Background: Di-Ras2 is a poorly characterized Ras-family GTPase specifically expressed in brain.
Results: Di-Ras2 co-purifies with SmgGDS from cytosol, and the affinity of Di-Ras2 for guanine-nucleotides is reduced when complexed with SmgGDS.
Conclusion: Di-Ras2 exits as a complex with SmgGDS in cytosol with lowered affinity for guanine nucleotides.
Significance: Di-Ras2 activity may be atypically regulated by complex formation with SmgGDS.
Keywords: guanine nucleotide exchange factor (GEF), protein complex, protein isoprenylation, Ras protein, Rho (Rho GTPase), small GTPase, SmgGDS
Abstract
The Ras family of small GTPases function in a wide variety of biological processes as “molecular switches” by cycling between inactive GDP-bound and active GTP-bound forms. Di-Ras1 and Di-Ras2 were originally identified as small GTPases forming a distinct subgroup of the Ras family. Di-Ras1/Di-Ras2 mRNAs are detected predominantly in brain and heart tissues. Biochemical analysis of Di-Ras1/Di-Ras2 has revealed that they have little GTPase activity and that their intrinsic guanine-nucleotide exchange rates are much faster than that of H-Ras. Yet little is known about the biological role(s) of Di-Ras1/Di-Ras2 or of how their activities are regulated. In the present study we found that endogenous Di-Ras2 co-purifies with SmgGDS from rat brain cytosol. Size-exclusion chromatography of purified recombinant proteins showed that Di-Ras2 forms a high affinity complex with SmgGDS. SmgGDS is a guanine nucleotide exchange factor with multiple armadillo repeats and has recently been shown to specifically activate RhoA and RhoC. In contrast to the effect on RhoA, SmgGDS does not act as a guanine nucleotide exchange factor for Di-Ras2 but instead tightly associates with Di-Ras2 to reduce its binding affinity for guanine nucleotides. Finally, pulse-chase analysis revealed that Di-Ras2 binds, in a C-terminal CAAX motif-dependent manner, to SmgGDS immediately after its synthesis. This leads to increased Di-Ras2 stability. We thus propose that isoprenylated Di-Ras2 forms a tight complex with SmgGDS in cytosol immediately after its synthesis, which lowers its affinity for guanine nucleotides.
Introduction
The Ras-family of small GTPases (Ras GTPases) function as molecular switches in diverse cellular events including cell growth and differentiation (1–3). Ras GTPases contain a well conserved domain (G-domain) responsible for guanine-nucleotide/Mg2+ binding and GTP hydrolysis and also have a C-terminal isoprenylation motif (CAAX motif: C, A, and X represent cysteine, an aliphatic amino acid, and any amino acid, respectively). Ras GTPases cycle between GDP-bound inactive and GTP-bound active conformations, a process is regulated by various accessory molecules. Activation of Ras GTPases is controlled by guanine-nucleotide exchange factors (GEFs),2 which catalyze the GDP/GTP exchange reactions. Activated Ras GTPases interact with their downstream effector molecules to transduce upstream signals and are then converted to GDP-bound inactive forms via hydrolysis of bound GTP, which is accelerated by GTPase activating proteins (GAPs) (4).
Di-Ras1 and Di-Ras2 were originally identified as small GTPases forming a distinct subgroup of Ras GTPases (5, 6). They both possess the G-domain and CAAX motif that are conserved among Ras GTPases but show some distinct biochemical properties. For example, the intrinsic nucleotide exchange reaction of Di-Ras1/Di-Ras2 proceeds much faster than that of H-Ras (6, 7). In addition, Di-Ras1/Di-Ras2 show little intrinsic GTPase activity (6, 7), which is probably due to substitutions of the amino acids responsible for GTPase activity; the amino acids corresponding to Ala-59 and Gln-61 in Ha-Ras are substituted to Thr-63 and Ser-65 in Di-Ras1/Di-Ras2, respectively. Thus, considerable amounts of GTP-bound forms of Di-Ras1/Di-Ras2 are detected in living cells when they are over-expressed, which is in contrast to the predominant GDP-bound form of H-Ras (6). Although the regulation of Di-Ras1/Di-Ras2 activity remains to be determined, Rap1GAP1 and Rap1GAP2 have been shown to stimulate GTPase activity of Di-Ras in vitro (7).
The biological function of Di-Ras proteins remains elusive. A previous study reported that overexpression of Di-Ras1 inhibited oncogenic H-Ras-induced cellular transformation and transactivation of the transcription factor Elk-1 (5), raising the possibility that Di-Ras1 could antagonize H-Ras-mediated signaling by competing for Ras effectors. However, Di-Ras proteins fail to interact with the Ras binding domain of Raf or the Ras association domain of RalGDS (6, 7). Northern blot analysis has shown that Di-Ras1 mRNA is expressed specifically in brain and heart, whereas Di-Ras2 mRNA is specifically expressed in brain (6). We have recently reported that that a Caenorhabditis elegans Di-Ras homolog (drn-1) is expressed specifically in neuronal cells and is involved in the modulation of synaptic activity (8).
In the present study we sought to characterize the biochemical properties of endogenous Di-Ras proteins. To this end we partially purified Di-Ras2 from rat brain cytosol and found that Di-Ras2 exists as a cytosolic complex with SmgGDS. SmgGDS was originally identified as a GEF for Rap1A and has guanine-nucleotide exchange activity on a number of small GTPases, including various Ras and Rho-family GTPases (9–13). Intriguingly, SmgGDS binds tightly to Di-Ras2 without showing GEF activity for Di-Ras2. The affinities of Di-Ras2 for guanine nucleotides are reduced when Di-Ras2 forms a complex with SmgGDS, and the complex formation enhances the protein stability of Di-Ras2. We thus propose that cytosolic Di-Ras2 is in a low affinity state for guanine nucleotides because it forms a complex with SmgGDS.
Experimental Procedures
Antibodies
Monoclonal antibodies against Di-Ras1 or Di-Ras2 were generated using a previously reported method (14). Briefly, female WKY/Ncrj rats were immunized in the hind footpads with purified recombinant Di-Ras1 or Di-Ras2 proteins (produced in Escherichia coli). Interiliac lymph node cells from these rats were then fused with the mouse myeloma cell line PAI to produce hybridoma cells. Hybridoma cell culture media were then screened by ELISA to select clones that produced antibodies specific for Di-Ras1 or Di-Ras2. The specificity and titer of the selected clones were then determined by Western blotting. Other antibodies used in this study were as follows: anti-GAPDH (Millipore), anti-SmgGDS (anti-Rap1GDS1) (Sigma), anti-N-Ras (Santa Cruz), anti-R-Ras (BD Pharmingen), anti-Na+/K+ ATPase α-subunit (Upstate Biotechnology), and anti-FLAG (M2, Sigma).
Purification of Di-Ras2 and an Associated 55-kDa Protein from Rat Brain Cytosol
Whole brain (∼40 g) of adult male Donryu rats was washed with a 5-fold volume of ice-cold TBS (20 mm Tris·HCl (pH 7.5), 150 mm NaCl) and homogenized in 400 ml of a buffer consisting of 10 mm Tris·HCl (pH 7.5), 2 mm MgCl2, 1 mm DTT, 1 μg/ml aprotinin, and 0.025 mg/ml 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF). The homogenate was centrifuged at 800 × g for 10 min, and the supernatant was then further centrifuged at 30,000 × g for 30 min. The resulting supernatant was ultracentrifuged at 100,000 × g for 90 min, and the clear supernatant (cytosol fraction, ∼350 ml) was stored at −80 °C until use. For purification of Di-Ras2, the cytosol fraction (160 ml) was applied to a Q-Sepharose HP column (GE Healthcare, 2.2 × 15 cm, 60-ml bed volume) equilibrated with 240 ml of a common purification buffer, TMD (consisting of 20 mm Tris·HCl (pH 7.5), 2 mm MgCl2, and 1 mm DTT), containing 40 mm NaCl. The column was washed with ∼60 ml of the equilibration buffer and then eluted with a 300-ml linear gradient of 40–225 mm NaCl at a flow rate of 5 ml/min. The obtained fractions were subjected to dot-blot analysis using anti-Di-Ras2 antibody. The fractions containing Di-Ras2 (∼50 ml eluted at ∼130 mm NaCl) were concentrated to ∼6 ml using an Amicon Ultra-15 ml 30-kDa Centrifugal Filters (Merck Millipore) and then applied to Sephacryl S-300 HR column (GE Healthcare, 2.6 × 60 cm, 320-ml bed volume) equilibrated with TMD containing 150 mm NaCl. Proteins were eluted from the column at a flow rate of 2 ml/min. The fractions containing Di-Ras2 (∼15 ml) were mixed with KPi to obtain a final concentration of 20 mm and applied to a ceramic-hydroxyapatite column (Bio-Rad, 1.0 × 4 cm, 3-ml bed volume) equilibrated with TMD containing 50 mm NaCl and 20 mm KPi. The column was washed with 5 ml of the equilibration buffer and eluted with a 20-ml linear gradient of 20–150 mm KPi at a flow rate of 1 ml/min. The fractions containing Di-Ras2 (∼4 ml eluted at 70∼100 mm KPi) were diluted with a 2–3-fold volume of TMD and applied to a Mono Q column (GE Healthcare, 0.5 × 3 cm, 0.6 ml-bed volume) equilibrated with TMD containing 30 mm NaCl. The column was washed with 2 ml of the equilibration buffer and then eluted with a 10-ml linear gradient of 30–200 mm NaCl at a flow rate of 0.6 ml/min. Di-Ras2 was eluted from the column at ∼100 mm NaCl. For a phenyl-Sepharose HP column (GE Healthcare), the fractions containing Di-Ras2 were diluted with an equal volume of TMD containing 500 mm (NH4)2SO4 and applied to the hydrophobic column (0.5 × 2.5 cm, 0.5-ml bed volume) equilibrated with TMD containing 250 mm (NH4)2SO4. The column was washed with 5 ml of the equilibration buffer and eluted with a 5-ml linear gradient of 250–0 mm (NH4)2SO4 and an additional 20 ml of TMD at a flow rate of 0.5 ml/min. Di-Ras2 was eluted from the column at the gradient end to 0 mm (NH4)2SO4 as a broad peak, and proteins were analyzed by SDS-PAGE. A 55-kDa band co-migrating with Di-Ras2 in various purification steps including the phenyl-Sepharose chromatography (see Fig. 2 and Table 1) was excised from the acrylamide gel and analyzed by mass spectrometry.
FIGURE 2.
Di-Ras2 is co-purified with SmgGDS from rat brain cytosol. A, Di-Ras2 was partially purified from rat brain cytosol as described in “Experimental Procedures.” Fractions containing Di-Ras2 from Q-Sepharose (lane 1), Sephacryl S-300 (lane 2), ceramic-hydroxyapatite (lane 3), and Mono Q (lane 4) were analyzed by SDS-PAGE and CBB staining (left panel). The same fractions were also subjected to Western blot analysis using anti-SmgGDS (right, upper panel) or anti-Di-Ras2 (right, lower panel) antibodies. The density of protein bands in Mono Q fraction (lane 4) was analyzed with ImageJ software (lane 5). B–D, representative chromatograms of Q-Sepharose (B), Sephacryl S-300 (C), and phenyl-Sepharose (D). Amounts of Di-Ras2 and SmgGDS were quantified by Western blot and/or dot-blot analyses using GST-Di-Ras2 and GST-SmgGDS, respectively, as standards. E, pulldown assay. CBB staining of purified proteins was used for the assay (left panel). Di-Ras2 was incubated with His-TF or His-TF-SmgGDS and then further incubated with Ni2+-Sepharose 6 Fast Flow beads. The proteins associated with the beads were then subjected to SDS-PAGE/CBB staining (right panel). F, guanine nucleotide content in purified protein fractions. The content of GDP and GTP in the Mono Q-purified Di-Ras2·SmgGDS complex (a, 150 pmol) and recombinant GST-Di-Ras2 monomer (b, 250 pmol) was spectroscopically analyzed by monitoring the absorbance at 254 nm of eluate from an anion-exchange column. For comparison, defined amounts of GDP and GTP (each 100, 200, and 400 pmol) were also analyzed (c).
TABLE 1.
Summary of purification of Di-Ras2·SmgGDS from rat brain cytosol
The immunoreactive amounts of Di-Ras2 and SmgGDS were measured by SDS-PAGE/Western blot and/or dot-blot analyses using recombinant GST-fused Di-Ras2 (moleculare mass 50 kDa) and GST-fused SmgGDS (moleculare mass 80 kDa), respectively, as standard proteins.
| Purification steps | Fraction volume | Total proteins |
Amount | Immunoreactive proteins: Di-Ras2·SmgGDS |
||||
|---|---|---|---|---|---|---|---|---|
| Amount | Concentration | Concentration | Specific activity | -Fold purification | Recovery | |||
| ml | mg | mg/ml | pmol | pmol/ml | pmol/mg | × | % | |
| Cytosol | 160 | 480 | 3.0 | 7200/NSa | 45/NSa | 15/NSa | 1/NSa | 100/NSa |
| Q-Sepharose HP | 50 | 20 | 0.40 | 4800/4100 | 96/81 | 240/202 | 16 (1)b/1 | 67 (100)b/100 |
| Sephacryl S-300 HR | 15 | 3.75 | 0.25 | 2040/1910 | 136/127 | 544/508 | 36 (2.3) b/2.5 | 28 (43)b/47 |
| Hydroxyapatite (CHT) | 4.0 | 0.72 | 0.18 | 960/828 | 240/207 | 1330/1150 | 89 (5.6)b/5.7 | 13 (20)b/20 |
| Mono Q | 1.4 | 0.067 | 0.048 | 322/251 | 230/179 | 4810/3750 | 320 (20)b/19 | 4.5 (6.7)b/6.1 |
a NS, not shown. Data are not shown, as immunoreactive SmgGDS of the cytosol was distributed in various fractions of Q-Sepharose chromatography (see Fig. 2B).
b Data are indicated as control values obtained in the purification step of Q-Sepharose, as Di-Ras2 and SmgGDS were co-purified after the chromatography of Q-Sepharose HP.
Spectroscopic Analysis of Guanine Nucleotide Content in Purified Proteins
Protein solutions containing Di-Ras2 were heat-denatured at ∼90 °C for 3 min and separated from the denatured proteins by centrifugation with an Amicon Ultra 0.5-ml 30-kDa centrifugal filters (Merck Millipore). The filters were further washed with 20 mm Tris·HCl (pH 7.5), and both filtrates were combined in the total volume of 1 ml and applied to Mono Q column (0.5 × 5 cm, 1 ml-bed volume) equilibrated with 20 mm Tris·HCl (pH 7.5) and 50 mm LiCl. The column was washed with the equilibration buffer, and guanine nucleotides were eluted with a 5.25-ml linear gradient of 50–500 mm LiCl at a flow rate of 0.75 ml/min. The eluate was monitored at 254 nm; GDP and GTP were eluted from the column at ∼230 and ∼300 mm LiCl, respectively, with >90% recovery.
Preparation of Mouse Brain Cytosol and Membrane Fractions
Whole brain from adult male C57BL/6J mice was washed with TBS and homogenized in a buffer consisting of 10 mm Tris·HCl (pH 7.5), 10% (w/v) sucrose, 1 μg/ml aprotinin, and 0.5 mm AEBSF. The homogenate was centrifuged at 800 × g for 10 min, and the supernatant was further centrifuged at 30,000 × g for 30 min. The pellet (membrane fraction) was washed, resuspended in the homogenization buffer, and stored at −80 °C until use. The supernatant from the first 30,000 × g spin was ultracentrifuged at 100,000 × g for 90 min, and the clear supernatant (cytosol fraction) and the residual pellet (microsome fraction) were stored at −80 °C until use.
Purification of Recombinant Di-Ras2, RhoA, and SmgGDS Proteins Produced in E. coli
The following cDNAs corresponding to the indicated residues were subcloned into pGEX6P-1 (GE Healthcare) for the production of recombinant proteins in E. coli: mouse Di-Ras1 (NCBI reference sequence NP_660252.1, amino acid residues 1–194), mouse Di-Ras2 (NCBI reference sequence NP_001019645.1, amino acid residues 1–195), human Di-Ras2 (NCBI reference sequence NP_060064.2, amino acid residues 1–195), human RhoA (NCBI reference sequence NP_001655.1, amino acid residues 1–189) and human SmgGDS (NCBI reference sequence NP_001093898.1, amino acid residues 1–559). The cDNA of mouse SmgGDS (NCBI reference sequence NP_663519.2, amino acid residues 1–558) was subcloned into pCold-TF (TaKaRa). Point mutants in human SmgGDS were generated by PCR-based site-directed mutagenesis. GST fusion proteins were induced in E. coli BL21-CodonPlus DE3 (Stratagene) harboring the corresponding plasmid with 0.1 mm isopropyl β-d-1-thiogalactopyranoside at 20 °C for 16 h. Recombinant proteins were purified with glutathione-Sepharose 4B beads (GE Healthcare) and then digested with PreScission Protease (GE Healthcare) to remove GST moieties. The digested proteins were applied to a Sephadex G-25 gel filtration column (GE Healthcare) equilibrated with another common buffer, TMDN (consisting of 50 mm Tris·HCl (pH 7.5), 5 mm MgCl2, 1 mm DTT, and 100 mm NaCl) for SmgGDS or TMDN containing 0.1% (w/v) Lubrol PX (Nakalai Tesque) for Di-Ras2 and RhoA. The fractions containing recombinant proteins were collected and stored at −80 °C until use. His-tagged trigger-factor fusion proteins of SmgGDS (His-TF-SmgGDS) were induced at 15 °C for 24 h and purified with Ni2+-Sepharose 6 Fast Flow beads (GE Healthcare). His-TF-SmgGDS proteins were applied to a Sephadex G-25 gel filtration column (GE Healthcare) equilibrated with TMDN, and the fractions containing recombinant proteins were stored at −80 °C until use.
Pulldown Assay Using Purified Proteins
Recombinant Di-Ras2 was incubated with His-TF (as a negative control) or His-TF-SmgGDS and further incubated with Ni2+-Sepharose 6 Fast Flow beads at 4 °C for 2 h. The beads were washed with an ice-cold wash buffer consisting of 20 mm Tris·HCl (pH 7.5), 100 mm NaCl, and 10 mm MgCl2, and the proteins bound to the beads were analyzed by SDS-PAGE and Coomassie Brilliant Blue (CBB) staining.
Guanine-nucleotide Binding Assays
[35S]GTPγS (a non-hydrolysable GTP analog) or [3H]GDP binding assays were performed as described previously (15). In brief, purified small GTPases (5–10 pmol) were incubated at 30 °C with 1 μm [35S]GTPγS (20,000 cpm/pmol) or 5 μm [3H]GDP (4000–6000 cpm/pmol) in the presence or absence of SmgGDS (40 pmol) in 50 μl of TMDN containing 0.002% (w/v) Lubrol PX and 100 μg/ml BSA. After incubation at 30 °C for various time periods, the amount of [35S]GTPγS or [3H]GDP bound to the proteins was determined by a nitrocellulose-filter binding method as described previously (6).
Guanine-nucleotide Dissociation Assays
RhoA or Di-Ras2 (5–10 pmol) was incubated for the appropriate time periods (6 h and 30 min for RhoA and Di-Ras2, respectively) with [35S]GTPγS (1 μm) or [3H]GDP (5 μm) in 40 μl of TMDN containing 0.002% (w/v) Lubrol PX and 100 μg/ml BSA. SmgGDS or vehicle was then added to the reaction mixture and incubated for various time periods, and the amount of [35S]GTPγS or [3H]GDP bound to the proteins was then determined by the nitrocellulose-filter binding method described above.
Analysis of Complex Formation by Size-exclusion Chromatography
Di-Ras2 (0.6 μm) or RhoA (0.6 μm) was incubated with 5 μm GTPγS in the presence or absence of SmgGDS (4 μm) at 30 °C for 30 min in 500 μl of TMDN containing 0.015% (w/v) Lubrol PX and 150 μg/ml BSA. The reaction mixture was separated by Superdex 200 prep grade (GE Healthcare) column chromatography (1.6 × 50 cm, 80-ml bed volume). Eluted fractions were analyzed by SDS-PAGE followed by immunoblotting.
Cell Culture and Transfection
HEK293T cells were cultured in DMEM supplemented with 10% (v/v) FBS in a humidified atmosphere of 5% CO2 at 37 °C. The cells were transfected with 2 or 5 μg (for 6- or 10-cm dishes, respectively) of plasmid DNA using Lipofectamine 2000 and incubated for 24–48 h for subsequent experiments.
Analysis of Guanine Nucleotides Associated with Di-Ras2 in Living Cells
HEK293T cells were transfected with a FLAG-tagged Di-Ras2 expression vector with or without a Myc-tagged SmgGDS expression vector. Guanine nucleotides associated with FLAG-Di-Ras2 were analyzed as described previously (6). In brief, 48 h after transfection, the cells were labeled for 2 h with 32Pi (1.85 MBq/dish) in phosphate-free DMEM. The labeled cells were lysed with 0.5 ml of ice-cold TMDN containing 1% (w/v) Lubrol PX, 2 μg/ml aprotinin, and 0.5 mm AEBSF, and the cell lysates were incubated with anti-FLAG M2 agarose affinity gel (Sigma) at 4 °C for 15 min. After washing the affinity gel with ice-cold TMDN containing 0.1% (w/v) Lubrol PX, the associated nucleotides were separated by thin-layer chromatography followed by detection and quantitation using a BAS-1500 imaging analyzer (Fuji film).
Pulse-chase Experiments
Pulse-chase analysis was performed basically as described previously (16). In brief, HEK293T cells expressing FLAG-Di-Ras2 with or without Myc-SmgGDS were pulse-labeled for 1 h with EasyTag Express-[35S] Protein Labeling Mix (PerkinElmer Life Sciences) in methionine/cysteine-free DMEM (2.775 MBq/dish) and chased for up to 48 h. At each time point cells were lysed with 0.5 ml of an ice-cold lysis buffer and clarified followed by incubation with anti-FLAG M2-agarose affinity gel at 4 °C for 2 h. After washing, the immunocomplexes were eluted with an ice-cold wash buffer containing 200 μg/ml FLAG peptide and separated by SDS-PAGE. The gel was analyzed using Typhoon FLA9000 phosphorimaging (GE Healthcare).
Results
Di-Ras2 Protein Is Detected in Both the Cytosol and Membrane Fractions of Brain Tissues
We previously reported that the mRNAs of human Di-Ras1 and Di-Ras2 are expressed in brain (6). To investigate the protein expression of Di-Ras1 and Di-Ras2, we generated monoclonal antibodies against Di-Ras1 and Di-Ras2, among which clones 6B10 and 2D6 specifically recognized Di-Ras1 and Di-Ras2, respectively, with comparable titers (Fig. 1A). Western blot analysis of rat tissue extracts with the antibody 2D6 detected Di-Ras2 specifically in rat brain extracts (Fig. 1B), whereas Di-Ras1 was not detected in these extracts (data not shown). We then conducted centrifugation-based subcellular fractionation of mouse brain to determine the distribution of Di-Ras2. N-Ras and R-Ras are detected only in membrane fractions (17, 18); however, considerable amounts of Di-Ras2 were detected in the cytosol as well as in the membrane fraction (Fig. 1C).
FIGURE 1.
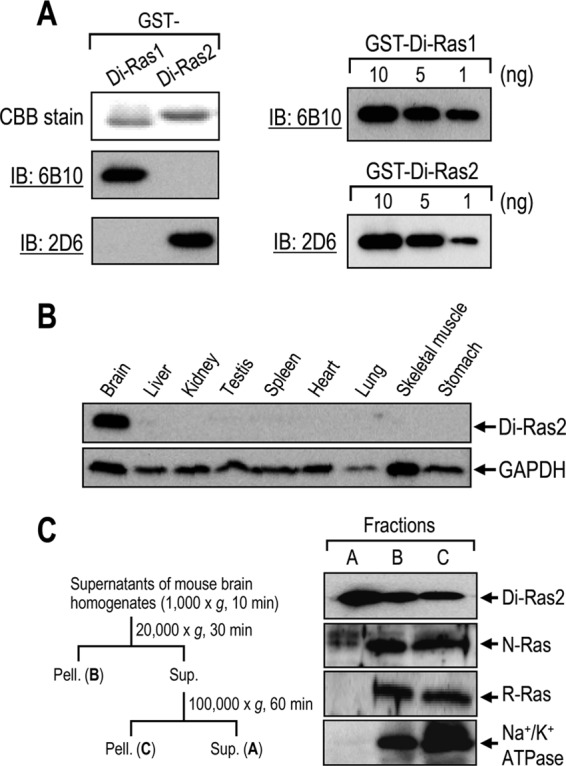
Di-Ras2 is detected specifically in brain tissues. A, characterization of anti-Di-Ras monoclonal antibodies. GST-Di-Ras1 and GST-Di-Ras2 were subjected to SDS-PAGE/CBB staining and Western blot analysis using clone 6B10 or 2D6 (left panel). The indicated amounts of GST-Di-Ras fusion proteins were subjected to Western blot analysis (right panel). IB, immunoblot. B, Western blot analysis of rat tissue extracts using anti-Di-Ras2 (upper panel) or anti-GAPDH (lower panel) antibodies. C, mouse brain homogenates were subjected to fractionation by centrifugation followed by Western blot analysis using anti-Di-Ras2, anti-N-Ras, anti-R-Ras or anti-Na+/K+-ATPase antibodies. Pell., pellet; Sup., supernatant.
Identification of SmgGDS as a Binding Protein of Di-Ras2
The abundant existence of Di-Ras2 in the cytosol fraction motivated us to investigate the biochemical properties of cytoplasmic Di-Ras2. To this end we partially purified Di-Ras2 from rat brain cytosol by sequential column chromatography using Q-Sepharose, Sephacryl S-300, ceramic-hydroxyapatite, and Mono Q on the basis of the immunoreactivity of 2D6 (Table 1). During the course of the purification, we noticed an ∼55-kDa protein co-purifying with Di-Ras2 (Fig. 2, A–D). The 55-kDa protein band was excised from SDS-PAGE gels and subjected to MALDI-TOF mass spectrometry analysis, which identified the protein as rat SmgGDS (accession number NP_001101198.1).
Most Di-Ras2 in the cytosol was eluted from the first Q-Sepharose column as a symmetric peak at ∼130 mm NaCl (Fig. 2B), which also contained SmgGDS with the same specific activity as Di-Ras2 (Table 1). However, more than a half of the cytoplasmic SmgGDS was separately recovered from the column at 150∼200 mm NaCl. This suggests that the latter SmgGDS exists as monomeric and/or other forms different from the former Di-Ras2·SmgGDS complex. In the next gel filtration (Sephacryl S-300) column (Fig. 2C), the cytoplasmic Di-Ras2 behaved as an ∼100-kDa protein associated with SmgGDS, and the monomeric form of Di-Ras2 or SmgGDS was not detected. Thus, Di-Ras2 and SmgGDS were co-purified from brain cytosol with the same specific activities and recoveries after the first step of Q-Sepharose chromatography. The theoretical specific activity of Di-Ras2 (23 kDa)-SmgGDS (55 kDa) heterodimer could be calibrated as 12.5 nmol/mg for the 78-kDa heterodimer. The present final Mono Q fraction, which contained each ∼4 nmol/mg Di-Ras2 and SmgGDS, implied that the protein purity of the heterodimer was ∼30%. This purity was well correlated with the combined amount of CBB-stained 55-kDa SmgGDS (24%) and 23-kDa Di-Ras2 (9%) bands in Fig. 2A (lane 5). Table 1 also revealed that Di-Ras2 (15 pmol/mg in cytosol) constitutes 0.035% (as 23-kDa monomer) or 0.12% (as 78-kDa heterodimer) of the total brain cytoplasmic proteins and that the small GTPase is not an abundant protein, unlike αβγ-trimeric G proteins, which constitute several percentages of membrane proteins in the same tissues. The partially purified heterodimer consisting of Di-Ras2 and SmgGDS could be further clarified by hydrophobic (phenyl-Sepharose) column chromatography almost to homogeneity (Fig. 2D). Direct interaction between Di-Ras2 and SmgGDS was confirmed by pulldown assay using E. coli-produced recombinant proteins; Di-Ras2 bound to SmgGDS fused to trigger factor (TF) but not to TF only (as a negative control) (Fig. 2E).
Because most native GTP-binding proteins and their recombinants are expected to be purified as a GDP- or GTP-bound form, we analyzed the content of guanine nucleotides, which are expected to be present in the partially purified Di-Ras2·SmgGDS complex of Mono Q fraction. The protein fraction was heat-denatured, and the solution was separated from the denatured proteins. Guanine nucleotides in the solution were spectroscopically analyzed with an anion-exchange (Mono Q) column (Fig. 2F). Intriguingly, no significant amount of GDP or GTP (at least >20% of its stoichiometry) was detected in the fraction of the partially purified Di-Ras2·SmgGDS complex. In sharp contrast, recombinant GST-Di-Ras2 monomer purified from E. coli retained stoichiometric amounts of GDP and GTP (GDP:GTP = 1:2). These results indicate that the Di-Ras2·SmgGDS complex may be characterized as a low affinity state for GDP and GTP as compared with Di-Ras2 monomer, although we cannot totally exclude the possibility that guanine nucleotides were released from the complex during the purification steps. Collectively, these results indicate that Di-Ras2 exists not only as a heterodimer tightly associated with SmgGDS in a 1:1-molar ratio but also as a low affinity state for guanine nucleotides in rat brain cytosol.
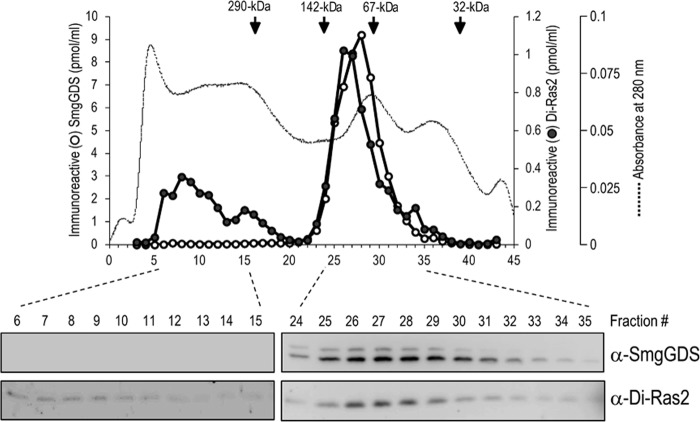
We also analyzed Di-Ras2 in the membrane fraction of rat brain. Cholate extracts of membranes from rat brain were subjected to gel filtration (Superdex 200 pg) column, and the eluted fractions were analyzed by Western blotting. Intriguingly, besides the elution as a peak with an apparent molecular mass of ∼100 kDa, a fraction of Di-Ras2 behaved as a >290-kDa protein in which no detectable SmgGDS was found (Fig. 3). These results suggest that Di-Ras2 interacts with other molecules than SmgGDS and/or self-interacts to form large protein complexes in membrane fractions.
FIGURE 3.
A fraction of d-Ras2 extracted from rat brain membranes is found in a high molecular weight complex without SmgGDS. Rat brain membranes were extracted with 1% sodium cholate, and the extracts were applied to Superdex 200 pg column equilibrated with a buffer (20 mm Tris·HCl (pH 8.0), 2 mm MgCl2, 1 mm DTT, 100 mm NaCl, 1 μg/ml aprotinin, and 1% sodium cholate). The eluted fractions were subjected to Western blot analysis using anti-SmgGDS or anti-Di-Ras2 antibodies, and the density of protein bands was analyzed with ImageQuant software (GE Healthcare).
Affinities of Di-Ras2 for Guanine Nucleotides Are Reduced in the Presence of SmgGDS
Because the protein amount of purified Di-Ras2 was limited due to its low abundance in rat brain, further biochemical analyses were performed with E. coli-produced recombinant proteins. SmgGDS was initially characterized as a GEF for multiple small GTPases, although a recent study has suggested that it specifically activates RhoA and RhoC (19). We investigated the effect of SmgGDS on the guanine-nucleotide binding activity of Di-Ras2. SmgGDS stimulated the binding of GTPγS and GDP to RhoA as reported previously, whereas it reduced the binding to Di-Ras2 (Fig. 4A). In general, GEFs preferentially bind to nucleotide-free, but not GDP or GTP-bound, G-proteins. Indeed, SmgGDS has been shown to form a complex with nucleotide-free RhoA in vitro. Because Di-Ras2 could bind to SmgGDS (Fig. 2), we hypothesized that the reduction of guanine-nucleotide binding to Di-Ras2 caused by SmgGDS might be due to a decrease in the affinity of Di-Ras2 for guanine nucleotides when associated with SmgGDS. To test this, SmgGDS was added to Di-Ras2 or RhoA after the binding of guanine nucleotides to the proteins had reached an equilibrium state. The amount of guanine nucleotides bound to Di-Ras2 decreased immediately after the addition of SmgGDS and then reached another equilibrium state, whereas the guanine nucleotides bound to RhoA were unaffected (Fig. 4B). We further tested whether Di-Ras2 and SmgGDS can form a complex under the same conditions used in GTPγS or GDP binding assays. Size exclusion chromatography analysis showed that Di-Ras2, but not RhoA, co-migrated with SmgGDS under the same conditions as the GTPγS binding assay (Fig. 5). These results suggest that the affinity of Di-Ras2 for guanine nucleotides is reduced upon complex formation with SmgGDS.
FIGURE 4.
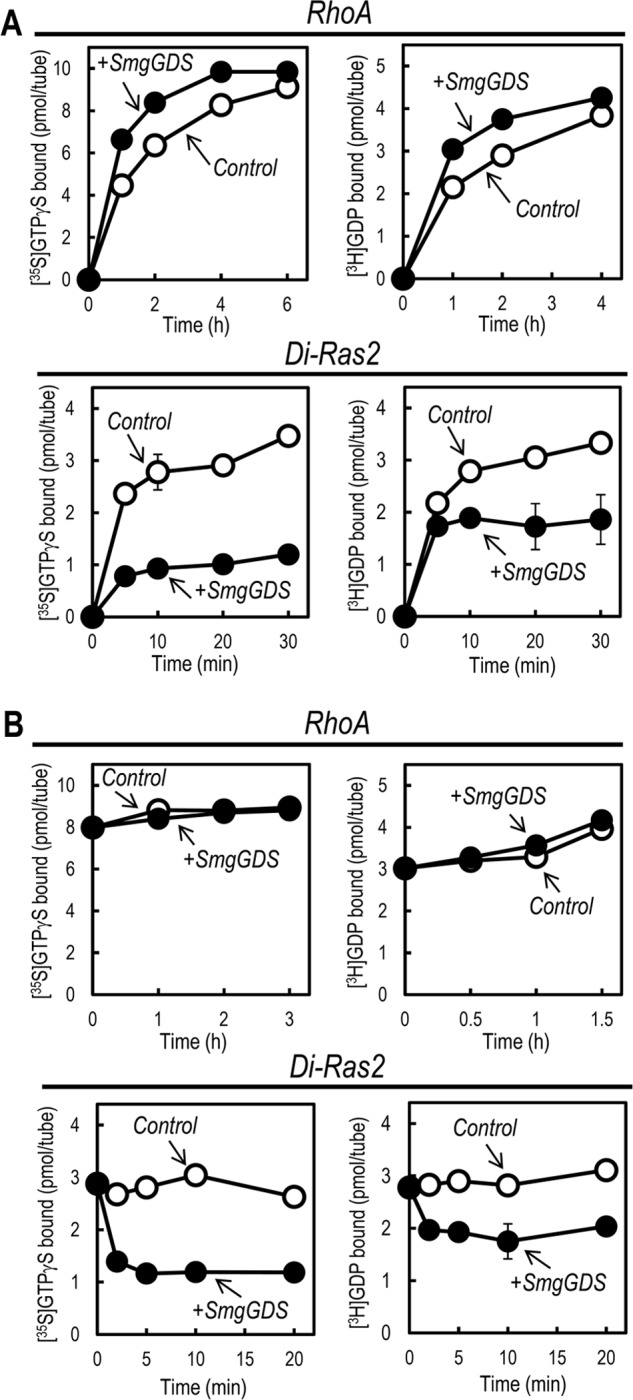
Binding affinities of Di-Ras2 for guanine nucleotides are reduced in the presence of SmgGDS. A, guanine-nucleotide binding assay for RhoA and Di-Ras2 in the presence or absence of SmgGDS. B, guanine-nucleotide dissociation assay for RhoA and Di-Ras2 in the presence or absence of SmgGDS.
FIGURE 5.
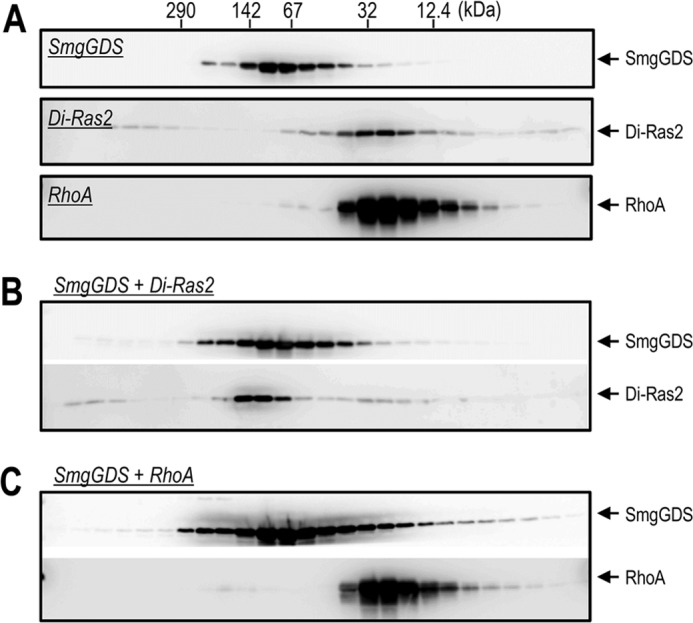
Di-Ras2 forms a high affinity complex with SmgGDS. A, purified recombinant proteins were separated by size-exclusion column chromatography, and the fractions were analyzed by SDS-PAGE and Western blotting. B and C, Di-Ras2 or RhoA were incubated with 5 μm GTPγS in the presence of SmgGDS at 30 °C for 30 min. The reaction mixtures were subjected to the same analysis as described in A.
An Amino Acid Residue of SmgGDS Responsible for RhoA Activation Is Also Involved in the Interaction with Di-Ras2
A recent study has reported mutational analysis of conserved amino acid residues of SmgGDS (19); mutation of Asn-338 in the C-terminal region to Ala (N338A) abolishes the GEF activity for RhoA, whereas mutating the N-terminal conserved region (R112A/N116A/Y119A) of SmgGDS does not affect its ability to activate RhoA (Fig. 6A, left panel, and Ref. 19). We first tested the effects of these mutations on the ability of SmgGDS to reduce guanine-nucleotide binding to Di-Ras2. Although SmgGDS/R112A/N116A/Y119A still retained an ability to reduce GTPγS binding to Di-Ras2 similarly to wild-type SmgGDS, SmgGDS/N338A lost this ability (Fig. 6A, right panel). We next investigated complex formation between Di-Ras2 and SmgGDS by size exclusion chromatography and found that SmgGDS/R112A/N116A/Y119A could form a complex with Di-Ras2, but SmgGDS/N338A could not (Fig. 6B). The reduced interaction between SmgGDS/N338A and Di-Ras2 was also observed in living cells; the amounts of co-immunoprecipitated FLAG-Di-Ras2 with Myc-SmgGDS/N338A were decreased compared with those with Myc-SmgGDS/WT in HEK293 cells (Fig. 6C). Together, these results suggest that Asn-338 of SmgGDS is important not only for RhoA activation but also for the stable interaction with Di-Ras2.
FIGURE 6.

A single amino acid substitution in SmgGDS results in the loss of not only activation of RhoA but also interaction with Di-Ras2. A, RhoA (5–10 pmol, left panel) or Di-Ras2 (5–10 pmol, right panel) was incubated with [35S]GTPγS (1 μm) in the presence or absence of wild-type and mutant forms of SmgGDS (40 pmol), and the [35S]GTPγS binding was determined at the indicated time points. B, Di-Ras2 was incubated with 5 μm GTPγS in the presence or absence of wild-type or mutant forms of SmgGDS at 30 °C for 30 min. The reaction mixtures were then subjected to size-exclusion chromatography analysis, and the fractions were analyzed by Western blotting. C, FLAG-Di-Ras2 was transiently expressed with Myc-SmgGDS wild-type (WT) or N338A. Two days after transfection, cells were subjected to immunoprecipitation (IP) using anti-Myc antibody 9E10 followed by Western blotting with anti-FLAG or anti-Myc antibodies.
SmgGDS Reduces Guanine-nucleotide Binding to Di-Ras2 in Living Cells
We further investigated if the guanine nucleotide-bound status of Di-Ras2 is affected by SmgGDS in living cells. We searched a number of mammalian cell lines for Di-Ras2 expression to analyze the effect of SmgGDS on endogenous Di-Ras2 in living cells; however, as far as we tested, there were no cell lines in which Di-Ras2 proteins were detected (Fig. 7A). Thus, HEK293 cells were transiently transfected with FLAG-Di-Ras2 alone or together with Myc-SmgGDS and metabolically labeled with 32Pi. The guanine nucleotides associated with FLAG-Di-Ras2 were then analyzed by thin-layer chromatography. Co-expression of Myc-SmgGDS had no apparent effect on the ratio of GDP/GTP associated with FLAG-Di-Ras2; however, it reduced the amounts of GDP and GTP bound to FLAG-Di-Ras2 (Fig. 7, B and C). These results, together with in vitro analysis using purified recombinant proteins, suggest that the affinity of Di-Ras2 for guanine nucleotides is reduced when it interacts with SmgGDS in living cells.
FIGURE 7.
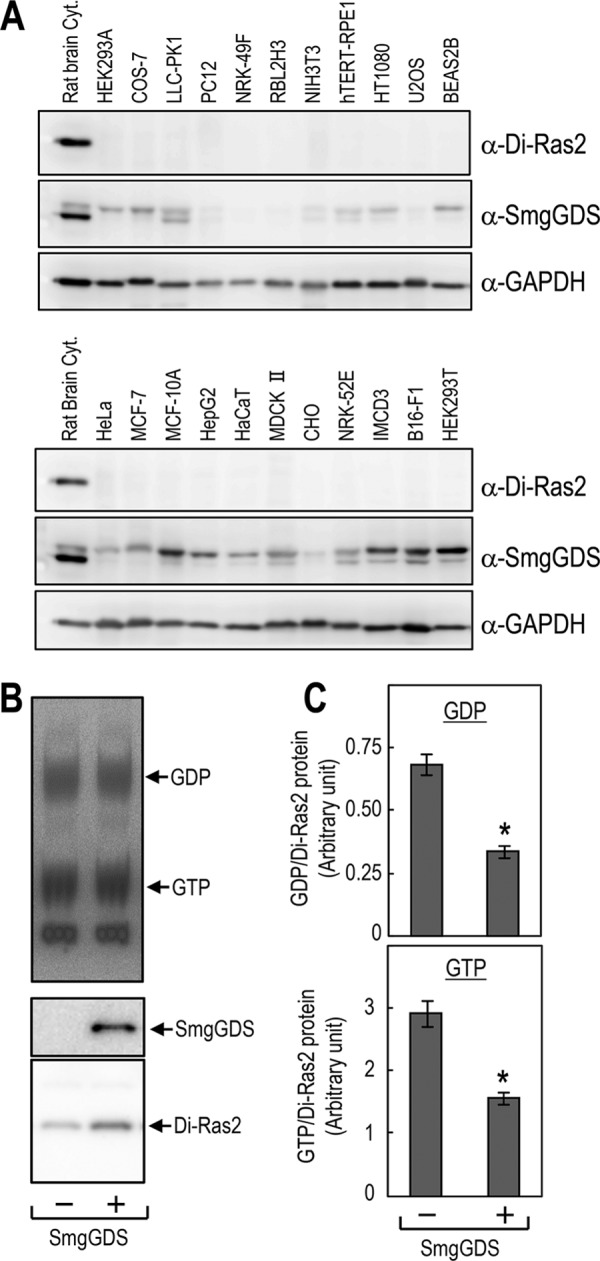
The amounts of guanine-nucleotides bound to Di-Ras2 are decreased in HEK293T cells overexpressing SmgGDS. A, Western blot analysis of extracts from various cell lines using anti-Di-Ras2, anti-SmgGDS, or anti-GAPDH antibodies. Rat brain cytosol was used as a positive control. B, FLAG-Di-Ras2 was transiently expressed with or without Myc-SmgGDS in HEK293T cells. Two days after transfection, cells were metabolically labeled with 32Pi and subjected to immunoprecipitation using an anti-FLAG antibody. The guanine nucleotides and proteins associated with the immunocomplexes were then analyzed by thin layer chromatography (upper panel) and Western blotting (middle and lower panels), respectively. C, quantitation of the amounts of guanine nucleotides associated with the immunocomplexes. n = 3; *, p < 0.05 by Student's t test.
Di-Ras2 Is Stabilized by Interaction with SmgGDS
In the course of Di-Ras2 expression experiments in HEK293 cells, we noticed that Di-Ras2 protein levels, when co-expressed with SmgGDS, were reproducibly higher compared with those when Di-Ras2 was expressed alone (for example, Fig. 7B, lower panel). Because Di-Ras2 was co-purified with SmgGDS from brain cytosol, we hypothesized that Di-Ras2 might be stabilized by forming a complex with SmgGDS in vivo. We performed pulse-chase experiments and showed that the half-life of Di-Ras2 was ∼16 h in control cells (Fig. 8A). When Di-Ras2 was co-expressed with SmgGDS, it interacted with SmgGDS shortly after protein synthesis (Fig. 8A, time = 0), and the half-life of Di-Ras2 was prolonged to ∼33 h. Previous studies have shown that SmgGDS preferentially interacts with isoprenylated but not non-isoprenylated, small GTPases in living cells (20, 21). Because Di-Ras2 has a C-terminal isoprenylation site (CAAX motif), we investigated the effect of CAAX deletion from Di-Ras2 on the interaction with SmgGDS. Pulse-chase experiments showed that CAAX-deleted Di-Ras2 formed substantially less complex with SmgGDS (Fig. 8B). Furthermore, no apparent enhancement in stability was observed for Di-Ras2ΔC4 by co-expression of SmgGDS (Fig. 8C). These results suggest that isoprenylated Di-Ras2 forms a complex with SmgGDS immediately after its synthesis in living cells, which leads to increased Di-Ras2 stability.
FIGURE 8.
The CAAX motif-dependent stabilization of Di-Ras2 by SmgGDS. A, HEK293T cells were transfected with mock, FLAG-Di-Ras2, or a combination of FLAG-Di-Ras2 and Myc-SmgGDS. Two days after transfection, the cells were pulse-labeled with 35S-labeled methionine/cysteine, chased for the indicated time periods, and subjected to SDS-PAGE. The dried gel was analyzed using phosphorimaging (left panel), and the relative band intensities of FLAG-Di-Ras2 were quantified (right panel). B, HEK293T cells were transfected with wild-type FLAG-Di-Ras2 or the CAAX motif deletion mutant of FLAG-Di-Ras2 (Di-Ras2ΔC4) together with Myc-SmgGDS. Two days after transfection, the cells were pulse-labeled with 35S-labeled methionine/cysteine, chased for the indicated time periods, and subjected to SDS-PAGE followed by image analysis as described above (left panel). Protein expression in the transfected cells was analyzed by Western blotting using anti-FLAG or anti-Myc antibodies (right panel). C, HEK293T cells were transfected with mock, FLAG-Di-Ras2ΔC4, or a combination of FLAG-Di-Ras2ΔC4 and Myc-SmgGDS and then subjected to pulse-chase analysis as described above.
Discussion
In the present study we have shown that SmgGDS is a binding partner of Di-Ras2 in brain tissues and in cultured cells. Subcellular fractionation by centrifugation revealed that considerable amounts of Di-Ras2 could be recovered from brain cytosol, which is in sharp contrast to the predominant membrane localization of general Ras-family GTPases. Di-Ras2 was co-purified with SmgGDS from rat brain cytosol in a 1:1 molar ratio, and recombinant Di-Ras2 and SmgGDS proteins formed a high affinity complex. The guanine-nucleotide affinities of Di-Ras2 were reduced when it bound to SmgGDS in vitro and in living cells. Di-Ras2 associated with SmgGDS immediately after protein synthesis, the interaction being dependent on the CAAX motif, and the interaction enhanced the stability of Di-Ras2. We thus propose that Di-Ras2, presumably its isoprenylated form, makes a tight complex with SmgGDS in the cytosol to be in a low affinity state for guanine nucleotides.
We previously reported Di-Ras1 and Di-Ras2 as small GTPases that form a distinct subgroup of Ras-family GTPases (6). Human Di-Ras1 and Di-Ras2 mRNAs are expressed in brain and a C. elegans Di-Ras homolog (drn-1) is also expressed specifically in neuronal cells (6, 8). Consistent with these findings, we detected Di-Ras2 protein specifically in brain tissues. As far as we tested, Di-Ras1 protein was undetectable in brain. These results suggest that Di-Ras2 is the major isoform of Di-Ras proteins in brain tissues, although we cannot rule out the possibility that Di-Ras1 protein is expressed in a restricted brain region and/or at a particular time.
Cytoplasmic Di-Ras2 was co-purified with SmgGDS, and the recombinant proteins formed a tight complex, suggesting that Di-Ras2 exists as a complex with SmgGDS in brain cytosol. Although initial studies have shown that SmgGDS can function as a GEF for a wide range of small GTPases, a recent study has indicated that SmgGDS specifically activates RhoA and RhoC (19); SmgGDS catalyzes the unloading of a fluorescent analog of GDP (Mant-GDP) from RhoA and also the loading of Mant-GDP in a concentration-dependent manner. We found that SmgGDS stimulated dissociation of guanine nucleotides from Di-Ras2, as observed for RhoA; however, SmgGDS failed to stimulate loading of guanine nucleotides to Di-Ras2. In general, GEFs for small GTPases can form high affinity complexes with guanine-nucleotide-free small GTPases. Indeed, SmgGDS forms a complex with guanine-nucleotide-free, but not GDP or GTP-bound, RhoA in vitro (19). It is thus likely that SmgGDS tightly associates with a guanine-nucleotide free form of Di-Ras2 after stimulation of guanine-nucleotide release from Di-Ras2. Considering that not all of the guanine nucleotides were released from E. coli-produced Di-Ras2, even in conditions when nearly all Di-Ras2 seems to be associated with SmgGDS, SmgGDS may also be able to form a complex with a guanine nucleotide-bound form of Di-Ras2. Nevertheless, these results indicate that the affinity of Di-Ras2 for guanine nucleotides is reduced when it forms a complex with SmgGDS.
Di-Ras1 and Di-Ras2 have a potential C-terminal isoprenylation site (CAAX motif), and indeed, Di-Ras1 has been shown to be farnesylated (5). Previous reports have shown that SmgGDS prefers isoprenylated small GTPases when interacting with them in living cells (20, 21). Consistent with this, CAAX motif deletion of Di-Ras2 resulted in a marked decrease in complex formation with SmgGDS in HEK293T cells (Fig. 8B, left panel). In addition, co-expression of SmgGDS had no apparent effect on the stability of the CAAX motif deletion mutant (Fig. 8C). Considering that protein isoprenylation generally occurs immediately after biosynthesis (24), these results suggest that isoprenylated, but not non-isoprenylated, Di-Ras2 binds to SmgGDS, which leads to the stabilization of Di-Ras2 in living cells. In this regard it should be noted here that rat brain Di-Ras2·SmgGDS complex was long sustained without dissociation during several steps of column chromatography (see Fig. 2) and that GTP binding activity was not significantly detected in the partially purified protein complex (data not shown). Thus, tight association of isoprenylated Di-Ras2 with SmgGDS may also lead to a very low affinity state for guanine nucleotides. Intriguingly, a recent study has pointed out that a preference of SmgGDS for isoprenylated small GTPases is lost in vitro; SmgGDS can bind to non-isoprenylated small GTPases in vitro binding assays using recombinant proteins (21). Indeed, we have shown that recombinant non-isoprenylated Di-Ras2 was able to form a tight complex with SmgGDS (Fig. 5B). SmgGDS binding to non-isoprenylated Di-Ras2 might be because the SmgGDS concentration was sufficiently high to enable stable complex formation with Di-Ras2 in the in vitro binding assay. Alternatively, post-translational modifications and/or unknown binding partners might regulate the interaction between isoprenylated Di-Ras2 and SmgGDS in living cells.
SmgGDS is a unique GEF in that it does not possess any of the catalytic domains found in other GEFs. Instead, SmgGDS contains multiple repeats of an armadillo motif, which is known to be involved in protein-protein interaction (22). Although the crystal structure of SmgGDS remains to be determined, a recent study using homology modeling has revealed that at least two regions of SmgGDS are involved in the activation of RhoA (19). One of these regions is a groove of the armadillo repeat superhelical structure. SmgGDS/N338A mutation in the groove of the superhelical structure abolishes the ability to activate RhoA (19). We found that this mutation rendered SmgGDS unable to interact with Di-Ras2, implicating the groove in not only RhoA but also Di-Ras2 interaction. The other region responsible for RhoA activation is an electronegative patch comprised of acidic amino acid residues, which may be involved in the interaction with the C-terminal polybasic region of RhoA (23). Because the C-terminal region of Di-Ras2 contains some basic amino acids (Lys-187 to Lys-195), these residues might also be involved in the interaction with SmgGDS. Future high resolution structural analysis of a SmgGDS-Di-Ras2 complex will be required to understand the molecular basis underlying the tight interaction between SmgGDS and Di-Ras2.
The physiological significance of the interaction of SmgGDS with small GTPases is still unclear. Recently, besides its role as a GEF, several possible functions of SmgGDS have been proposed (20, 21, 23). It is speculated that SmgGDS may be involved in the regulation of the release of newly isoprenylated small GTPases from protein farnesyl transferase and/or the subsequent CAAX processing of small GTPases in the ER. SmgGDS is also expected to regulate the transport of fully matured (processed) small GTPases from the ER to the plasma membrane or to mediate their extraction from the plasma membrane. Thus, interaction of Di-Ras2 with SmgGDS might regulate the post-translational modifications and/or trafficking of Di-Ras2. SmgGDS might also have a role in solubilizing and stabilizing isoprenylated Di-Ras2, because a considerable amount of cytosolic Di-Ras2 was detected in a complex with SmgGDS. Alternatively, the interaction may be involved in the regulation of Di-Ras2 activity. It should be noted that Di-Ras1 and Di-Ras2 favor an active GTP-bound form because of their spontaneous guanine-nucleotide exchange activity together with their low GTPase activity (6, 7). This self-activating property of Di-Ras2 is analogous to that observed for fast-cycling Ras GTPase mutants with transforming activity in which the intrinsic GDP/GTP exchange rate is enhanced (25, 26). Considering that the tight association of Di-Ras2 with SmgGDS renders the Di-Ras2 guanine-nucleotide affinity reduced, the complex formation might be important for the suppression of spontaneous activation of Di-Ras2. This hypothesis is well consistent with our previous findings that Di-Ras1/Di-Ras2 are predominantly GTP-bound forms in living cells when overexpressed (6); because overexpression of Di-Ras1 and Di-Ras2 leads to high levels of production of those proteins (relative to endogenous SmgGDS), Di-Ras1 and Di-Ras2 are likely to be “SmgGDS-free” states and thus become able to bind GTP, which is ∼10 times abundant than GDP in cytosol. Considering that a fraction of Di-Ras2 was found in a high molecular weight complex without SmgGDS in rat brain membranes (Fig. 3), Di-Ras2 may dissociate from SmgGDS to become a GTP-bound form, thereby interacting with as yet an unidentified binding partner(s) and/or self-associating in the membranes.
A recent study has reported a unique regulation of Rnd GTPase, whose biochemical properties are similar to those of Di-Ras, via interaction with 14-3-3. The study showed that 14-3-3 proteins act on phosphorylated Rnd proteins to facilitate their translocation from membranes to cytoplasm and consequently to inhibit their function (27). There might be post-translational modification and/or unknown interactor(s) regulating the interaction between Di-Ras2 and SmgGDS. Clarifying how the association/dissociation of Di-Ras2 and SmgGDS is regulated is an important issue for understanding the molecular basis of the regulation of Di-Ras function.
Author Contributions
Y. O., T. K., and K. K. designed the study and wrote the paper. S. E. performed and analyzed the experiments shown in Figs. 3 and 7. A. E. contributed to the experiments shown in Figs. 2 and 4. N. U. contributed to the preparation of anti-Di-Ras mAbs and the experiments shown in Fig. 1. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Drs. Toma and Shimizu (University of Tokyo) for helpful discussions and the present/former members of the Katada laboratory for useful suggestions.
This work was supported in part by research grants from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (to K. K. and T. K.) and the Japan Society for the Promotion of Science (JSPS) (to K. K. and T. K.). The authors declare that they have no conflicts of interest with the contents of this article.
- GEF
- guanine-nucleotide exchange factor
- GAP
- GTPase activating protein
- TF
- trigger factor
- AEBSF
- 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride
- TF
- trigger factor
- CBB
- Coomassie Brilliant Blue
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
References
- 1. Colicelli J. (2004) Human RAS superfamily proteins and related GTPases. Sci. STKE 2004, RE13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macara I. G., Lounsbury K. M., Richards S. A., McKiernan C., Bar-Sagi D. (1996) The Ras superfamily of GTPases. FASEB J. 10, 625–630 [DOI] [PubMed] [Google Scholar]
- 3. Takai Y., Sasaki T., Matozaki T. (2001) Small GTP-binding proteins. Physiol. Rev. 81, 153–208 [DOI] [PubMed] [Google Scholar]
- 4. Bos J. L., Rehmann H., Wittinghofer A. (2007) GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 [DOI] [PubMed] [Google Scholar]
- 5. Ellis C. A., Vos M. D., Howell H., Vallecorsa T., Fults D. W., Clark G. J. (2002) Rig is a novel Ras-related protein and potential neural tumor suppressor. Proc. Natl. Acad. Sci. U.S.A. 99, 9876–9881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kontani K., Tada M., Ogawa T., Okai T., Saito K., Araki Y., Katada T. (2002) Di-Ras, a distinct subgroup of ras family GTPases with unique biochemical properties. J. Biol. Chem. 277, 41070–41078 [DOI] [PubMed] [Google Scholar]
- 7. Gasper R., Sot B., Wittinghofer A. (2010) GTPase activity of Di-Ras proteins is stimulated by Rap1GAP proteins. Small GTPases 1, 133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tada M., Gengyo-Ando K., Kobayashi T., Fukuyama M., Mitani S., Kontani K., Katada T. (2012) Neuronally expressed Ras-family GTPase Di-Ras modulates synaptic activity in Caenorhabditis elegans. Genes Cells 17, 778–789 [DOI] [PubMed] [Google Scholar]
- 9. Chuang T. H., Xu X., Quilliam L. A., Bokoch G. M. (1994) SmgGDS stabilizes nucleotide-bound and -free forms of the Rac1 GTP-binding protein and stimulates GTP/GDP exchange through a substituted enzyme mechanism. Biochem. J. 303, 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiraoka K., Kaibuchi K., Ando S., Musha T., Takaishi K., Mizuno T., Asada M., Ménard L., Tomhave E., Didsbury J. (1992) Both stimulatory and inhibitory GDP/GTP exchange proteins, smg GDS and rho GDI, are active on multiple small GTP-binding proteins. Biochem. Biophys. Res. Commun. 182, 921–930 [DOI] [PubMed] [Google Scholar]
- 11. Mizuno T., Kaibuchi K., Yamamoto T., Kawamura M., Sakoda T., Fujioka H., Matsuura Y., Takai Y. (1991) A stimulatory GDP/GTP exchange protein for smg p21 is active on the post-translationally processed form of c-Ki-ras p21 and rhoA p21. Proc. Natl. Acad. Sci. U.S.A. 88, 6442–6446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vikis H. G., Stewart S., Guan K. L. (2002) SmgGDS displays differential binding and exchange activity towards different Ras isoforms. Oncogene 21, 2425–2432 [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto T., Kaibuchi K., Mizuno T., Hiroyoshi M., Shirataki H., Takai Y. (1990) Purification and characterization from bovine brain cytosol of proteins that regulate the GDP/GTP exchange reaction of smg p21s, ras p21-like GTP-binding proteins. J. Biol. Chem. 265, 16626–16634 [PubMed] [Google Scholar]
- 14. Tamatani T., Miyasaka M. (1990) Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int. Immunol. 2, 165–171 [DOI] [PubMed] [Google Scholar]
- 15. Kawazu M., Ueno T., Kontani K., Ogita Y., Ando M., Fukumura K., Yamato A., Soda M., Takeuchi K., Miki Y., Yamaguchi H., Yasuda T., Naoe T., Yamashita Y., Katada T., Choi Y. L., Mano H. (2013) Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc. Natl. Acad. Sci. U.S.A. 110, 3029–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonifacino J. S. (2001) Metabolic labeling with amino acids. Curr. Protoc. Cell Biol. Chapter 7, Unit 7.1 [DOI] [PubMed] [Google Scholar]
- 17. Hansen M., Prior I. A., Hughes P. E., Oertli B., Chou F. L., Willumsen B. M., Hancock J. F., Ginsberg M. H. (2003) C-terminal sequences in R-Ras are involved in integrin regulation and in plasma membrane microdomain distribution. Biochem. Biophys. Res. Commun. 311, 829–838 [DOI] [PubMed] [Google Scholar]
- 18. Whyte D. B., Kirschmeier P., Hockenberry T. N., Nunez-Oliva I., James L., Catino J. J., Bishop W. R., Pai J. K. (1997) K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 272, 14459–14464 [DOI] [PubMed] [Google Scholar]
- 19. Hamel B., Monaghan-Benson E., Rojas R. J., Temple B. R., Marston D. J., Burridge K., Sondek J. (2011) SmgGDS is a guanine nucleotide exchange factor that specifically activates RhoA and RhoC. J. Biol. Chem. 286, 12141–12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berg T. J., Gastonguay A. J., Lorimer E. L., Kuhnmuench J. R., Li R., Fields A. P., Williams C. L. (2010) Splice variants of SmgGDS control small GTPase prenylation and membrane localization. J. Biol. Chem. 285, 35255–35266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schuld N. J., Vervacke J. S., Lorimer E. L., Simon N. C., Hauser A. D., Barbieri J. T., Distefano M. D., Williams C. L. (2014) The chaperone protein SmgGDS interacts with small GTPases entering the prenylation pathway by recognizing the last amino acid in the CAAX motif. J. Biol. Chem. 289, 6862–6876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peifer M., Berg S., Reynolds A. B. (1994) A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76, 789–791 [DOI] [PubMed] [Google Scholar]
- 23. Williams C. L. (2003) The polybasic region of Ras and Rho family small GTPases: a regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell. Signal. 15, 1071–1080 [DOI] [PubMed] [Google Scholar]
- 24. Repko E. M., Maltese W. A. (1989) Post-translational isoprenylation of cellular proteins is altered in response to mevalonate availability. J. Biol. Chem. 264, 9945–9952 [PubMed] [Google Scholar]
- 25. Quilliam L. A., Zhong S., Rabun K. M., Carpenter J. W., South T. L., Der C. J., Campbell-Burk S. (1995) Biological and structural characterization of a Ras transforming mutation at the phenylalanine-156 residue, which is conserved in all members of the Ras superfamily. Proc. Natl. Acad. Sci. U.S.A. 92, 1272–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reinstein J., Schlichting I., Frech M., Goody R. S., Wittinghofer A. (1991) p21 with a phenylalanine 28→leucine mutation reacts normally with the GTPase activating protein GAP but nevertheless has transforming properties. J. Biol. Chem. 266, 17700–17706 [PubMed] [Google Scholar]
- 27. Riou P., Kjær S., Garg R., Purkiss A., George R., Cain R. J., Bineva G., Reymond N., McColl B., Thompson A. J., O'Reilly N., McDonald N. Q., Parker P. J., Ridley A. J. (2013) 14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif to inhibit G proteins. Cell 153, 640–653 [DOI] [PMC free article] [PubMed] [Google Scholar]