Background: The antidiabetic drug and mitochondrial NADH:ubiquinone oxidoreductase (complex I) inhibitor metformin has anti-inflammatory activity.
Results: Complex I inhibition decreases LPS-induced IL-1β and boosts IL-10. Mitochondrial ROS may be a signal driving LPS-induced IL-1β.
Conclusion: Complex I has a role in the induction of cytokines by LPS.
Significance: This study provides insight into the anti-inflammatory action of metformin and reveals a role for complex I dysfunction in inflammatory signaling.
Keywords: AMP-activated kinase (AMPK), complex I, IL-1, LPS, metformin
Abstract
Metformin, a frontline treatment for type II diabetes mellitus, decreases production of the pro-form of the inflammatory cytokine IL-1β in response to LPS in macrophages. We found that it specifically inhibited pro-IL-1β production, having no effect on TNF-α. Furthermore, metformin boosted induction of the anti-inflammatory cytokine IL-10 in response to LPS. We ruled out a role for AMP-activated protein kinase (AMPK) in the effect of metformin because activation of AMPK with A769662 did not mimic metformin here. Furthermore, metformin was still inhibitory in AMKPα1- or AMPKβ1-deficient cells. The activity of NADH:ubiquinone oxidoreductase (complex I) was inhibited by metformin. Another complex I inhibitor, rotenone, mimicked the effect of metformin on pro-IL-1β and IL-10. LPS induced reactive oxygen species production, an effect inhibited by metformin or rotenone pretreatment. MitoQ, a mitochondrially targeted antioxidant, decreased LPS-induced IL-1β without affecting TNF-α. These results, therefore, implicate complex I in LPS action in macrophages.
Introduction
Type 2 diabetes mellitus (T2DM)2 is a metabolic disease with a major pathological inflammatory component (1). T2DM is characterized by pancreatic β cell dysfunction and insulin resistance, resulting in impaired glucose uptake and utilization in muscle and increased glucose output by the liver. Inflammation participates in the pathogenesis of T2DM by contributing to both insulin resistance and pancreatic β cell dysfunction. In particular, the proinflammatory cytokine IL-1β induces apoptosis in pancreatic β cells (2). Furthermore, JNK, which can be activated in response to IL-1β, plays a role in insulin resistance in obesity (3). Blockade of IL-1β using a neutralizing antibody (4) or an IL-1β receptor antagonist (5) improves the clinical profile in T2DM. We have previously reported on the importance of the NLRP3 inflammasome in T2DM, where the amyloid protein islet amyloid polypeptide is sensed by NLRP3, leading to caspase-1 activation and production of mature IL-1β (6). Metformin, a frontline drug for the treatment of T2DM, decreases glucose production and improves insulin sensitivity (7). However, its beneficial effects may also be due to its anti-inflammatory action.
Metformin directly inhibits complex I (NADH:ubiquinone oxidoreductase) of the mitochondrial electron transport chain (8, 9). Complex I is the first of four complexes that comprise the electron transport chain, located in the inner mitochondrial membrane. In a series of redox reactions, electrons are transferred sequentially along the electron transport chain to the final electron acceptor, molecular oxygen. The energy released by this process is used to pump protons to generate an electrochemical gradient across the inner mitochondrial membrane. This generates a proton motive force that is used to synthesize ATP via ATP synthase. An interesting property of the electron transport chain is that it can also flow in reverse. Electrons entering at complex II (succinate dehydrogenase) can flow backwards to complex I (10, 11), generating reactive oxygen species (ROS) (12). Metformin has been proposed to inhibit this process (13). ROS have been implicated in LPS-induced IL-1β mRNA production in macrophages (14).
Another consequence of complex I inhibition by metformin is the activation of the intracellular energy sensor AMP-activated protein kinase (AMPK) (15). Decreased complex I activity leads to an overall reduction in ATP production by oxidative phosphorylation, therefore increasing the cellular AMP/ATP and ADP/ATP ratios. AMP or ADP binding to AMPK makes this kinase more susceptible to stimulatory phosphorylation by its upstream kinases (16, 17) and inhibits its dephosphorylation by protein phosphatase 2C (PP2C) α. The beneficial effects of metformin in T2DM were originally thought to be mediated by AMPK. Metformin decreases the transcription of gluconeogenic genes via AMPK (18, 19), and mice lacking liver kinase B1 (LKB1), an upstream kinase that activates AMPK, fail to lower their glucose levels in response to metformin (20). It is now appreciated, however, that many of the effects of metformin are AMPK-independent. For example, the effects of metformin on hepatic glucose output are preserved in AMPK-deficient mice (20). Furthermore, metformin blocks antigen-induced T cell proliferation independently of AMPK (21).
Because IL-1β has been implicated in the pathogenesis of T2DM, we considered whether metformin might block its induction. We found that metformin inhibits LPS-induced IL-1β production and boosts levels of the anti-inflammatory cytokine IL-10. These effects occur independently of AMPK activation. We implicated complex I in the induction of IL-1β, with a particular role for ROS in this event. Our work, therefore, provides insights into the anti-inflammatory action of metformin and identifies a role for complex I of the electron transport chain in the production of a proinflammatory cytokine (IL-1β) while limiting an anti-inflammatory cytokine (IL-10).
Experimental Procedures
Materials
Metformin, rotenone, glutamate, malate, diethyl succinate, digitonin, glucose, oligomycin, ATP, and ADP were purchased from Sigma. A769662 was from Abcam. Ultrapure rough LPS (from Escherichia coli, serotype EH100) was purchased from Alexis. R848 and CpG were from Invivogen. RNeasy Plus mini kits were from Qiagen. Mouse IL-10 and TNF-α ELISA Duoset kits and 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution were purchased from R&D Biosystems. The mouse β-actin antibody was from Sigma. Goat anti-mouse IL-1β was from R&D Biosystems. ACC, phospho-ACC, AMPKα, AMPKβ, and phospho-AMPKα (Thr-172) antibodies were from Cell Signaling Technology. Secondary horseradish peroxidase-conjugated anti-mouse IgG, anti-rabbit IgG, and anti-goat IgG were from Jackson ImmunoResearch Laboratories Inc. CellROX and Aqua live/dead FACS stains were from Molecular Probes. XF assay buffer, Seahorse cell culture plates, utility plates, and calibrant solution were from Seahorse Biosciences. MitoQ was a gift from Prof. Rob Smith (University of Otago, New Zealand).
Cell Culture
Wild type C57/Bl6 mice were from Harlan UK. Mice were euthanized in a CO2 chamber, and death was confirmed by cervical dislocation. Femora were removed from mice. AMPKα1 knockout legs were a gift from Benoit Viollet (INSERM, Université Paris Descartes, Paris, France) and Sandrine Horman (Université Catholique de Louvain, Brussels, Belgium). AMPKβ1 knockout legs were a gift from Morgan Fullerton and Greg Steinberg (McMaster University, Hamilton, ON, Canada). IL-10 knockout legs were a gift from Kingston Mills (Trinity College, Dublin, Ireland). Bone marrow cells were extracted from the mouse bones and differentiated in the presence of 20% macrophage colony-stimulating factor for 6 days, and then they were counted and replated for experiments.
FACS Analysis of Cellular ROS
Cells were stained with 5 μm CellROX dye for the final 30 min of the LPS stimulation. The medium was then removed, and cells were stained with Aqua live/dead stain for a further 30 min. The supernatant was removed, and cells were scraped in 1 ml PBS, washed, and transferred to FACS tubes. Cells were then analyzed using a Dako CyAn flow cytometer, and data were analyzed using FlowJo software.
Seahorse Analysis of Metabolic Parameters
BMDMs were cultured in DMEM containing 10% FCS, penicillin/streptomycin, and 10% macrophage colony-stimulating factor during metformin pretreatment and LPS stimulation. The medium was then removed, and cells were washed with 500 μl of mitochondrial assay buffer. 500 μl of mitochondrial assay buffer supplemented with 4 mm ADP and 10 μg/ml digitonin was added to each well. 70 μl of electron transport chain complex substrate was added to the appropriate injector port. Glutamate and malate were used as complex I substrates, whereas succinate was used as a complex II substrate.
ELISA
For cytokine measurements, BMDMs were stimulated in triplicate as indicated. Supernatants were analyzed for IL-10 and TNF-α release using ELISA kits according to the instructions of the manufacturer.
Western Blotting
BMDMs were seeded in 12-well plates at 0.5 × 106 cells/ml. Protein samples were prepared by direct lysis of cells in 5× Laemmli sample buffer, followed by boiling. Protein samples were resolved on 8% or 12% SDS-PAGE gels and then transferred onto PVDF membrane using either a wet or semidry transfer system. Membranes were blocked in 5% (w/v) dried milk in tris-buffered saline-Tween for at least 1 h at room temperature. Membranes were incubated with primary antibody followed by the appropriate horseradish peroxidase-conjugated secondary antibody. They were developed by ECL using a GelDoc system.
RNA Isolation and Real-time PCR
Total RNA was extracted using an RNeasy kit (Qiagen). cDNA was prepared using 20–100 ng/ml total RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems) according to the instructions of the manufacturer. mRNA expression was measured on the 7900HT RT-PCR system (Applied Biosystems) using TaqMan or SYBR Green primers for mouse IL-1β, IL-10, TNF-α, 18S, or Rps18. -Fold changes in expression were calculated by the ΔΔCt method using mouse 18S or Rps18 as an endogenous control for mRNA expression. All -fold changes are expressed normalized to the untreated control.
Results
Metformin Specifically Inhibits LPS-induced IL-1β Production and Boosts IL-10 Induction
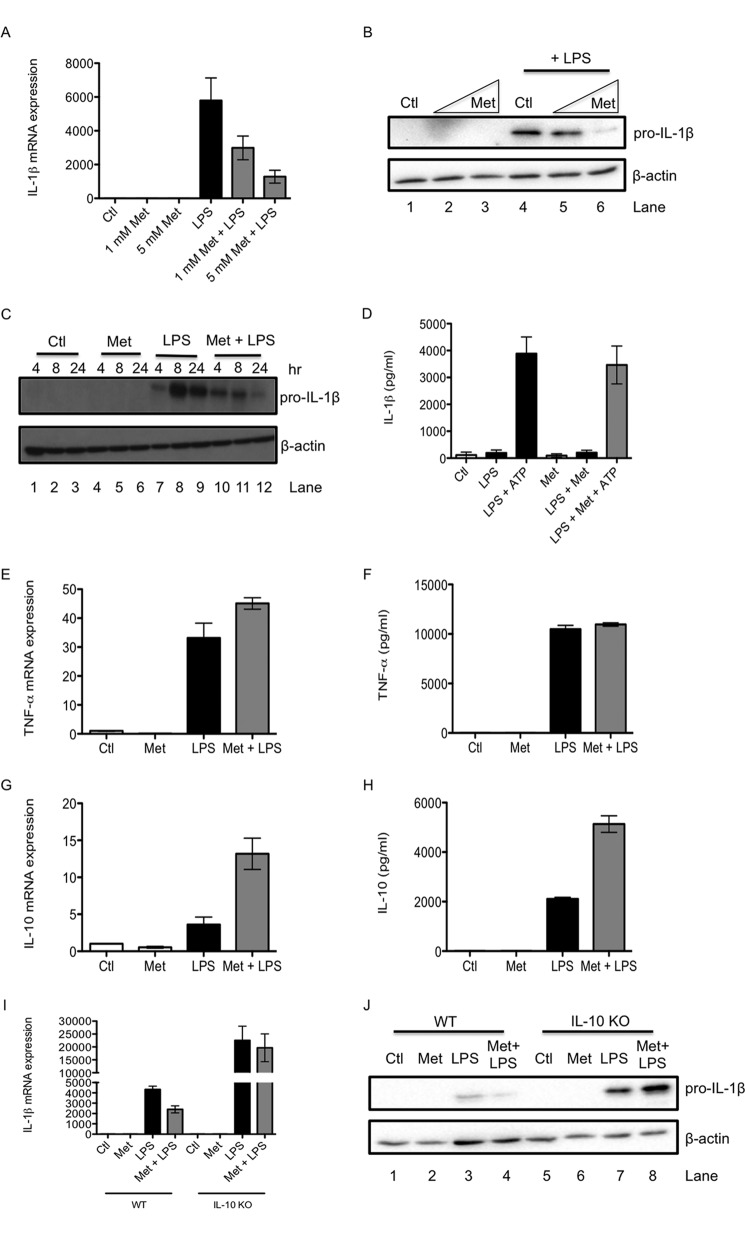
The effect of metformin on LPS-induced pro-IL-1β expression was first examined in primary murine bone marrow-derived macrophages (BMDMs). LPS increased pro-IL-1β mRNA expression at 24 h, an effect that was dose-dependently inhibited by metformin pretreatment (Fig. 1A). Furthermore, LPS increased pro-IL-1β protein levels (Fig. 1B, lane 4), and metformin reduced this induction (Fig. 1B, lanes 5 and 6). The effect of metformin on LPS-induced pro-IL-1β was also tested over a time course. LPS induced pro-IL-1β protein by 4 h, with pro-IL-1β levels being increased further at 8 and 24 h (Fig. 1C, lanes 7–9). Metformin was especially effective at decreasing pro-IL-1β at the later time points (Fig. 1C, lanes 11 and 12). Metformin had no effect on secretion of the mature form of IL-1β (Fig. 1D).
FIGURE 1.
Metformin decreases pro-IL-1β and increases IL-10 induction in response to LPS. WT BMDMs (A–C and E–H) were pretreated with metformin (Met, 1–5 mm) for 1 h before stimulation with LPS (100 ng/ml) for 24 h (A, B, E, F, and H), 4 h (G), or over the indicated time course (C). To activate the NLRP3 inflammasome, BMDMs were treated with LPS for 3 h, then with Met (5 mm) for 1 h, and, finally, with ATP (5 mm) for 1 h (D). WT and IL-10 KO BMDMs (I and J) were also pretreated with Met (5 mm) for 1 h before stimulation with LPS (100 ng/ml) for 24 h. mRNA was extracted from total cell lysates and analyzed by qPCR for IL-1β (A and I), TNF-α (E), and IL-10 (G) expression. Whole cell lysates were analyzed by Western blotting for pro-IL-1β and β-actin (B, C, and J). Supernatants were analyzed by ELISA for IL-1β (D), TNF-α (F), and IL-10 (H) production. The data shown in A and D—I are expressed as mean ± S.E. of three independent experiments, each performed in triplicate. The blots in B, C, and J are representative of three independent experiments. Ctl, control.
In a screen of other cytokines, we examined both the proinflammatory cytokine TNF-α and the anti-inflammatory cytokine IL-10. In contrast to pro-IL-1β, the induction of TNF-α by LPS was unaffected by metformin pretreatment. LPS potently induced TNF-α mRNA expression at 4 h, and metformin had no effect on this (Fig. 1E). Metformin also had no effect on LPS-induced TNF-α protein at 24 h (Fig. 1F).
For IL-10, LPS treatment resulted in a 2-fold increase in mRNA expression after 24 h. Intriguingly, this effect was boosted 3-fold by metformin pretreatment (Fig. 1G). LPS stimulation also induced IL-10 protein production at 24 h, and metformin further increased this LPS-induced IL-10 (Fig. 1H).
IL-10 May Mediate Some of the Effect of Metformin on Pro-IL-1β
To investigate whether there is a physiological role for the metformin-induced boost in IL-10, we tested the effect of metformin in IL-10 KO BMDMs. LPS induced pro-IL-1β to a greater extent in IL-10 KO macrophages than in WT macrophages at both mRNA (Fig. 1I) and protein levels (Fig. 1J, compare lane 7 with lane 3). Furthermore, metformin was no longer able to decrease LPS-induced pro-IL-1β mRNA expression (Fig. 1I) or protein production (Fig. 1J, compare lane 8 with lane 4) in IL-10 KO BMDMs.
Metformin Inhibits IL-1β and Boosts IL-10 Induced by TLR7/8 and TLR9 Ligands
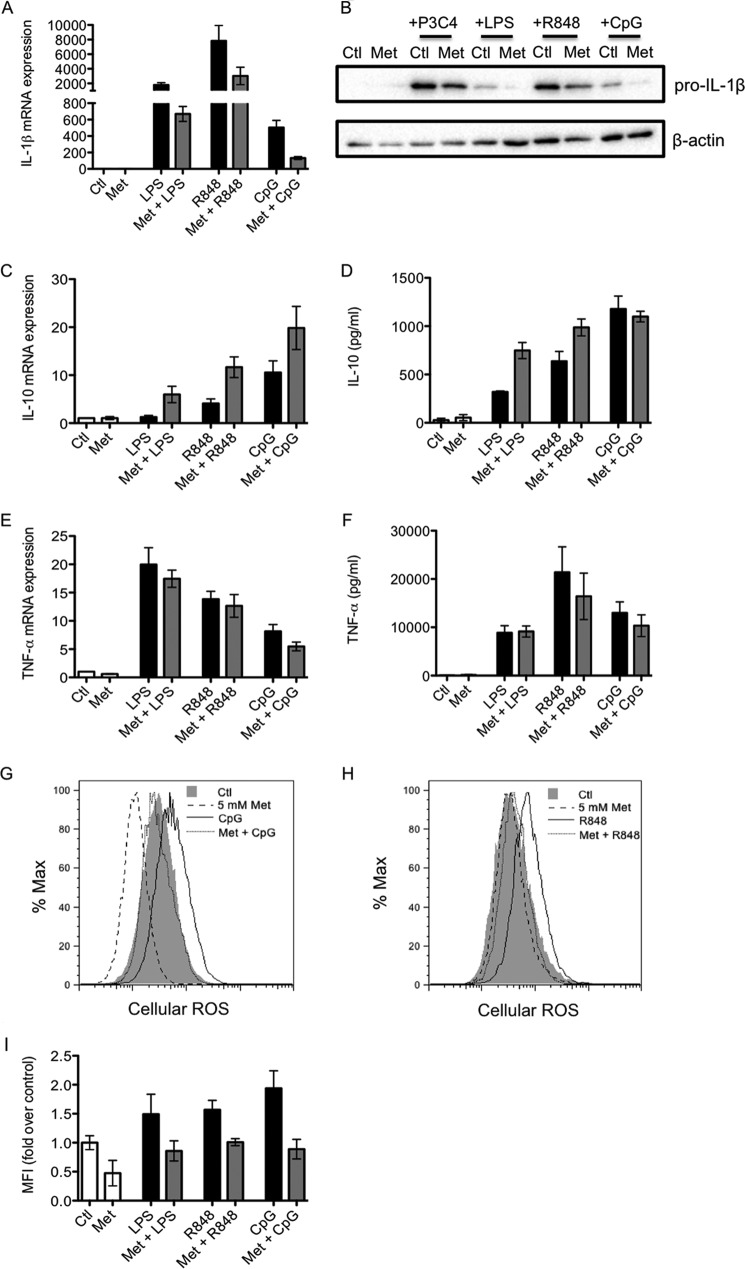
The effects of metformin on the cytokine response to other TLR ligands was also investigated. Metformin decreased pro-IL-1β mRNA (Fig. 2A) and protein (Fig. 2B, eighth and tenth lanes) in response to the TLR7/8 ligand R848 and the TLR9 ligand CpG. Metformin slightly decreased pro-IL-1β induced by the TLR1/2 ligand Pam3Csk4. Metformin also boosted R848- and CpG-induced IL-10 mRNA expression (Fig. 1C). Metformin did not boost CpG-induced IL-10 protein, but this may be due to the fact that metformin only begins to affect CpG-induced IL-10 mRNA at later time points. R848-induced IL-10 protein was boosted by metformin (Fig. 2D). Metformin had no effect on TNF-α induced by any of the TLR ligands tested (Fig. 2, E and F). Furthermore, metformin decreased the production of ROS induced by LPS, R848, and CpG (Fig. 2, G–I).
FIGURE 2.
Metformin alters ROS and cytokine production induced by TLR7/8 and TLR9 ligands. BMDMs were pretreated with metformin (Met, 5 mm) for 1 h before being stimulated with LPS (100 ng/ml), Pam3Csk4 (P3C4, 1 μg/ml), R848 (1 μg/ml), or CpG (1 μg/ml) for 24 h (A–D and F–I) or 4 h (E). mRNA was extracted from total cell lysates and analyzed by qPCR for IL-1β (A), IL-10 (C), or TNF-α (E) expression. Whole cell lysates were analyzed by Western blotting for pro-IL-1β and β-actin (B). Supernatants were analyzed by ELISA for IL-10 (D) and TNF-α (F) production. Mean fluorescence intensity (MFI) was measured in live cells by FACS to determine the production of reactive oxygen species (G–I). The data shown in A, C–F, and I represent the mean ± S.E. of three independent experiments, each performed in triplicate. The data shown in B, G, and H are representative of three independent experiments. Ctl, control.
Pharmacological Manipulation of AMPK Has No Effect on LPS-induced Pro-IL-1β or IL-10
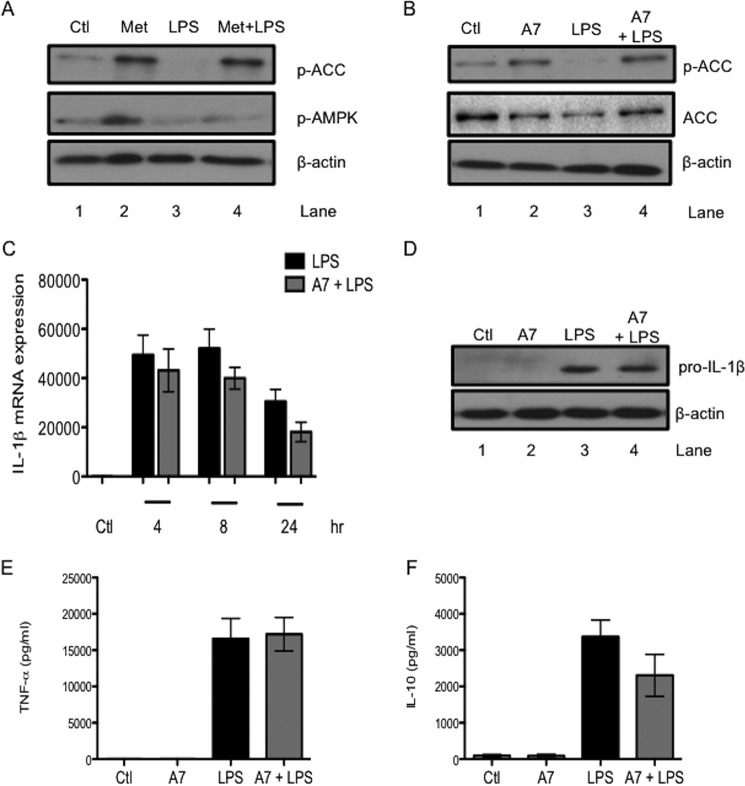
The role of AMPK, an established mediator of many of the effects of metformin, was investigated next. First, metformin was confirmed to activate AMPK in BMDMs. Metformin alone increased the phosphorylation of ACC (Fig. 3A, lane 2). The phosphorylation status of ACC correlates tightly with AMPK activity (22) and provides a technically clearer readout of AMPK activation than the more variable phospho-AMPK, although an increase in phospho-AMPK in response to metformin was observed (Fig. 3A, second panel, lane 2). LPS alone decreased basal ACC phosphorylation (Fig. 3A, lane 3). Importantly, metformin opposed this LPS-induced dephosphorylation of ACC, indicating that metformin could activate AMPK even in the presence of LPS (Fig. 3A, lane 4). The specific activator of AMPK, A769662, was employed to further probe the involvement of AMPK in the effects of metformin on LPS-stimulated BMDMs. A769662 (100 μm) alone increased the phosphorylation of ACC (Fig. 3B, lane 2). A769662 also opposed the LPS-induced dephosphorylation of ACC (Fig. 3B, lane 4). This effect was most pronounced with 1 h of A769662 pretreatment before 1 h of LPS stimulation.
FIGURE 3.
Pharmacological manipulation of AMPK reveals that AMPK has no role in the effects of metformin on LPS-induced cytokines. BMDMs were pretreated with Metformin (Met, 5 mm) or A769662 (A7, 100 μm) for 1 h before stimulation with LPS (100 ng/ml) for 1 h (A and B), for the indicated times (C), or for 24 h (D–F). Whole cell lysates were analyzed by Western blotting for p-ACC, total ACC, p-AMPK (A and B), pro-IL-1β (D), and β-actin (A, B, and D). mRNA was extracted from total cell lysates and analyzed by qPCR for IL-1β expression (C). Supernatants were analyzed by ELISA for TNF-α (E) and IL-10 (F) production. The blots in A, B, and D are representative of three independent experiments. The data in C, E, and F are expressed as mean ± S.E. of three independent experiments, each performed in triplicate. Ctl, control.
The effect of A769662 pretreatment on LPS-induced cytokines was investigated next. A769662 did not significantly decrease LPS-induced IL-1β mRNA expression after 24 h stimulation (Fig. 3C). LPS-induced pro-IL-1β protein was unaffected by A769662 (Fig. 3D). Furthermore, A769662 had no effect on LPS-induced TNF-α (Fig. 3E) or IL-10 (Fig. 3F) protein production. These results indicated that pharmacological activation of AMPK by A769662 did not mimic the effect of metformin here.
Metformin Is Still Capable of Decreasing LPS-induced Pro-IL-1β and Boosting IL-10 in AMPKα1 and AMPKβ1 Knockout Macrophages
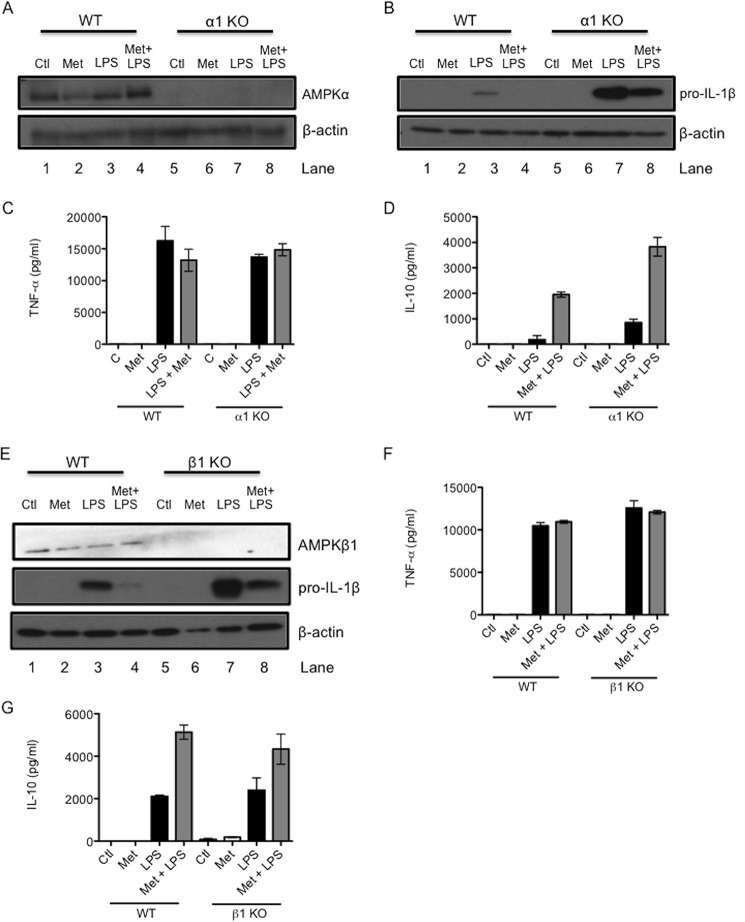
Because pharmacological agents can have off-target effects, genetic mutants of AMPK were next used to examine the role of AMPK in the effects of metformin on LPS-induced cytokines. BMDMs were generated from mice lacking the α1 subunit of AMPK (AMPKα1). These cells were confirmed to be deficient in AMPKα protein expression (Fig. 4A, right). When stimulated with LPS alone, AMPKα1 KO BMDMs produced dramatically more pro-IL-1β protein than WT BMDMs (Fig. 4B, compare lane 7 with lane 3), confirming previous studies indicating an anti-inflammatory role for AMPK (23). However, metformin still decreased LPS-induced pro-IL-1β in KO cells (Fig. 4B, lane 8). Metformin had no effect on TNF-α in either WT or KO cells (Fig. 4C) but boosted LPS-induced IL-10 protein in both cases (Fig. 4D).
FIGURE 4.
Genetic mutants of AMPK indicate that the effects of metformin on LPS-induced cytokines are independent of AMPK. AMPKα1-deficient (A–D) and AMPKβ1-deficient (E–G) BMDMs were pretreated with metformin (Met, 5 mm) for 1 h before stimulation with LPS (100 ng/ml) for 24 h. Whole cell lysates were analyzed by Western blotting for AMPKα1, pro-IL-1β, AMPKβ1, and β-actin (A, B, and E). The blots in A, B, and E are representative of three independent experiments. Supernatants were analyzed by ELISA for TNF-α (C and F) and IL-10 (D and G) production. The data in C, D, F, and G are expressed as mean ± S.E. of three independent experiments, each performed in triplicate. Ctl, control.
To bolster these results, BMDMs lacking the β1 subunit of AMPK (AMPKβ1) were investigated next. These cells were deficient in AMPKβ1 (Fig. 4E, lanes 5–8). Similar to AMPKα1 KO macrophages, LPS alone increased pro-IL-1β to a much greater extent in AMPKβ1 knockout BMDMs than in wild-type BMDMs (Fig. 4E, compare lane 7 with lane 3). Metformin inhibited LPS-induced pro-IL-1β protein in both WT and AMPKβ1 KO BMDMs (Fig. 4E, lanes 4 and 8, respectively). LPS-induced TNF-α remained unaffected by metformin treatment in either WT or KO macrophages (Fig. 4F). In addition, metformin boosted LPS-induced IL-10 in WT and AMPKβ1 KO macrophages (Fig. 4G).
These data suggested that AMPK was not required for the inhibitory effect of metformin on LPS-induced IL-1β production. Furthermore, they indicated that there was no role for AMPK in the metformin-induced increase in LPS-stimulated IL-10.
Metformin Inhibits Respiration Driven by Complex I of the Mitochondrial Electron Transport Chain
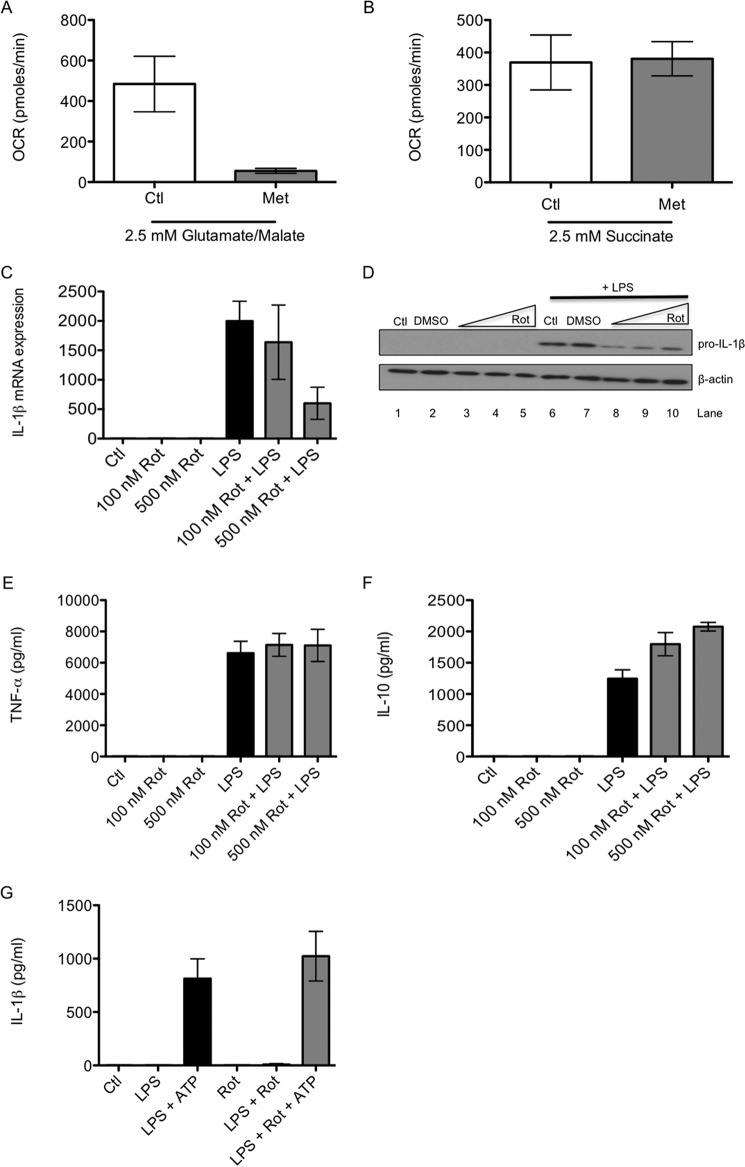
The role of complex I in the effects of metformin on LPS-induced cytokines was investigated next. First, metformin was confirmed to inhibit complex I in BMDMs. BMDMs were restricted to respiring either only on the complex I substrates glutamate and malate or the complex II substrate succinate. Metformin inhibited oxygen consumption in cells respiring using complex I (Fig. 5A) but not in those using complex II (Fig. 5B). This confirmed that metformin specifically inhibited complex I-mediated respiration in BMDMs.
FIGURE 5.
Inhibition of complex I mimics the effects of metformin on LPS-induced cytokines. BMDMs were treated with metformin (Met, 5 mm) for 24 h. The cell culture medium was then changed so that it contained only the complex I substrates glutamate and malate (A, 2.5 mm) or the complex II substrate succinate (B, 2.5 mm). Cells were permeabilized with digitonin (10 μg/ml) immediately prior to analysis. Oxygen consumption rate (OCR) was analyzed using a Seahorse XF-24 analyzer. BMDMs were also pretreated for 1 h with rotenone (Rot, 100–500 nm) before LPS stimulation (100 ng/ml) for 24 h (C–F). To activate the NLRP3 inflammasome, BMDMs were treated with LPS for 3 h, then with rotenone (500 nm) for 1 h, and then with ATP (5 mm) for another hour (G). mRNA was extracted from total cell lysates and analyzed by qPCR for IL-1β expression (C). Whole cell lysates were analyzed by Western blotting for pro-IL-1β and β-actin (D). Supernatants were analyzed by ELISA for TNF-α (E), IL-10 (F), and IL-1β (G) production. The blot in D is representative of three independent experiments. The data in A–C and E–G are expressed as mean ± S.E. of three independent experiments, each performed in triplicate. Ctl, control; DMSO, dimethyl sulfoxide.
Rotenone Mimics the Effect of Metformin on LPS-induced Cytokines
Rotenone, a specific inhibitor of complex I, was tested next. Rotenone pretreatment decreased LPS-induced IL-1β mRNA expression with nanomolar potency (Fig. 5C). Furthermore, rotenone pretreatment reduced the production of pro-IL-1β protein (Fig. 5D, lanes 8–10). Similarly to metformin, rotenone had no effect on TNF-α (Fig. 5E) but further increased LPS-induced IL-10 protein levels (Fig. 5F). Rotenone also had no effect on the secretion of mature IL-1β (Fig. 5G).
Metformin and Rotenone Decrease LPS-induced Reactive Oxygen Species
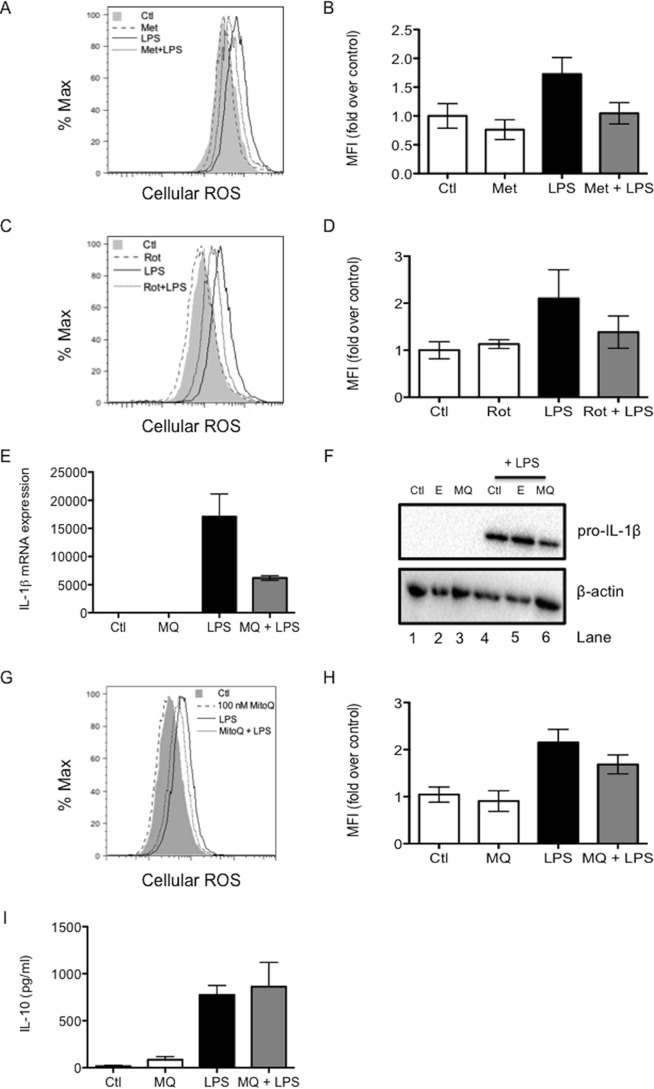
ROS are a potential signal coming from complex I to drive IL-1β, which might be modulated by metformin. The levels of ROS were measured by flow cytometry after 24 h of LPS stimulation with or without metformin pretreatment. LPS increased ROS after 24 h, an effect that was countered by metformin pretreatment (Fig. 6, A and B). In agreement with its effects on LPS-induced cytokines, rotenone also mimicked the effect of metformin on cellular ROS. Rotenone opposed the increase in ROS production after 24 h of LPS stimulation (Fig. 6, C and D).
FIGURE 6.
Metformin may decrease LPS-induced IL-1β by decreasing LPS-induced ROS production. BMDMs were pretreated with metformin (Met, 5 mm, A and B) or rotenone (Rot, 500 nm, C and D) for 1 h or with MitoQ (MQ; 50 nm for E, F, and I and 100 nm for G and H) for 3 h before stimulation with LPS (1 μg/ml for A–D, G, and H and 100 ng/ml for E, F, and I) for 24 h. Live cells were analyzed by FACS for the production of reactive oxygen species (A–D, G, and H). Mean fluorescence intensity (MFI) was quantified, and -fold induction was calculated relative to untreated cells. mRNA was extracted from total cell lysates and analyzed by qPCR for IL-1β expression (E). Whole cell lysates were analyzed by Western blotting for pro-IL-1β and β-actin (F). Supernatants were analyzed by ELISA for IL-10 (I) production. The experiments shown in A, C, F, and G are representative of three independent experiments. The data shown in B, D, E, H, and I represent the mean ± S.E. of three independent experiments, each performed in triplicate.
MitoQ Decreases LPS-induced Pro-IL-1β
On the basis of the ability of metformin and rotenone to decrease LPS-induced ROS, possibly originating from complex I, the effect of MitoQ, a specific mitochondrially targeted antioxidant, on cytokine induction by LPS was examined. MitoQ pretreatment of BMDMs decreased LPS-induced IL-1β mRNA expression (Fig. 6E) and protein production (Fig. 6F, lane 6) after 24 h. MitoQ also inhibited LPS-induced ROS (Fig. 6, G and H) but had no effect on IL-10 production (Fig. 6I). Taken together, these results implicate ROS generation by complex I in the induction of pro-IL-1β, with metformin inhibiting this process.
Discussion
In this study, we examined the effects of metformin on cytokine production in LPS-stimulated BMDMs. Metformin decreased LPS-induced production of the proinflammatory cytokine pro-IL-1β at both the mRNA and protein levels, in agreement with previous findings in human monocyte-derived macrophages (24). Metformin had no effect on the processed form of IL-1β, indicating that metformin specifically affects the proform of this cytokine. Moreover, metformin boosted LPS induction of the anti-inflammatory cytokine IL-10, an effect observed previously in spleen cells in experimental autoimmune encephalomyelitis (25). The decreased production of pro-IL-1β in combination with increased IL-10 indicates that metformin acted in an anti-inflammatory manner in LPS-activated macrophages. This effect was specific because metformin had no effect on another LPS-induced proinflammatory cytokine, TNF-α. This indicates that metformin is not cytotoxic, nor does it have a nonspecific effect on general cytokine production. This observation is in disagreement with other studies examining the effects of metformin on TNF-α in an inflammatory context. Metformin inhibited TNF-α production in a mouse model of endotoxemia induced by LPS injection (26) as well as in experimental autoimmune encephalomyelitis (25). This discrepancy may be due to differences in the effects of metformin in vivo versus in vitro, the in vivo effects of metformin on TNF-α being indirect. The boost in IL-10 may be physiologically significant because the absence of IL-10 did alter LPS-induced cytokine production as well as the anti-inflammatory action of metformin. LPS induced pro-IL-1β to a greater extent in IL-10 KO macrophages than in wild-type BMDMs, and metformin was unable to block LPS-induced pro-IL-1β in these cells. IL-10 may be involved in the regulation of IL-1β production by metformin in response to LPS, with the induction of IL-10 feeding back to inhibit pro-IL-1β induction. This may be why metformin is especially effective at inhibiting the induction of pro-IL-1β at later time points. Metformin decreased pro-IL-1β and boosted IL-10 in response to the TLR7/8 ligand R848 and the TLR9 ligand CpG. Therefore, the anti-inflammatory effect of metformin persists for a variety of TLR stimuli.
The cellular energy sensor AMPK is known to be an indirect target of metformin (27, 28) and mediates some of the effects of the drug (29). Furthermore, AMPK itself has anti-inflammatory activity and is a marker of M2 macrophages (23), which are generally anti-inflammatory. Therefore, the role of AMPK in the effect of metformin on LPS-induced cytokines was investigated using both pharmacological and genetic manipulation of this enzyme. Activation of AMPK by metformin in macrophages was confirmed. LPS decreased AMPK activity, indicating macrophage skewing toward a proinflammatory state. Sag et al. (23) have previously demonstrated the LPS-induced inhibition of AMPK in the context of such macrophage polarization. Metformin rescued this LPS-induced decrease in AMPK activity. Metformin is capable of restoring AMPK activity in mice with experimental autoimmune encephalomyelitis (25) as well as in a mouse model of diet-induced obesity (30).
To elucidate the involvement of AMPK in LPS activation of macrophages, the specific AMPK activator A769662 was exploited. This pharmacological agent binds to the β subunit of AMPK and promotes allosteric activation of the kinase while inhibiting its dephosphorylation (31). A769662 activated AMPK in BMDMs and was capable of rescuing the LPS-induced decrease in AMPK activity in this system. Despite this, A769662 had no effect on LPS-induced pro-IL-1β. It slightly decreased IL-1β mRNA after 24 h of LPS stimulation but had no effect on pro-IL-1β protein. In addition, A769662 did not alter LPS-induced IL-10 levels. These discrepancies between the effects of metformin and A769662 on LPS-activated macrophages suggested that AMPK activation was not responsible for the effect of metformin on LPS-induced IL-1β and IL-10 in BMDMs. The impact of A769662 on these cytokines has not yet been investigated elsewhere, but it does inhibit the palmitate-induced activation of JNK, a kinase involved in inflammatory cytokine production in macrophages (32). This effect of A769662 is dependent on AMPK.
There are disadvantages associated with the use of pharmacological agents to probe biological systems because these agents may have off-target, nonspecific, or unknown effects and may be cytotoxic (although A769662 seems to be highly specific). Confusingly, both AMPK activation and inhibition using synthetic agents have been shown to decrease LPS-induced liver injury (33). To clarify the results obtained by pharmacologically manipulating AMPK, genetic AMPK mutants were employed (34). A total AMPK knockout mouse is embryonic lethal, so two different AMPK knockout cell types were used, with each type deficient in a particular subunit of AMPK. First, BMDMs lacking AMPKα1 were used. AMPKα is the catalytic subunit of AMPK, responsible for its kinase activity. Pro-IL-1β expression after LPS stimulation alone was dramatically higher in KO cells than in the WT cells, indicating that AMPK has an anti-inflammatory role, countering LPS-induced cytokine production. Despite this dramatic enhancement of IL-1β production, metformin was still capable of decreasing LPS-induced pro-IL-1β in the AMPKα1 KO BMDMs. This implies that at least some of the effect of metformin on LPS-induced pro-IL-1β is independent of AMPK activation. Furthermore, metformin still boosted LPS-induced IL-10 in AMPK−/− cells. This argues that there may be some role for AMPK in the macrophage response to LPS alone but not in the effects of metformin on LPS-induced cytokine production in BMDMs. AMPK may affect inflammatory MAPK or JAK-STAT signaling pathways or may drive M2 macrophage metabolism and an associated anti-inflammatory state. TNF-α levels were similar in WT and KO cells and were unaffected by metformin in either cell type, suggesting that AMPK is not involved in the regulation of TNF-α by LPS or metformin. This disagrees with a study by Carroll et al. (35) that showed that in response to LPS, macrophages derived from AMPKα1 KO mice exhibit increased production of TNF-α. Metformin has also been shown to decrease LPS-induced TNF-α production in primary murine macrophages, but the time course of metformin treatment is very different to that used in our study (36).
Results obtained using BMDMs deficient in AMPKβ1 agreed with the observations seen with AMPKα1 KO cells. AMPKβ1 is a regulatory subunit responsible for binding glycogen and linking the α and γ subunits of AMPK. The anti-inflammatory agent salicylate promotes AMPK activity by binding to the β subunit (37). Similarly to AMPKα1 KO BMDMs, LPS alone markedly increased pro-IL-1β in AMPKβ1 KO macrophages compared with WT controls. Moreover, metformin still decreased pro-IL-1β in AMPKβ1 KO macrophages. This further argues against the involvement of AMPK in the regulation of pro-IL-1β by metformin. Metformin also boosted LPS-induced IL-10 protein in WT and AMPKβ1-deficient BMDMs, indicating that the effect of metformin on IL-10 is AMPK-independent. Metformin had no effect on LPS-induced TNF-α in AMPKβ1−/− BMDMs. AMPKβ1 KO macrophages exhibit increased production of IL-1β and TNF-α in response to palmitate as a proinflammatory stimulus (32). A role for AMPKβ1 in TNF-α production may be specific for palmitate. In wild-type cells, palmitate is broken down by β-oxidation, removing it as a proinflammatory stimulus. The lack of AMPK leads to decreased β-oxidation of palmitate, meaning it remains in its immunostimulatory form longer.
After ruling out a role for AMPK in the effect of metformin on LPS-induced IL-1β, other possible targets of metformin responsible for this effect were investigated. A direct target of metformin is NADH:ubiquinone oxidoreductase, otherwise known as complex I of the mitochondrial transport chain (8, 9). Metformin inhibits this complex in hepatocytes (15). Metformin specifically decreased the activity of complex I in BMDMs while leaving complex II unaffected, confirming the role of metformin as a complex I inhibitor. An early study proposing complex I as a direct target of metformin found that, in hepatoma cells, metformin inhibited mitochondrial oxidation of the complex I substrates glutamate and malate but had no effect on the oxidation of the complex II substrate succinate (8).
Rotenone, a specific and established inhibitor of complex I, was used to investigate the possibility that complex I inhibition was responsible for the effect of metformin on LPS-induced IL-1β. Rotenone mimicked the effects of metformin on LPS-induced cytokines, inhibiting IL-1β and boosting IL-10, implicating complex I in their regulation by metformin. Like metformin, rotenone had no effect on mature IL-1β, therefore suggesting that the effect of complex I inhibition is on the induction of the pro-form of IL-1β.
A possible signal coming from complex I to drive IL-1β production could be ROS (12). There is evidence that ROS are involved in LPS-induced IL-1β mRNA production in macrophages (14). LPS increased ROS levels at 24 h, an effect inhibited by both metformin and rotenone. Metformin also inhibited ROS induced by R848 and CpG. Both metformin and rotenone are capable of reducing ROS produced at complex I of the mitochondria in rat liver (13). Interestingly, this ROS production is due to the reverse electron flux along the respiratory chain from complex II back to complex I, making this process an intriguing prospect for further study. The rate of ROS production by complex I during reverse electron transport is significantly greater than that during forward electron transport (38). It is possible that metformin and rotenone may be decreasing ROS production because of reverse electron transport.
MitoQ, an antioxidant specifically targeted to the mitochondria (39), was used to further investigate the effect of mitochondrial ROS on IL-1β production. MitoQ decreased LPS-induced IL-1β mRNA expression after 24 h of LPS stimulation and inhibited pro-IL-1β protein production at the same time point. MitoQ can decrease IL-1β and TNF-α mRNA expression in mice with experimental autoimmune encephalomyelitis (40) as well as levels of IL-1β mRNA and mature IL-1β protein in a mouse model of dextran sulfate sodium-induced colitis (41). MitoQ decreased ROS at this time point. MitoQ did not, however, boost IL-10 production, suggesting that ROS are not a target for metformin or rotenone in their effects on IL-10. It is possible that the inhibition of reverse electron transport by metformin is somehow involved in IL-10 inhibition. This will be the subject of further study.
These data, therefore, indicate a role for ROS derived from complex I in the production of IL-1β. How exactly ROS induce IL-1β production is under investigation. What is clear is that complex I dysfunction can act as a signal for inflammation, promoting IL-1β production while limiting IL-10. Targeting of complex I may, therefore, prove to be useful therapeutically in the control of inflammatory diseases.
Author Contributions
B. K. designed, performed, and analyzed the experiments and wrote the paper. G. M. T. designed experiments. M. P. M. conceived ideas and provided advice and reagents. L. A. J. O. designed experiments, conceived ideas, and oversaw the research program. All authors reviewed the final version of the manuscript.
Acknowledgments
We thank Dr. Viollet and Dr. Horman for AMPKα1 knockout mice, Dr. Fullerton and Prof. Steinberg for AMPKβ1 knockout mice, and Prof. Mills for IL-10-deficient mice. We also thank Prof. Smith for MitoQ.
This work was supported by the European Research Council and Science Foundation Ireland. The authors declare that they have no conflicts of interest with the contents of this article.
- T2DM
- type II diabetes mellitus
- ROS
- reactive oxygen species
- AMPK
- AMP-activated protein kinase
- ACC
- acetyl-CoA carboxylase
- BMDM
- bone marrow-derived macrophage
- TLR
- Toll-like receptor.
References
- 1. Donath M. Y., Shoelson S. E. (2011) Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 [DOI] [PubMed] [Google Scholar]
- 2. Bendtzen K., Mandrup-Poulsen T., Nerup J., Nielsen J. H., Dinarello C. A., Svenson M. (1986) Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science 232, 1545–1547 [DOI] [PubMed] [Google Scholar]
- 3. Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 4. Moran A., Bundy B., Becker D. J., DiMeglio L. A., Gitelman S. E., Goland R., Greenbaum C. J., Herold K. C., Marks J. B., Raskin P., Sanda S., Schatz D., Wherrett D. K., Wilson D. M., Krischer J. P., Skyler J. S., Type 1 Diabetes TrialNet Canakinumab Study Group, Pickersgill L., de Koning E., Ziegler A. G., Böehm B., Badenhoop K., Schloot N., Bak J. F., Pozzilli P., Mauricio D., Donath M. Y., Castaño L., Wägner A., Lervang H. H., Perrild H., Mandrup-Poulsen T., and AIDA Study Group (2013) Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 381, 1905–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larsen C. M., Faulenbach M., Vaag A., Vølund A., Ehses J. A., Seifert B., Mandrup-Poulsen T., Donath M. Y. (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 [DOI] [PubMed] [Google Scholar]
- 6. Masters S. L., Dunne A., Subramanian S. L., Hull R. L., Tannahill G. M., Sharp F. A., Becker C., Franchi L., Yoshihara E., Chen Z., Mullooly N., Mielke L. A., Harris J., Coll R. C., Mills K. H., Mok K. H., Newsholme P., Nuñez G., Yodoi J., Kahn S. E., Lavelle E. C., O'Neill L. A. (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hundal R. S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S. E., Schumann W. C., Petersen K. F., Landau B. R., Shulman G. I. (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Owen M. R., Doran E., Halestrap A. P. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 [PMC free article] [PubMed] [Google Scholar]
- 9. El-Mir M. Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 [DOI] [PubMed] [Google Scholar]
- 10. Chance B., Hollunger G. (1961) The interaction of energy and electron transfer reactions in mitochondria: IV: the pathway of electron transfer. J. Biol. Chem. 236, 1562–1568 [PubMed] [Google Scholar]
- 11. Chance B., Hollunger G. (1961) The interaction of energy and electron transfer reactions in mitochondria: I: general properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J. Biol. Chem. 236, 1534–1543 [PubMed] [Google Scholar]
- 12. Murphy M. P. (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Batandier C., Guigas B., Detaille D., El-Mir M. Y., Fontaine E., Rigoulet M., Leverve X. M. (2006) The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J. Bioenerg. Biomembr. 38, 33–42 [DOI] [PubMed] [Google Scholar]
- 14. Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. (2011) Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187, 613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stephenne X., Foretz M., Taleux N., van der Zon G. C., Sokal E., Hue L., Viollet B., Guigas B. (2011) Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 54, 3101–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Mäkelä T. P., Alessi D. R., Hardie D. G. (2003) Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 18. Kim Y. D., Park K. G., Lee Y. S., Park Y. Y., Kim D. K., Nedumaran B., Jang W. G., Cho W. J., Ha J., Lee I. K., Lee C. H., Choi H. S. (2008) Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57, 306–314 [DOI] [PubMed] [Google Scholar]
- 19. Lee J. M., Seo W. Y., Song K. H., Chanda D., Kim Y. D., Kim D. K., Lee M. W., Ryu D., Kim Y. H., Noh J. R., Lee C. H., Chiang J. Y., Koo S. H., Choi H. S. (2010) AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner. J. Biol. Chem. 285, 32182–32191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 120, 2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zarrouk M., Finlay D. K., Foretz M., Viollet B., Cantrell D. A. (2014) Adenosine-mono-phosphate-activated protein kinase-independent effects of metformin in T cells. PloS ONE 9, e106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park S. H., Gammon S. R., Knippers J. D., Paulsen S. R., Rubink D. S., Winder W. W. (2002) Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J. Appl. Physiol. 92, 2475–2482 [DOI] [PubMed] [Google Scholar]
- 23. Sag D., Carling D., Stout R. D., Suttles J. (2008) Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 181, 8633–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee H. M., Kim J. J., Kim H. J., Shong M., Ku B. J., Jo E. K. (2013) Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62, 194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nath N., Khan M., Paintlia M. K., Singh I., Hoda M. N., Giri S. (2009) Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J. Immunol. 182, 8005–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsoyi K., Jang H. J., Nizamutdinova I. T., Kim Y. M., Lee Y. S., Kim H. J., Seo H. G., Lee J. H., Chang K. C. (2011) Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br. J. Pharmacol. 162, 1498–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meng S., Cao J., He Q., Xiong L., Chang E., Radovick S., Wondisford F. E., He L. (2015) Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J. Biol. Chem. 290, 3793–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fullerton M. D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z. P., O'Neill H. M., Ford R. J., Palanivel R., O'Brien M., Hardie D. G., Macaulay S. L., Schertzer J. D., Dyck J. R., van Denderen B. J., Kemp B. E., Steinberg G. R. (2013) Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woo S. L., Xu H., Li H., Zhao Y., Hu X., Zhao J., Guo X., Guo T., Botchlett R., Qi T., Pei Y., Zheng J., Xu Y., An X., Chen L., Chen L., Li Q., Xiao X., Huo Y., Wu C. (2014) Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PloS ONE 9, e91111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Göransson O., McBride A., Hawley S. A., Ross F. A., Shpiro N., Foretz M., Viollet B., Hardie D. G., Sakamoto K. (2007) Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J. Biol. Chem. 282, 32549–32560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galic S., Fullerton M. D., Schertzer J. D., Sikkema S., Marcinko K., Walkley C. R., Izon D., Honeyman J., Chen Z. P., van Denderen B. J., Kemp B. E., Steinberg G. R. (2011) Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Invest. 121, 4903–4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo Y., Zhang Y., Hong K., Luo F., Gu Q., Lu N., Bai A. (2014) AMPK inhibition blocks ROS-NFκB signaling and attenuates endotoxemia-induced liver injury. PloS ONE 9, e86881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Viollet B., Athea Y., Mounier R., Guigas B., Zarrinpashneh E., Horman S., Lantier L., Hebrard S., Devin-Leclerc J., Beauloye C., Foretz M., Andreelli F., Ventura-Clapier R., Bertrand L. (2009) AMPK: lessons from transgenic and knockout animals. Front. Biosci. 14, 19–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carroll K. C., Viollet B., Suttles J. (2013) AMPKα1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. J. Leukocyte Biol. 94, 1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim J., Kwak H. J., Cha J. Y., Jeong Y. S., Rhee S. D., Kim K. R., Cheon H. G. (2014) Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J. Biol. Chem. 289, 23246–23255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hawley S. A., Fullerton M. D., Ross F. A., Schertzer J. D., Chevtzoff C., Walker K. J., Peggie M. W., Zibrova D., Green K. A., Mustard K. J., Kemp B. E., Sakamoto K., Steinberg G. R., Hardie D. G. (2012) The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336, 918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lambert A. J., Brand M. D. (2004) Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem. 279, 39414–39420 [DOI] [PubMed] [Google Scholar]
- 39. Murphy M. P., Smith R. A. (2007) Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 47, 629–656 [DOI] [PubMed] [Google Scholar]
- 40. Mao P., Manczak M., Shirendeb U. P., Reddy P. H. (2013) MitoQ, a mitochondria-targeted antioxidant, delays disease progression and alleviates pathogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Biochim. Biophys. Acta 1832, 2322–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dashdorj A., Jyothi K. R., Lim S., Jo A., Nguyen M. N., Ha J., Yoon K. S., Kim H. J., Park J. H., Murphy M. P., Kim S. S. (2013) Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 11, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]