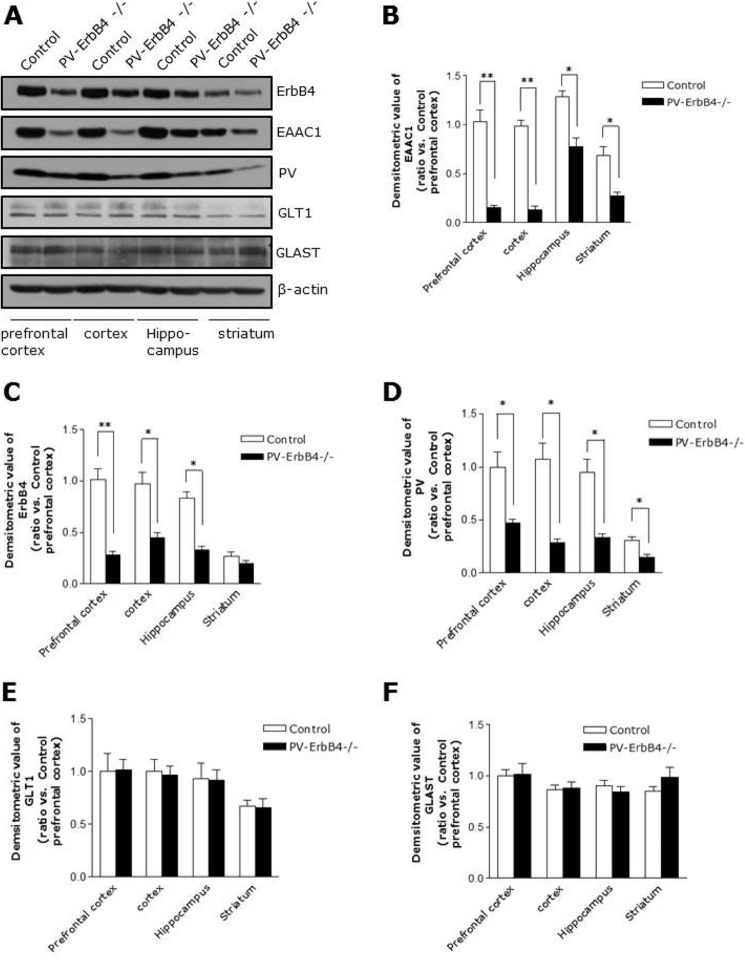
EAAC1 Is Remarkably Reduced in PV-ErbB4−/− Mice
We checked the protein level of EAAC1 in PV-ErbB4−/− mice to investigate the involvement of ErbB4 in vivo. ErbB4 is predominantly located in PV-positive interneurons (7, 9, 10, 26). PV-ErbB4−/− mice are PV-specific ErbB4 knock-out mice. In PV-Cre mice, the expression of Cre recombinase is activated at postnatal day 13 (27, 28). As shown in Fig. 7, A and C, ErbB4 was reduced in the various brain areas. In addition, PV was also reduced in the brains of PV-ErbB4−/− mice (Fig. 7, A and D), which is in agreement with previous studies (9), Importantly, EAAC1 was dramatically reduced but not GLT1 and GLAST in several brain regions (prefrontal cortex, cortex, hippocampus, and striatum) of PV-ErbB4−/− mice (Fig. 7, A–F).
FIGURE 7.
Reduced levels of EAAC1 in PV-ErbB4−/− mice. A, reduced levels of ErbB4, PV and EAAC1 (but not GLT1 and GLAST) in the brains of PV-ErbB4−/− mice. The prefrontal cortex, cortex, hippocampus, and striatum were collected from PV-ErbB4−/− mice and control littermates (PV-ErbB4+/+). The lysates (40 μg of protein) were used for Western blot analysis. Western blots of protein extracts probed with specific ErbB4 antibodies as well as with EAAC1, PV, GLT1, GLAST, and β-actin antibodies as specificity and loading controls. B, quantification analysis of EAAC1 expression data in A. n = 10. *, p < 0.05; **, p < 0.01. C, quantification analysis of ErbB4 expression data in A. n = 10. *, p < 0.05; **, p < 0.01. D, quantification analysis of PV expression data in A. n = 10. *, p < 0.05. E, quantification analysis of GLT1 expression data in A. n = 10. F, quantification analysis of GLAST expression data in A. n = 10.
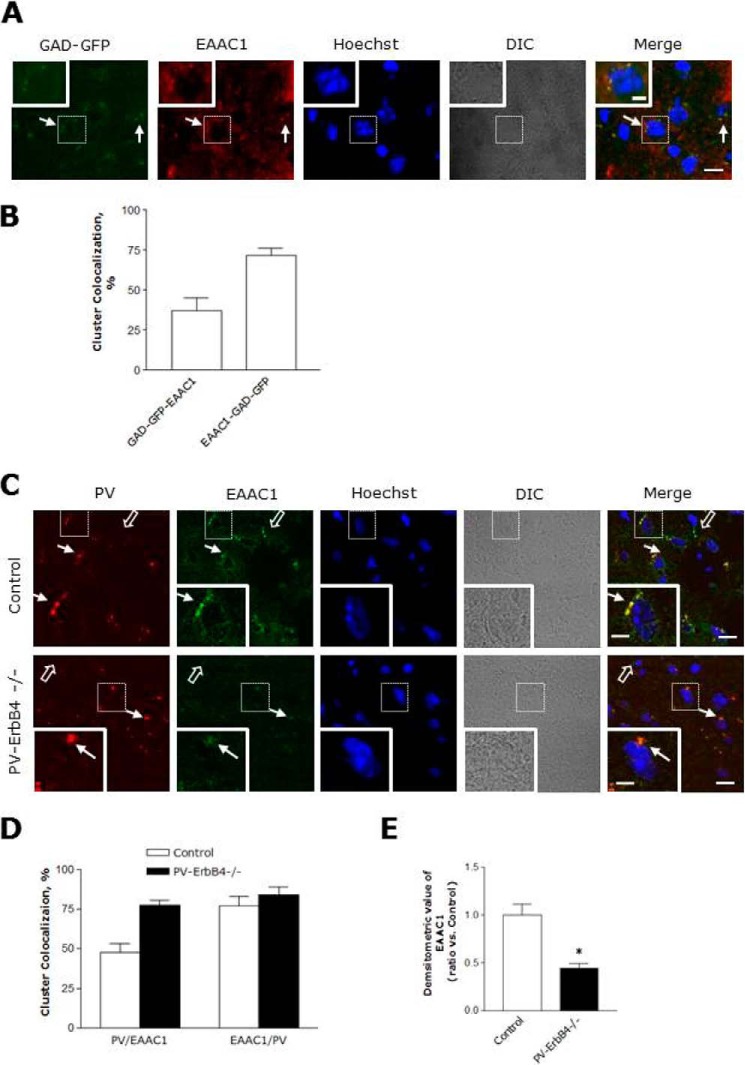
EAAC1 was found to be remarkably reduced in same region of PV-ErbB4−/− (Fig. 7, A and B), indicating that ErbB4 in PV-positive neurons is required for EAAC1 expression. In addition, we stained PFC sections of mice that express GFP in inhibitory neurons (GAD 67 mice, Jackson Lab) to examine the subcellular localization of EAAC1 in GABAergic neurons. EAAC1 immunoreactivity colocalized with GFP fluorescence when stained with either anti-mouse antibodies (Fig. 8, A and B). In total, 37% of GAD-GFP clusters were colocalized with EAAC1, and 72% of EAAC1 cluster was colocalized with GAD-GFP positive puncta-ring-like staining neurons in the PFC (Fig. 8, A and B). These observations suggest that EAAC1 is expressing at presynaptic GABAergic neurons in the PFC.
FIGURE 8.
Ablation of ErbB4 in PV-positive neurons prevented EAAC1 expression. Ablation of ErbB4 in PV neurons prevented EAAC1 expression. A, coronal sections of the PFC from GAD-GFP mice were stained with anti-EAAC1. The arrows indicate colocalization of EAAC1 and GAD-GFP. Scale bar, 20 μm; inset, enlarged areas. Scale bar, 5 μm. B, quantification analysis of the colocalization of EAAC1 with GAD-GFP. The results are presented as the means ± S.E. C, coronal sections of the PFC of control (PV-ErbB4+/+; top panels) and PV-ErbB4−/− (bottom panels) mice were stained with anti-PV and anti-EAAC1. Immunoreactivity was visualized via Oyster 550 fluorescence-labeled anti-PV and Alexa 488-conjugated secondary antibodies for anti-EAAC1. Scale bar, 15 μm; inset, enlarged areas. Scale bar, 5 μm. D, quantification analysis EAAC1 clusters with PV and EAAC1 clusters with PV in C. E, quantification analysis of EAAC1 immunoreactivity in C. The results are presented as the means ± S.E.
In addition, we stained coronal sections of the PFC of control (PV-ErbB4+/+; top panels) and PV-ErbB4−/− (bottom panels) mice with anti-PV and anti-EAAC1. In total, 48 and 78% of EAAC1 clusters colocalized with PV in the control and PV-ErbB4−/− positive puncta-ring like staining neurons in the PFC, respectively (Fig. 8, C and D). On the other hand, the colocalization of PV clusters with EAAC1 did not significantly differ. However, EAAC1 immunoreactivity decreased in the PFC of the PV-ErbB4−/− mice (Fig. 8E). These results indicate that the reduction in EAAC1 expression is not only observed in PV neurons but also in non-PV neurons. Taken together, these results indicate that ErbB4 expression in PV-positive interneurons may be critical for EAAC1 expression.
Discussion
A prominent hypotheses as to the underlying pathophysiology of schizophrenia is that disturbances in GABAergic and glutamatergic neurotransmission play causal factors. Patients with schizophrenia have been shown to have decreased expression of GAD 67 mRNA (29) and of the GABA transporter GAT-1 (30) in the PFC. The expression of PV is also reduced in the brains of schizophrenia patients (31). Accumulating evidence supports the action of NRG1/ErbB4 signaling as a stimulator of GABAergic transmission, which may contribute to compensating for abnormal GABAergic circuitry. In humans, reducing glutamatergic transmission can mimic schizophrenia (32), while enhancing glutamatergic transmission can alleviate symptoms (33). Patients with the disease show decreased excitatory synaptic function in hippocampal and cortical regions (34–36). NRG1 functions by activating the ErbB family of tyrosine kinase receptors, including ErbB2, ErbB3, and ErbB4. ErbB2 cannot bind to NRG1 but forms an activated heterodimer with ErbB3 or ErbB4. ErbB3, and ErbB4 can bind to NRG1; in this case, ErbB4 homodimers take on the activating form, whereas ErbB3 is kinase-inactive (9). Recent studies have provided strong evidence that NRG1/ErbB4 signaling is a schizophrenia susceptibility pathway. NRG1/ErbB4 signaling is a schizophrenia susceptibility pathway. NRG1 and ErbB4 have been identified as candidate genes for schizophrenia (9). In animal models, mice lacking of NRG1, or ErbB gene cause various behavioral deficits including hyperactivity, prepulse inhibition (PPI), social behavior, and working memory (1, 37–39). In particular, the lack of ErbB4 in PV-positive neurons also showed schizophrenia-like behavioral abnormalities (9) similar to the traits exhibited by NRG1 or ErbB4 mutant mice. These genetic studies have suggested a link between NRG1/ErbB4 signaling and schizophrenia.
In the brain, glutamate serves as an excitatory neurotransmitter, a metabolic substrate for GABA, and as an amino acid for general cellular metabolism. Glutamate transporters maintain low extracellular glutamate and influence the kinetics of glutamate receptor activation. The function of EAAC1 in the CNS has not yet been established. Recent studies indicate that EAAC1 functions as a cysteine transporter, maintains neuronal glutathione metabolism and has a unique anti-apoptotic activity in injured neurons (40). Knock-out of EAAC1 in rodent leads to the development of epilepsy, resulting from the reduced synthesis of the neurotransmitter GABA (16), dicarboxylic aminoaciduria, and significant motor impairment (15). Changes in the glutamate transport activity of EAAC1 are associated with LTP and fear conditioning (41). Increased EAAC1 transcripts and proteins were reported in schizophrenic subjects (dorsolateral prefrontal and anterior cingulated cortex) (42). In contrast, decreased SLC1A1 transcript expression in the striatum was observed in schizophrenia (and in bipolar disorder) (43) and confirmed in patients with schizophrenia in a later study (44). This study provides evidence that EAAC1 may play an important role in synaptic plasticity, maintenance, or regulation. Despite advances in the field, a mechanism has not been identified that can link GABAergic and glutamatergic neurotransmission to NRG1 signaling.
In this study, we investigated the function of NRG1 signaling in the regulation of neuronal glutamate transporters in the brain. The predominant neuronal glutamate transporter, EAAC1, may play a role in regulating GABA synthesis because glutamate is a precursor to GABA. We demonstrated that NRG1 regulates glutamate uptake by up-regulating EAAC1 in neuronal cells. NRG1 showed no effect on the protein level of astroglial transporters (GLAST and GLT1). These results provide a direct link between NRG1 and the glutamatergic system in neuronal cells. Inhibition of ErbB4 signaling by the specific ErbB4 inhibitor AG1478 or by knocking-down ErbB4 by siRNA prevented NRG1 from up-regulating EAAC1 expression and glutamate uptake in neuronal cells. Moreover, EAAC1 immunoreactivity was increased by NRG1 and colocalized with ErbB4 in primary cortical neurons. Therefore, we propose that NRG1 regulates EAAC1 by the direct activation of ErbB4 receptors. Altered NRG1-ErbB4 signaling is shown to contribute to NMDA hypofunction in schizophrenia (45–47). Based on the previous findings above, our findings support the notion that up-regulation of EAAC1 by NRG1 may provide a potential mechanism of glutamatergic hypofunction in schizophrenia.
We found an interesting relationship between ErbB4 and EAAC1 in PV-positive interneurons. The specific absence of ErbB4 in PV interneurons inhibited EAAC1 expression, indicating a crucial role of ErbB4 in PV-positive interneurons in the regulation of EAAC1 expression. EAAC1 is localized to the dendrite and soma of many neurons. Rare presynaptic localization is restricted to GABA terminals (16). A previous study found that ErbB4 is located in presynaptic GABAergic neurons in the PFC and that ErbB4 is mainly expressed in PV-positive interneurons, which are involved in neurotransmission and synaptic plasticity (9). In accordance with these results, we found that EAAC1 was expressed in presynaptic GABAergic neurons, including PV-positive neurons in the PFC. The presence of EAAC1 in GAD-GFP-positive neurons and its colocalization with PV provides anatomical evidence that supports this notion. In addition, the ratio of PV-positive clusters co-expressed with EAAC1 was increased in the PFC of PV-ErbB4−/− mice compared with controls, and EAAC1 immunoreactivity decreased in the PFC of PV-ErbB4−/− mice. These results suggest that EAAC1 expression remained decreased in the remaining PV clusters, which decreased in PV-ErbB4−/− mice. However, a recent study has revealed that the spine density and the number of excitatory synapse are reduced in PV-ErbB4−/− mice (47), which may contribute to the reduction in EAAC1 expression in the mouse model. Therefore, further study is required to determine whether it is a direct effect or whether it is an effect caused by the changes of synapse in PV-ErbB4−/− mice. Nevertheless, we found that NRG1/ErbB4 signaling is critical to EAAC1 expression and function. More work is required to determine the mechanism of protein levels and transport activity through NRG1/ErbB4 signaling.
In conclusion, our results reveal a novel function of NRG1/ErbB4 in the modulation of EAAC1 expression and function. These results suggest that the regulation of EAAC1 by NRG1 may participate in normal GABA or glutamate transmission and that the alterations in EAAC1 may also contribute to the GABAergic or glutamatergic dysfunction implicated in schizophrenia. These results contribute to a better understanding of how abnormal NRG1/ErbB4 signaling may be involved in the pathogenesis of schizophrenia.
Author Contributions
H. N. Y., T. K. B., and R. S. W. designed research. H. N. Y., K. H. N., and D. Y. S. performed research. W. K. P. and H. S. K. analyzed data. T. K. B., T. K. B., and R. S. W. wrote the manuscript.
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Grant Number NRF-2013R1A1A4A01012426. The authors declare that they have no conflicts of interest with the contents of this article.
- NRG1
- neuregulin1
- GABA
- γ-aminobutyric acid
- EGF
- epidermal growth factor
- LTP
- long-term potentiation
- PFC
- prefrontal cortex
- PV
- parvalbumin
- EAAC1
- excitatory amino-acid carrier 1
- siRNA
- small interfering RNA
- IP
- immunoprecipitation
- GAD
- glutamic acid decarboxylase.
References
- 1. Mei L., Xiong W. C. (2008) Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nature Reviews. Neuroscience 9, 437–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwon O. B., Longart M., Vullhorst D., Hoffman D. A., Buonanno A. (2005) Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J. Neurosci. 25, 9378–9383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwon O. B., Paredes D., Gonzalez C. M., Neddens J., Hernandez L., Vullhorst D., Buonanno A. (2008) Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc. Natl. Acad. Sci. U.S.A. 105, 15587–15592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang Y. Z., Won S., Ali D. W., Wang Q., Tanowitz M., Du Q. S., Pelkey K. A., Yang D. J., Xiong W. C., Salter M. W., Mei L. (2000) Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron 26, 443–455 [DOI] [PubMed] [Google Scholar]
- 5. Pitcher G. M., Beggs S., Woo R. S., Mei L., Salter M. W. (2008) ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport 19, 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gu Z., Jiang Q., Fu A. K., Ip N. Y., Yan Z. (2005) Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J. Neurosci. 25, 4974–4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vullhorst D., Neddens J., Karavanova I., Tricoire L., Petralia R. S., McBain C. J., Buonanno A. (2009) Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J. Neurosci. 29, 12255–12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woo R. S., Li X. M., Tao Y., Carpenter-Hyland E., Huang Y. Z., Weber J., Neiswender H., Dong X. P., Wu J., Gassmann M., Lai C., Xiong W. C., Gao T. M., Mei L. (2007) Neuregulin-1 enhances depolarization-induced GABA release. Neuron 54, 599–610 [DOI] [PubMed] [Google Scholar]
- 9. Wen L., Lu Y. S., Zhu X. H., Li X. M., Woo R. S., Chen Y. J., Yin D. M., Lai C., Terry A. V., Jr., Vazdarjanova A., Xiong W. C., Mei L. (2010) Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc. Natl. Acad. Sci. U.S.A. 107, 1211–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yau H. J., Wang H. F., Lai C., Liu F. C. (2003) Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cerebral Cortex 13, 252–264 [DOI] [PubMed] [Google Scholar]
- 11. Mathews G. C., Diamond J. S. (2003) Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. J. Neurosci. 23, 2040–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothstein J. D., Dykes-Hoberg M., Pardo C. A., Bristol L. A., Jin L., Kuncl R. W., Kanai Y., Hediger M. A., Wang Y., Schielke J. P., Welty D. F. (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 [DOI] [PubMed] [Google Scholar]
- 13. He Y., Janssen W. G., Rothstein J. D., Morrison J. H. (2000) Differential synaptic localization of the glutamate transporter EAAC1 and glutamate receptor subunit GluR2 in the rat hippocampus. J. Comp. Neurol. 418, 255–269 [PubMed] [Google Scholar]
- 14. Holmseth S., Dehnes Y., Huang Y. H., Follin-Arbelet V. V., Grutle N. J., Mylonakou M. N., Plachez C., Zhou Y., Furness D. N., Bergles D. E., Lehre K. P., Danbolt N. C. (2012) The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J. Neurosci. 32, 6000–6013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peghini P., Janzen J., Stoffel W. (1997) Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 16, 3822–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sepkuty J. P., Cohen A. S., Eccles C., Rafiq A., Behar K., Ganel R., Coulter D. A., Rothstein J. D. (2002) A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J. Neurosci. 22, 6372–6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bianchi M. G., Bardelli D., Chiu M., Bussolati O. (2014) Changes in the expression of the glutamate transporter EAAT3/EAAC1 in health and disease. Cell. Mol. Life Sci. 71, 2001–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lortet S., Canolle B., Masmejean F., Nieoullon A. (2008) Plasma membrane expression of the neuronal glutamate transporter EAAC1 is regulated by glial factors: evidence for different regulatory pathways associated with neuronal maturation. Neurochemistry International 52, 1373–1382 [DOI] [PubMed] [Google Scholar]
- 19. Song C. H., Furuoka H., Kim C. L., Ogino M., Suzuki A., Hasebe R., Horiuchi M. (2008) Effect of intraventricular infusion of anti-prion protein monoclonal antibodies on disease progression in prion-infected mice. J. Gen. Virol. 89, 1533–1544 [DOI] [PubMed] [Google Scholar]
- 20. Rothstein J. D., Martin L., Levey A. I., Dykes-Hoberg M., Jin L., Wu D., Nash N., Kuncl R. W. (1994) Localization of neuronal and glial glutamate transporters. Neuron 13, 713–725 [DOI] [PubMed] [Google Scholar]
- 21. Conti F., DeBiasi S., Minelli A., Rothstein J. D., Melone M. (1998) EAAC1, a high-affinity glutamate tranporter, is localized to astrocytes and gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cerebral Cortex 8, 108–116 [DOI] [PubMed] [Google Scholar]
- 22. Bianchi M. G., Gazzola G. C., Tognazzi L., Bussolati O. (2008) C6 glioma cells differentiated by retinoic acid overexpress the glutamate transporter excitatory amino acid carrier 1 (EAAC1). Neuroscience 151, 1042–1052 [DOI] [PubMed] [Google Scholar]
- 23. Shimamoto K., Sakai R., Takaoka K., Yumoto N., Nakajima T., Amara S. G., Shigeri Y. (2004) Characterization of novel L-threo-β-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol. Pharmacol. 65, 1008–1015 [DOI] [PubMed] [Google Scholar]
- 24. Ryu J., Yu H. N., Cho H., Kim H. S., Baik T. K., Lee S. J., Woo R. S. (2012) Neuregulin-1 exerts protective effects against neurotoxicities induced by C-terminal fragments of APP via ErbB4 receptor. J. Pharmacol. Sci. 119, 73–81 [DOI] [PubMed] [Google Scholar]
- 25. Woo R. S., Lee J. H., Kim H. S., Baek C. H., Song D. Y., Suh Y. H., Baik T. K. (2012) Neuregulin-1 protects against neurotoxicities induced by Swedish amyloid precursor protein via the ErbB4 receptor. Neuroscience 202, 413–423 [DOI] [PubMed] [Google Scholar]
- 26. Fisahn A., Neddens J., Yan L., Buonanno A. (2009) Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cerebral Cortex 19, 612–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. del Río J. A., de Lecea L., Ferrer I., Soriano E. (1994) The development of parvalbumin-immunoreactivity in the neocortex of the mouse. Brain research. Dev. Brain Res. 81, 247–259 [DOI] [PubMed] [Google Scholar]
- 28. Hof P. R., Glezer II, Condé F., Flagg R. A., Rubin M. B., Nimchinsky E. A., Vogt Weisenhorn D. M. (1999) Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J. Chem. Neuroanatomy 16, 77–116 [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto T., Volk D. W., Eggan S. M., Mirnics K., Pierri J. N., Sun Z., Sampson A. R., Lewis D. A. (2003) Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 23, 6315–6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohnuma T., Augood S. J., Arai H., McKenna P. J., Emson P. C. (1999) Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor α-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience 93, 441–448 [DOI] [PubMed] [Google Scholar]
- 31. Lewis D. A., Hashimoto T., Volk D. W. (2005) Cortical inhibitory neurons and schizophrenia. Nature Reviews. Neuroscience 6, 312–324 [DOI] [PubMed] [Google Scholar]
- 32. Pietraszek M. (2003) Significance of dysfunctional glutamatergic transmission for the development of psychotic symptoms. Polish Journal of Pharmacology 55, 133–154 [PubMed] [Google Scholar]
- 33. Javitt D. C. (2004) Glutamate as a therapeutic target in psychiatric disorders. Molecular Psychiatry 9, 984– 997, 979 [DOI] [PubMed] [Google Scholar]
- 34. Bressan R. A., Pilowsky L. S. (2000) Imaging the glutamatergic system in vivo–relevance to schizophrenia. Eur. J. Nuclear Med. 27, 1723–1731 [DOI] [PubMed] [Google Scholar]
- 35. Abbott C., Bustillo J. (2006) What have we learned from proton magnetic resonance spectroscopy about schizophrenia? A critical update. Curr. Opin. Psych. 19, 135–139 [DOI] [PubMed] [Google Scholar]
- 36. Pilowsky L. S., Bressan R. A., Stone J. M., Erlandsson K., Mulligan R. S., Krystal J. H., Ell P. J. (2006) First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol. Psych. 11, 118–119 [DOI] [PubMed] [Google Scholar]
- 37. Stefansson H., Sigurdsson E., Steinthorsdottir V., Bjornsdottir S., Sigmundsson T., Ghosh S., Brynjolfsson J., Gunnarsdottir S., Ivarsson O., Chou T. T., Hjaltason O., Birgisdottir B., Jonsson H., Gudnadottir V. G., Gudmundsdottir E., Bjornsson A., Ingvarsson B., Ingason A., Sigfusson S., Hardardottir H., Harvey R. P., Lai D., Zhou M., Brunner D., Mutel V., Gonzalo A., Lemke G., Sainz J., Johannesson G., Andresson T., Gudbjartsson D., Manolescu A., Frigge M. L., Gurney M. E., Kong A., Gulcher J. R., Petursson H., Stefansson K. (2002) Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 71, 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang J. Z., Si T. M., Ruan Y., Ling Y. S., Han Y. H., Wang X. L., Zhou M., Zhang H. Y., Kong Q. M., Liu C., Zhang D. R., Yu Y. Q., Liu S. Z., Ju G. Z., Shu L., Ma D. L., Zhang D. (2003) Association study of neuregulin 1 gene with schizophrenia. Mol. Psych. 8, 706–709 [DOI] [PubMed] [Google Scholar]
- 39. Law A. J., Lipska B. K., Weickert C. S., Hyde T. M., Straub R. E., Hashimoto R., Harrison P. J., Kleinman J. E., Weinberger D. R. (2006) Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc. Natl. Acad. Sci. U.S.A. 103, 6747–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiryu-Seo S., Gamo K., Tachibana T., Tanaka K., Kiyama H. (2006) Unique anti-apoptotic activity of EAAC1 in injured motor neurons. EMBO J. 25, 3411–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levenson J., Weeber E., Selcher J. C., Kategaya L. S., Sweatt J. D., Eskin A. (2002) Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nature Neuroscience 5, 155–161 [DOI] [PubMed] [Google Scholar]
- 42. Bauer D., Gupta D., Harotunian V., Meador-Woodruff J. H., McCullumsmith R. E. (2008) Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophrenia Research 104, 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCullumsmith R. E., Meador-Woodruff J. H. (2002) Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology 26, 368–375 [DOI] [PubMed] [Google Scholar]
- 44. Nudmamud-Thanoi S., Piyabhan P., Harte M. K., Cahir M., Reynolds G. P. (2007) Deficits of neuronal glutamatergic markers in the caudate nucleus in schizophrenia. Journal of Neural Transmission 281–285 [DOI] [PubMed] [Google Scholar]
- 45. Hahn C. G., Wang H. Y., Cho D. S., Talbot K., Gur R. E., Berrettini W. H., Bakshi K., Kamins J., Borgmann-Winter K. E., Siegel S. J., Gallop R. J., Arnold S. E. (2006) Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nature Medicine 12, 824–828 [DOI] [PubMed] [Google Scholar]
- 46. Pitcher G. M., Kalia L. V., Ng D., Goodfellow N. M., Yee K. T., Lambe E. K., Salter M. W. (2011) Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nature Medicine 17, 470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yin D. M., Sun X. D., Bean J. C., Lin T. W., Sathyamurthy A., Xiong W. C., Gao T. M., Chen Y. J., Mei L. (2013) Regulation of spine formation by ErbB4 in PV-positive interneurons. J. Neurosci. 33, 19295–19303 [DOI] [PMC free article] [PubMed] [Google Scholar]