Background: DHA is known as an endogenous ligand for GPR120.
Results: DHA increased the phosphorylation of AMPK and induced glucose uptake in skeletal muscle cells. GPR120 is involved in DHA-mediated glucose uptake.
Conclusion: DHA exerts a benign metabolic role through the AMPK pathway.
Significance: DHA, a ligand for GPR120, is potential drug candidate for diabetes.
Keywords: AMP-activated kinase (AMPK), diabetes, glucose transport, metabolism, signal transduction
Abstract
Docosahexaenoic acid (DHA) is an endogenous ligand of G protein-coupled receptor 120 (GPR120). However, the mechanisms underlying DHA action are poorly understood. In this study, DHA stimulated glucose uptake in the skeletal muscles in an AMP-activated protein kinase (AMPK)-dependent manner. GPR120-mediated increase in intracellular Ca2+ was critical for DHA-mediated AMPK phosphorylation and glucose uptake. In addition, DHA stimulated GLUT4 translocation AMPK-dependently. Inhibition of AMPK and Ca2+/calmodulin-dependent protein kinase kinase blocked DHA-induced glucose uptake. DHA and GW9508, a GPR120 agonist, increased GPR120 expression. DHA-mediated glucose uptake was not observed in GPR120 knockdown conditions. DHA increased AMPK phosphorylation, glucose uptake, and intracellular Ca2+ concentration in primary cultured myoblasts. Taken together, these results indicated that the beneficial metabolic role of DHA was attributed to its ability to regulate glucose via the GPR120-mediated AMPK pathway in the skeletal muscles.
Introduction
AMP-activated protein kinase (AMPK)2 is an evolutionarily conserved and ubiquitously expressed kinase that plays an important role in cellular energy homeostasis. It is a heterotrimeric complex containing a catalytic subunit (α) and 2 regulatory subunits (β and γ) and is activated by Thr172 phosphorylation in the α subunit by AMPK kinase upon energy depletion (1). When the AMP:ATP ratio increases, AMP binds to the γ subunit and induces a conformational change in the heterotrimer, thus exposing the α subunit to upstream kinases. Liver kinase B1 (LKB1) and Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKKβ) are the upstream kinases of AMPK (2–5). AMPK activation through physiological stimulation such as muscle contraction or through pharmacological activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside significantly increases glucose uptake mediated by glucose transporter type 4 (GLUT4) translocation (6, 7). GLUT4 is highly expressed in the adipose tissues and skeletal muscles (8, 9), and it mediates the removal of circulating glucose. Thus, it is a key regulator of systemic glucose homeostasis (10). Antidiabetic drugs such as metformin and rosiglitazone stimulate the AMPK pathway (11). Thus, AMPK activation is a unique and potential target for treating metabolic diseases such as obesity and diabetes.
G protein-coupled receptors (GPCRs) are important signaling molecules that perform various cellular functions. They share common structural motifs, such as seven transmembrane helices, and activate heterotrimeric G proteins such as Gαs, Gαi, and Gαq. GPCR activation stimulates many cellular responses via second messengers such as cAMP (12), phospholipase C (13), and mitogen-activated protein kinase (14). Regulation of GPCR signal selectivity and specificity is highly complex and involves the activation of various pathways that eventually lead to biological responses. Fatty acids (FAs) act as GPCR ligands. Short chain fatty acids activate GPR41 and GPR43 (15), medium chain FAs activate GPR 84 (16), and long chain FAs activate GPR40 (17) and GPR120 (18). Controversies exist regarding the relationship between lipid components and metabolic diseases such as diabetes and obesity. Some studies indicate that saturated FAs induce obesity and diabetes (19), whereas long chain polyunsaturated omega-3 FAs protect against these diseases.
Although functions of GPCRs in the islet system are relatively well known, their functions in nonislet tissues such as the skeletal muscles are unknown. The skeletal muscle is a major tissue through which GPCRs regulate glucose. In this study, we examined the role of DHA, a GPR120 ligand, in regulating glucose in the skeletal muscles.
Experimental Procedures
Reagents
Glucose, STO-609 (CaMKK inhibitor), GW9508 (GPR120 agonist), and 1,7-bis [4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione (DHA) were purchased from Sigma-Aldrich. Polyclonal antibodies against phospho-AMPKα, phospho-ACC, AMPKα, ACC, and β-actin were purchased from Millipore (Billerica, MA). Polyclonal anti-GPR120 antibody was purchased from Abcam (Cambridge, MA). Compound C was provided by Merck. Hybond ECL nitrocellulose membranes were obtained from GE Healthcare.
Cell Culture
Mouse C2C12 and rat L6 myoblasts were maintained in DMEM supplemented with 10% (v/v) FBS and 1% (v/v) antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin) at 37 °C in humidified atmosphere of 5% CO2. For differentiation into myotubes, rat L6 myoblasts (density, 2 × 104 cells/ml) were seeded into 12-well plates (for glucose uptake). After 24 h (>80% confluence), the medium was replaced by DMEM containing 2% (v/v) FBS for 7 days.
Western Blotting
Cells were grown in 6-well plates until they reached 60–70% confluence. Next, the cells were subjected to serum starvation for 24 h before treatment with selected agents at 37 °C. The cells were then lysed in 100 μl of lysis buffer (0.5% deoxycholate, 0.1% SDS, 1% Nonidet P-40, 150 mm NaCl, and 50 mm Tris-HCl, pH 8.0) containing proteinase inhibitors (0.5 μm aprotinin, 1 μm phenylmethylsulfonyl fluoride, and 1 μm leupeptin; Sigma-Aldrich). Proteins were separated on SDS-PAGE (8%) and were transferred onto a polyvinylidene difluoride membrane. The blots were incubated with primary antibodies at 4 °C. Next, the blots were probed with HRP-conjugated secondary antibodies. The blots were then visualized using ECL solution (GE Healthcare). The membrane was scanned, and densitometry analysis was performed with an ImageJ analysis.
RT-PCR
First strand cDNA was synthesized using 1 μg of total RNA from C2C12 cells at 55 °C for 20 min by using Thermoscript II One-Step RT-PCR kit (Life Technologies). The cDNA was amplified using GeneAmp PCR System 9700 (Applied Biosystems, Warrington, UK). PCR was performed using 34 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and amplification at 72 °C for 60 s. Next, PCR samples stained with ethidium bromide. Band intensities were quantified using Gene Genius gel documentation system (Syngene). PCRs were performed using the following primers: GLUT4 sense, 5′-TTGGAGAGAGAGCGTCCAAT-3′; GLUT4 antisense, 5′-CTCAAAGAAGGCCACAAAGC-3′; GPR120 sense, 5′-AGAGGCTTACGCTGAGCTTG-3′; GPR120 antisense, 5′-AAGAAAAGGGATGGCCAGAT-3′; β-actin sense, 5′-CAGGAGGAGCAATGATCTTGA-3′; and β-actin antisense, 5′-ACTACCTCATGAAGATCCTCA-3′.
Measurement of Intracellular Ca2+
Ca2+ concentration was measured by detecting fluorescence of Ca2+-sensitive indicator fluo-3 AM under a confocal microscope (Zeiss LSM 510 Meta). The cells were treated with fluo-3 AM for 45 min. Culture plates were observed under 20× objective. Signal was detected at an excitation of 488 nm. The images were digitalized and analyzed using Zen program software.
2-Deoxyglucose Uptake
Glucose uptake was analyzed by measuring the uptake of 2-deoxy-d[H3]-glucose (2-DG) in differentiated L6 myotubes. The cells were starved in serum-free DMEM for 3 h. After DHA treatment, the cells were incubated in KRB (20 mm HEPES, pH 7.4, 130 mm NaCl, 1.4 mm KCl, 1 mm CaCl2, 1.2 mm MgSO4, and 1.2 mm KH2PO4) containing 0.5 μCi of 2-DG at 37 °C for 15 min. The reaction was terminated by washing twice with ice-cold PBS. The cells were lysed in 0.5 n NaOH, and 400 μl of cell lysate was mixed with 3.5 ml of scintillation mixture. Radioactivity was measured by scintillation counting.
AMPKα2, GPR120, and CaMKKβ Silencing
Cells were transiently transfected with AMPKα2, GPR120, and CaMKKβ siRNAs by using Lipofectamine 2000 (Invitrogen, Life Technologies). For transfection, 5 μl of siRNAs and 5 μl of Lipofectamine 2000 were diluted using 95 μl of reduced serum medium (Opti-MEM; Invitrogen, Life Technologies) and were mixed. The mixture was incubated for 30 min and was added dropwise to each culture well. The medium was replaced with a fresh complete medium at 4 h.
Myc-GLUT4 Translocation Assay
Cell surface expression of myc-GLUT4 was quantified using an antibody-coupled colorimetric absorbance assay, as described previously. After DHA stimulation, myc-GLUT4 L6 myotubes stably expressing myc-GLUT4 were incubated with polyclonal anti-myc antibody (1:1000) for 60 min, fixed with 4% paraformaldehyde for 10 min, and incubated with HRP-conjugated goat anti-rabbit IgG (1:1000) for 1 h. The cells were washed six times with PBS and were incubated in 1 ml of o-phenylenediamine (0.4 mg/ml) for 30 min. Absorbance of the supernatant was measured at 492 nm.
Preparation of Primary Myoblasts
Primary myoblasts were isolated from the fore limbs and hind limbs of three or four 5-day-old littermates. The dissected muscle was disaggregated in 4 ml of PBS containing 1.5 units/ml dispase II and 1.4 units/ml collagenase D (Roche) and was triturated with a 10-ml pipette every 5 min for 20 min. The cells were filtered through a 70-μm mesh (BD Biosciences) and were centrifuged at 1000 rpm for 5 min. The cell pellet was dissociated in 10 ml of F10 medium (Invitrogen, Life Technologies) and 10% cosmic calf serum (GE Healthcare; referred to as growth medium 1). Finally, the cells were preplated twice on non-collagen-coated plates for 1 h to deplete the population of fibroblasts.
Data Analysis
We used one-way analysis of variance and Holm-Sidak comparisons and the post hoc Fisher test to compare the potency of glucose uptake. The difference between the mean values was considered to be statistically significant when p < 0.05.
Results
DHA Increases Glucose Uptake in an AMPK-dependent Manner
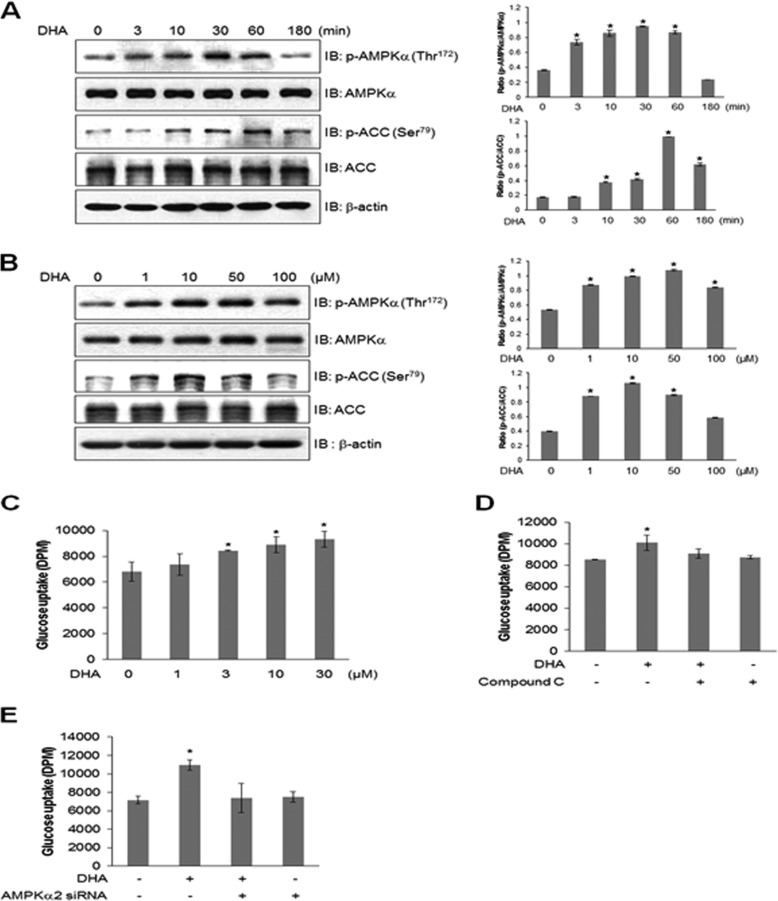
To determine the mechanism underlying the metabolic effects of DHA in C2C12 myoblasts, we assessed the activation of AMPK, a key regulator of glucose. DHA administration induced time- and dose-dependent increases in AMPK and its downstream ACC phosphorylation (Fig. 1, A and B). AMPK phosphorylation began at 3 min and reached a maximum level at 30 min and returned to the basal level at 3 h. Maximal AMPK phosphorylation was observed at 10 μm DHA concentration. We assessed the effects of DHA on glucose uptake in rat L6 myotubes. L6 myotubes showed higher glucose uptake than C2C12 cells, suggesting that L6 myotubes are the most promising model for investigating glucose uptake. Accordingly, the effect of DHA on glucose uptake in differentiated L6 cells was determined. DHA increased glucose uptake (Fig. 1C). Pretreatment with 2 μm AMPK inhibitor (compound C) blocked DHA-induced glucose uptake, suggesting that AMPK played a role in DHA-induced glucose uptake (Fig. 1D). Knockdown of AMPKα2 suppressed DHA-induced glucose uptake (Fig. 1E). These results indicated that AMPKα2 was involved in DHA-induced glucose uptake.
FIGURE 1.
DHA stimulates glucose uptake through AMPK. A, cells were stimulated with DHA for the indicated times and were analyzed by Western blotting with antibodies against phospho-AMPK and phospho-ACC. AMPK and ACC served as controls. The results are representative of four independent experiments. *, p < 0.05 versus basal conditions. B, cells were stimulated with various doses of DHA, and cell lysates were analyzed by Western blotting with antibodies against phospho-AMPK and phospho-ACC. AMPK and ACC served as controls. The results are representative of four independent experiments. *, p < 0.05 versus basal conditions. C, L6 myoblasts were differentiated and stimulated with various doses of DHA for 1 h, and 2-DG uptake was assayed. *, p < 0.05 compared with control. D, L6 myoblasts were differentiated and stimulated using 50 μm DHA for 1 h in the presence of compound C, and 2-DG uptake was assayed. *, p < 0.05 compared with DHA-treated cells. E, L6 myoblasts were transiently transfected with AMPKα2 siRNA for 2 days and stimulated using 50 μm DHA for 1 h, and 2-DG uptake was assayed. *, p < 0.05 compared with control. IB, immunoblot.
Ca2+ Mediates DHA-induced AMPK Phosphorylation and Glucose Uptake
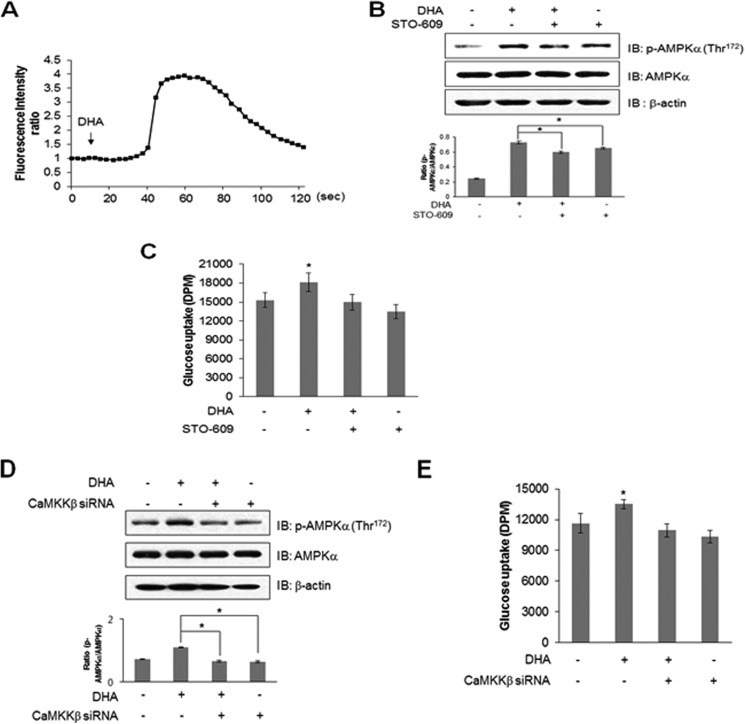
Intracellular Ca2+ levels were measured to characterize the upstream components of the AMPK pathway. Ca2+ was traced using fluo-3 AM. Fluorescence intensity indicates the degree of calcium concentration. DHA increased the intensity of green fluorescence (Fig. 2A), indicating an increase in intracellular Ca2+ concentration. This indicated that CaMKK was a candidate of upstream component of the AMPK pathway because it was activated by Ca2+/calmodulin binding. To confirm this, C2C12 cells were pretreated with STO-609, CaMKK inhibitor, before adding DHA. STO-609 blocked DHA-induced AMPK phosphorylation (Fig. 2B) and glucose uptake (Fig. 2C). Knockdown of CaMKKβ blocked DHA-induced AMPK phosphorylation (Fig. 2D). Knockdown of CaMKKβ blocked DHA-induced glucose uptake (Fig. 2E). These results confirmed that DHA increased glucose uptake via a Ca2+-mediated CaMKK/AMPK pathway.
FIGURE 2.
DHA increases AMPK phosphorylation by releasing intracellular Ca2+. A, C2C12 cells were pretreated with fluo-3 AM for 30 min and then with 100 μm DHA. Green fluorescence was detected using confocal microscopy and analyzed. B, C2C12 cells were pretreated with STO-609 for 30 min and then with 50 μm DHA for 3 h. The cell lysates were analyzed by Western blotting with antibody against phospho-AMPK. AMPK and β-actin served as controls. The results are representative of four independent experiments. *, p < 0.05 versus DHA-treated conditions. C, L6 myoblasts were differentiated for 7 days and were pretreated with STO-609 (2 μm) and DHA (50 μm) for 1 h. Uptake of 2-DG was assayed. *, p < 0.05 compared with control. D, C2C12 cells transiently transfected with CaMKKβ siRNA for 2 days and stimulated using 50 μm DHA for 1 h. The cell lysates were analyzed by Western blotting with antibody against phospho-AMPK. AMPK and β-actin served as controls. The results are representative of four independent experiments. *, p < 0.05 versus DHA-treated conditions. E, L6 myoblasts were transiently transfected with CaMKKβ siRNA for 2 days and stimulated using 50 μm DHA for 1 h, and 2-DG uptake was assayed. *, p < 0.05 compared with control. IB, immunoblot.
GPR120 Mediates DHA-induced AMPK Phosphorylation and Glucose Uptake
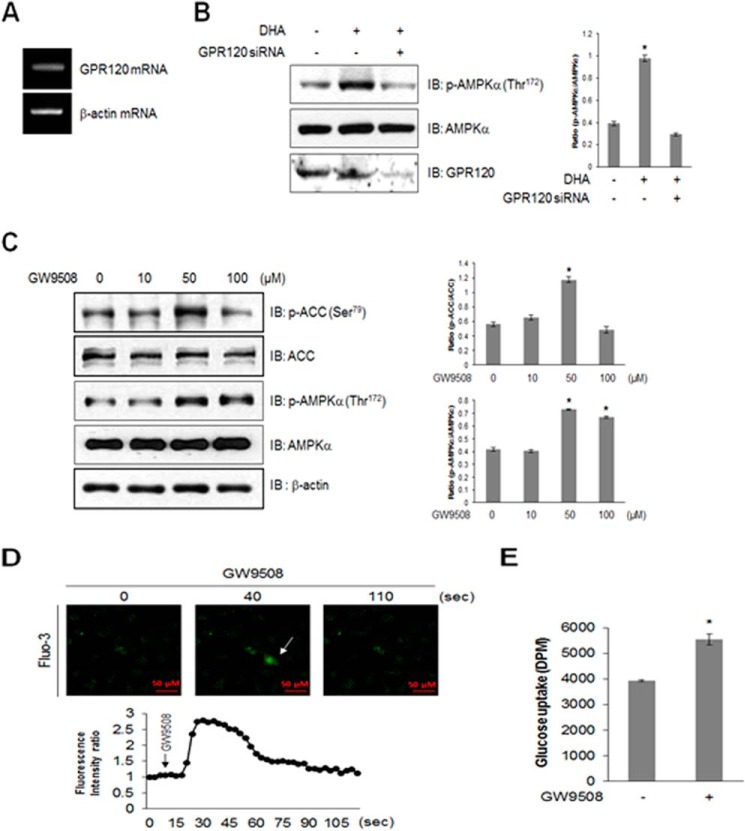
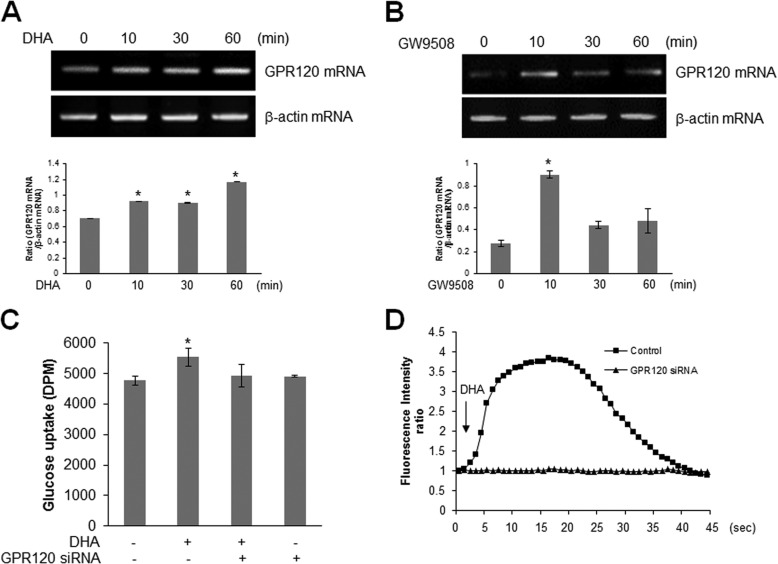
Because DHA is a GPR120 ligand, we hypothesized that DHA stimulated glucose uptake via the GPR120 pathway and examined the expression of GPR120. GPR120 was highly expressed in the skeletal muscle cells (Fig. 3A), and its knockdown blocked DHA-induced AMPK phosphorylation (Fig. 3B). GW9508, an endogenous ligand for GPR120, strongly activated AMPK phosphorylation (Fig. 3C) and increased Ca2+ concentration in the skeletal muscles (Fig. 3D). The cell indicated by an arrow in Fig. 3D was calcium increased cell by GW9508. Moreover, GW9508 induced glucose uptake (Fig. 3E). These results indicated that GPR120 was an important mediator of DHA-induced glucose uptake.
FIGURE 3.
GPR120 mediates DHA-induced AMPK phosphorylation. A, total mRNA was extracted from C2C12 cells, and RT-PCR was performed using GPR120-specific primers. PCR products were visualized under ultraviolet light. β-Actin was used as a positive control. B, C2C12 cells were transiently transfected with GPR120 siRNA for 48 h and stimulated with 50 μm DHA. Cell lysates were analyzed by Western blotting with antibodies against phospho-AMPKα, with AMPKα as a control. The results are representative of four independent experiments. *, p < 0.05 versus basal conditions. C, the cells were stimulated with GW9508 for indicated doses for 1 h, and cell lysates were analyzed using Western blotting with antibodies against phospho-AMPKα and phospho-ACC, with AMPKα, ACC, and β-actin as controls. The results are representative of four independent experiments. *, p < 0.05 versus basal conditions. D, C2C12 cells were pretreated with fluo-3 AM for 30 min and then with 100 μm GW9508. Green fluorescence was detected using confocal microscopy. E, L6 myoblasts were differentiated and stimulated with 50 μm GW9508 for 1 h. Uptake of 2-DG was assayed. *, p < 0.05 compared with control. IB, immunoblot.
DHA Stimulates GLUT4 Translocation in AMPK Dependently
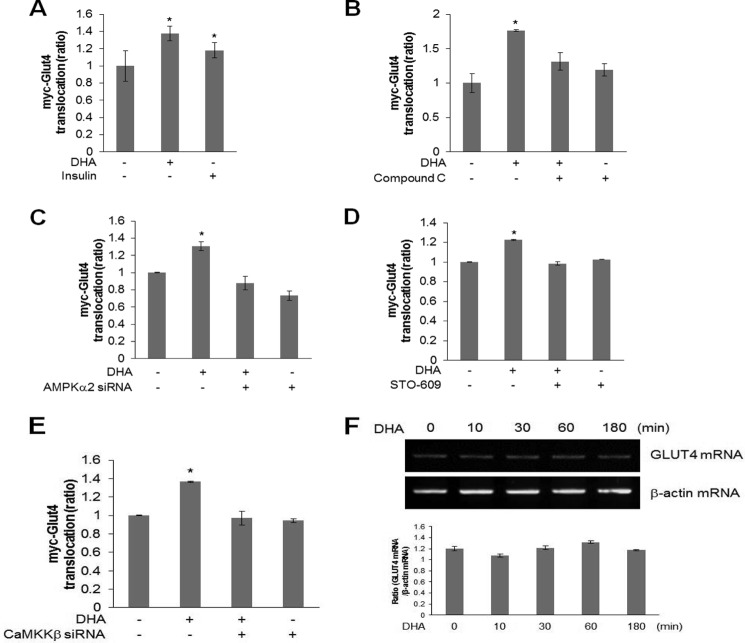
GLUT4 translocation was investigated to understand the role of DHA-mediated AMPK phosphorylation. Plasma membrane myc-GLUT4 increased in the presence of DHA, indicating that DHA stimulated GLUT4 translocation from the cytosol to the plasma membrane (Fig. 4A). Insulin was used as a positive control. This increase was not apparent in cells pretreated with compound C (Fig. 4B) and AMPKα2 knockdown (Fig. 4C). Inhibition with STO-609 and knockdown with CaMKKβ siRNA also blocked DHA-mediated GLUT4 translocation (Fig. 4, D and E). Effect of DHA on GLUT4 expression was evaluated based on the finding that DHA mediated glucose uptake. DHA did not increase GLUT4 mRNA levels in C2C12 cells (Fig. 4F), indicating that DHA regulated glucose uptake by stimulating GLUT4 translocation and not by inducing its expression.
FIGURE 4.
DHA stimulates GLUT4 translocation in AMPK dependently. A, myoblasts stably expressing myc-GLUT4 L6 were differentiated for 7 days. They were pretreated with DHA for 1 h and then treated with insulin for 15 min. Cell surface expression of GLUT4-myc was detected using antibody-coupled colorimetric absorbance assay. *, p < 0.05 compared with control. B, differentiated cells were pretreated with 2 μm compound C and then with DHA for 1 h. Cell surface expression of GLUT4-myc was detected using antibody-coupled colorimetric absorbance assay. *, p < 0.05 compared with control. C, differentiated cells were transfected with AMPKα2 for 2 days and then with DHA for 1 h. Cell surface expression of GLUT4-myc was detected using antibody-coupled colorimetric absorbance assay. *, p < 0.05 compared with control. D, differentiated cells were pretreated with 2 μm STO-609 and then with DHA for 1 h. Cell surface expression of GLUT4-myc was detected using antibody-coupled colorimetric absorbance assay. *, p < 0.05 compared with control cells. E, differentiated cells were transfected with CaMKKβ for 2 days and then with DHA for 1 h. Cell surface expression of GLUT4-myc was detected using antibody-coupled colorimetric absorbance assay. *, p < 0.05 compared with control. F, total mRNA was extracted from DHA-treated C2C12 cells, and RT-PCR was performed using GLUT4-specific primers. PCR products were visualized under ultraviolet light. The results are representative of four independent experiments. *, p < 0.05 versus basal conditions.
DHA Induces GPR120 Expression
GPR120 was regarded as a key signal for glucose uptake. We hypothesized that DHA may affect glucose regulation via GPR120. To determine the mechanism of DHA-mediated glucose uptake, we examined the effect of DHA on GPR120. GPR120 expression was increased in DHA-treated cells (Fig. 5A). To test the interrelation of GPR120, the effect of endogenous ligand GW9508 on GPR120 was investigated. GW9508 increased GPR120 expression (Fig. 5B). Further, GPR120 knockdown abrogated the increase in DHA-induced glucose uptake (Fig. 5C). Moreover, the increase of calcium caused by DHA was attenuated by knockdown of GPR120 (Fig. 5D). These results showed that GPR120 is involved in DHA-induced glucose uptake.
FIGURE 5.
DHA induces GPR120. A and B, total mRNA was extracted from DHA-treated (A) and GW9058-treated (B) C2C12 cells. RT-PCR was performed using GPR120-specific primers. PCR products were visualized under ultraviolet light. β-Actin was used as a positive control. The results are representative of four independent experiments. *, p < 0.05 versus basal conditions. C, L6 myotubes were transiently transfected with GPR120 siRNA and incubated with DHA for 1 h. Uptake of 2-DG was assayed. *, p < 0.05 compared with control. D, C2C12 cells were transfected with GPR120 siRNA for 2 days and then treated with 100 μm DHA. Green fluorescence was detected and analyzed.
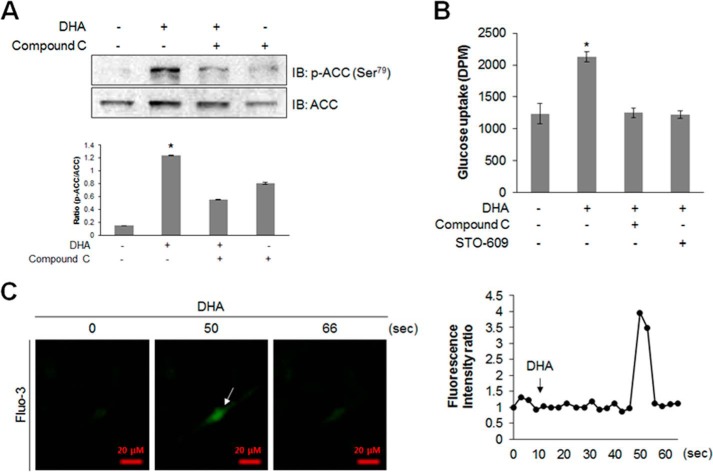
DHA Activates AMPK Pathway and Stimulates Glucose Uptake in Primary Cultured Myoblasts
To get insight into the role of DHA in vivo, we examined the effect of DHA on primary cultured myoblasts. DHA-mediated ACC phosphorylation was suppressed by compound C (Fig. 6A). Compound C and STO-609 abrogated the increase in DHA-induced glucose uptake (Fig. 6B). To confirm the components of the AMPK activation pathway, intracellular Ca2+ levels were measured in the presence of DHA. DHA increased intracellular Ca2+ levels in primary cultured myoblast cells (Fig. 6C). The cell indicated by an arrow in Fig. 6C was a calcium-responsive cell. These results indicated that DHA induced glucose uptake via AMPK pathway in primary cultured myoblasts.
FIGURE 6.
DHA activates AMPK and stimulates glucose uptake in primary cultured myoblasts. A, primary cultured myoblasts were stimulated with DHA for 1 h in the presence of compound C. Cell lysates were analyzed by Western blotting with antibodies against phospho-ACC, with ACC as the control. The results are representative of four independent experiments. *, p < 0.05 versus basal conditions. B, differentiated primary cultured myoblasts were incubated with DHA for 1 h in the presence of compound C (2 μm) or STO-609 (2 μm) and were assayed for glucose uptake. C, for detecting Ca2+, the cells were pretreated with fluo-3 AM (5 μm) for 45 min. Ca2+ response after DHA treatment was measured using confocal microscopy.
Discussion
The key finding of the present study was that DHA exerted metabolic effects in the skeletal muscle cells via GPR120-mediated AMPK pathways and that the hypoglycemic effect of DHA was attributed to the regulation of the AMPK activity. In our result, DHA stimulated glucose uptake, but this uptake was not as potent as AMPK phosphorylation. This discrepancy may be explained by the fact that AMPK phosphorylation is a necessary factor for glucose uptake, but it is not a sufficient factor for glucose uptake. It suggests that AMPK phosphorylation is not sufficient to trigger glucose uptake.
AMPK activation requires Thr172 phosphorylation in its catalytic α subunit. LKB1 and CaMKK are the upstream components of the AMPK pathway. LKB1, purified from rat liver, activates AMPK (20, 21). LKB1 mutation is associated with Peutz-Jeghers syndrome (22, 23), suggesting that AMPK acts as a tumor suppressor. Further, CaMKK phosphorylates and activates AMPK (4, 5, 24). CaMKK is activated by an increase in intracellular Ca2+, suggesting that AMPK activation may be mediated by Ca2+. GPCR-dependent signaling is potentially involved in AMPK activation. GPR40, GPR41, GPR43, GPR84, and GPR120 are activated by free fatty acids. GPR40 is involved in insulin secretion in the pancreatic β cells (17), and GPR41 and GPR43 are expressed in the colon (15). GPR84 is expressed in hematopoietic cells (16) and may be involved in insulin inflammatory response. GPR120 is highly expressed in the monocytes and adipose tissue (28) and is suggested to exert anti-inflammatory and insulin-sensitizing effects. Omega-3 FAs, which act as GPR120 agonists, cannot induce insulin sensitivity in GPCR knock-out mice. Ca2+ is an herb signal under GPCR activation. Many GPCRs induce intracellular Ca2+. However, their functional output depends on their type and the tissues in which they are expressed, indicating that GPCR ligands are critical for determining their roles. Of the various GPCR ligands, omega-3 FAs such as DHA and EPA exert potent insulin-sensitizing effects through GPR120.
Since the report on the involvement of GPR40 in free fatty acid-mediated insulin secretion (17), many studies have focused on GPCRs for developing antidiabetic drugs. Deletion and overexpression of GPR40 (29, 30) affect glucose-mediated insulin secretion in the pancreas, suggesting that GPR40 may regulate insulin secretion and mediate the effect of antidiabetic drugs. Clinical usefulness of GPCR agonists has been suggested in diabetes treatment. The GPCR119 agonists oleoyl lysophosphatidylcholine (31) and oleoylethanolamide (32) enhance glucose-induced insulin release from the pancreas, indicating their therapeutic potential. GPCR120 has attracted the attention of many researchers because of its usefulness for treating diabetes. Disruption of GPCR120 is associated with obesity (33). Although its clinical impact is much higher, it has only a few known endogenous ligands. Availability of endogenous ligands with suitable selectivity may potentiate the clinical application of GPCR120. In the present study, we showed that DHA, an endogenous GPCR120 ligand, exerted beneficial effects on glucose metabolism via AMPK-related pathways. Biological effects of DHA are known; however, its effects via GPCRs are unknown. In the present study, GPCR120 agonist GW9508 stimulated AMPK phosphorylation and glucose uptake. GPCR120 knockdown affected DHA-induced glucose regulation, suggesting that the beneficial metabolic effects of DHA on glucose metabolism were mediated by GPCR120. According to previous reports, physiological concentration of DHA ranged within 90 μm in human (34, 35). In our experiments, minimum effective dose was observed at 1 μm, and maximal effective dose was about 30 μm in cell culture systems. Because there is a slight difference between in vitro and in vivo, our experimental conditions are not much different from physiological conditions. Therefore, the results of DHA in cells do not exclude the biological significance of DHA in physiological conditions.
Previous articles showed that cytoplasmic calcium levels in several cell types, including skeletal muscle, promoted AMPK activation (36, 37). It has also been demonstrated that CaMKII contributed to AMPK activity in mammalian skeletal muscle cells (38, 39). It is known that calcium is necessary factor for AMPK phosphorylation but not essential factor for AMPK activation. However, other reports showed that the increase in AMPK phosphorylation is calcium-independent. Thus, it remains unclear whether calcium is an essential and sufficient factor for AMPK activation in skeletal muscle cells.
DHA is the most abundant omega-3 fatty acid in the brain and retina. In mice, DHA slowed the progression of Alzheimer neuropathology (40). In addition, DHA was found to inhibit growth of colon carcinoma cells (41, 42), reduce the risk of cardiovascular diseases (43), and decrease the inflammatory markers (25). These facts demonstrated that DHA has tissue-specific roles. On the other hand, DHA exerts its effect via GPR120. Free fatty acid receptors are expressed differentially, and they may play different functional roles. Immunoreactivity for GPR120 was found to be more abundant in the mouse large intestine, lung, and adipose tissues (26). High fat diet up-regulated GPR120 gene expression in skeletal muscles (27). Although many studies suggested benign effects of DHA on human health, there is not much molecular information of the role of DHA in skeletal muscles. In this study, we demonstrated that DHA exerted its benign metabolic roles in skeletal muscle cells, implying that DHA may become a promising molecular target for the development of treatment drug for diabetes. These findings provide insights into the beneficial metabolic role of DHA in the skeletal muscles and suggest that DHA is a promising agent for treating metabolic disorders such as obesity and diabetes.
Author Contributions
H. S. K. and N. K. conceived and designed the experiment; N. K., J. O. L., H. J. L., H. I. K., J. K. K., and Y. W. L. performed the experiments; S. J. K., S. K. L., and S. H. P. contributed reagents, materials, and analysis tools; N. K. wrote the paper; and H. S. K. reviewed and contributed to revisions of the manuscript.
This study was supported by Grant NRF-2013R1A2A2A05004796 from the National Research Foundation of Korea, which is funded by the Korean government. The authors declare that they have no conflicts of interest with the contents of this article.
- AMPK
- AMP-activated protein kinase
- DHA
- docosahexaenoic acid
- CaMKK
- Ca2+/calmodulin-dependent protein kinase kinase
- LKB1
- liver kinase B1
- GLUT4
- glucose transporter type 4
- GPCR
- G protein-coupled receptor
- FA
- fatty acid
- AM
- acetoxylmethl ester
- 2-DG
- 2-deoxy-d[H3]-glucose
- ACC
- acetyl-CoA carboxylase.
References
- 1. Hardie D. G., Carling D. (1997) The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259–273 [DOI] [PubMed] [Google Scholar]
- 2. Sakamoto K., McCarthy A., Smith D., Green K. A., Grahame Hardie D., Ashworth A., Alessi D. R. (2005) Deficiency of LK1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 24, 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koh H. J., Arnolds D. E., Fujii N., Tran T. T., Rogers M. J., Jessen N., Li Y., Liew C. W., Ho R. C., Hirshman M. F., Kulkarni R. N., Kahn C. R., Goodyear L. J. (2006) Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol. Cell. Biol. 26, 8217–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 5. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 6. Hayashi T., Hirshman M. F., Kurth E. J., Winder W. W., Goodyear L. J. (1998) Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47, 1369–1373 [DOI] [PubMed] [Google Scholar]
- 7. Mu J., Brozinick J. T., Jr., Valladares O., Bucan M., Birnbaum M. J. (2001) A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7, 1085–1094 [DOI] [PubMed] [Google Scholar]
- 8. Tsao T. S., Burcelin R., Katz E. B., Huang L., Charron M. J. (1996) Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes 45, 28–36 [DOI] [PubMed] [Google Scholar]
- 9. Tsao T. S., Li J., Chang KS., Stenbit A. E., Galuska D., Anderson J. E., Zierath J. R., McCarter R. J., Charron M. J. (2001) Metabolic adaptations in skeletal muscle overexpressing GLUT4: effects on muscle and physical activity. FASEB J. 15, 958–969 [DOI] [PubMed] [Google Scholar]
- 10. Rossetti L., Stenbit A. E., Chen W., Hu M., Barzilai N., Katz E. B., Charron M. J. (1997) Peripheral but not hepatic insulin resistance in mice with one disrupted allele of the glucose transporter type 4 (GLUT4) gene. J. Clin. Invest. 100, 1831–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fryer L. G., Parbu-Patel A., Carling D. (2002) The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277, 25226–25232 [DOI] [PubMed] [Google Scholar]
- 12. Ulloa-Aguirre A., Stanislaus D., Janovick J. A., Conn P. M. (1999) Structure-activity relationships of G protein-coupled receptors. Arch. Med. Res. 30, 420–435 [DOI] [PubMed] [Google Scholar]
- 13. Gether U. (2000) Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21, 90–113 [DOI] [PubMed] [Google Scholar]
- 14. Schulte G., Fredholm B. B. (2003) Signaling from adenosine receptors tomitogen-activated protein kinases. Cell Signal. 15, 813–827 [DOI] [PubMed] [Google Scholar]
- 15. Tazoe H., Otomo Y., Kaji I., Tanaka R., Karaki S. I., Kuwahara A. (2008) Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 59, 251–262 [PubMed] [Google Scholar]
- 16. Wang J., Wu X., Simonavicius N., Tian H., Ling L. (2006) Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J. Biol. Chem. 281, 34457–34464 [DOI] [PubMed] [Google Scholar]
- 17. Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., Tanaka H., Maruyama M., Satoh R., Okubo S., Kizawa H., Komatsu H., Matsumura F., Noguchi Y., Shinohara T., Hinuma S., Fujisawa Y., Fujino M. (2003) Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422, 173–176 [DOI] [PubMed] [Google Scholar]
- 18. Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11, 90–94 [DOI] [PubMed] [Google Scholar]
- 19. van Dijk S. J., Feskens E. J., Bos M. B., Hoelen D. W., Heijligenberg R., Bromhaar M. G., de Groot L. C., de Vries J. H., Müller M., Afman L. A. (2009) A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am. J. Clin. Nutr. 90, 1656–1664 [DOI] [PubMed] [Google Scholar]
- 20. Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Mäkelä T. P., Alessi D. R., Hardie D. G. (2003) Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13, 2004–2008 [DOI] [PubMed] [Google Scholar]
- 22. Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Höglund P., Järvinen H., Kristo P., Pelin K., Ridanpää M., Salovaara R., Toro T., Bodmer W., Olschwang S., Olsen A. S., Stratton M. R., de la Chapelle A., Aaltonen L. A. (1998) A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391, 184–187 [DOI] [PubMed] [Google Scholar]
- 23. Jenne D. E., Reimann H., Nezu J., Friedel W., Loff S., Jeschke R., Müller O., Back W., Zimmer M. (1998) Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 18, 38–43 [DOI] [PubMed] [Google Scholar]
- 24. Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066 [DOI] [PubMed] [Google Scholar]
- 25. Kelley D. S., Siegel D., Fedor D. M., Adkins Y., Mackey B. E. (2009) DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J. Nutr. 139, 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyauchi S., Hirasawa A., Iga T., Liu N., Itsubo C., Sadakane K., Hara T., Tsujimoto G. (2009) Distribution and regulation of protein expression of the free fatty acid receptor GPR120. Naunyn Schmiedebergs Arch. Pharmacol. 379, 427–434 [DOI] [PubMed] [Google Scholar]
- 27. Cornall L. M., Mathai M. L., Hryciw D. H., McAinch A. J. (2011) Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol. Biochem. 28, 949–958 [DOI] [PubMed] [Google Scholar]
- 28. Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alquier T., Peyot M. L., Latour M. G., Kebede M., Sorensen C. M., Gesta S., Ronald Kahn C., Smith R. D., Jetton T. L., Metz T. O., Prentki M., Poitout V. (2009) Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes 58, 2607–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagasumi K., Esaki R., Iwachidow K., Yasuhara Y., Ogi K., Tanaka H., Nakata M., Yano T., Shimakawa K., Taketomi S., Takeuchi K., Odaka H., Kaisho Y. (2009) Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 58, 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soga T., Ohishi T., Matsui T., Saito T., Matsumoto M., Takasaki J., Matsumoto S., Kamohara M., Hiyama H., Yoshida S., Momose K., Ueda Y., Matsushime H., Kobori M., Furuichi K. (2005) Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 326, 744–751 [DOI] [PubMed] [Google Scholar]
- 32. Ning Y., O'Neill K., Lan H., Pang L., Shan L. X., Hawes B. E., Hedrick J. A. (2008) Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br. J. Pharmacol. 155, 1056–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hudson B. D., Shimpukade B., Milligan G., Ulven T. (2014) The molecular basis of ligand interaction at free fatty acid receptor 4 (FFA4/GPR120). J. Biol. Chem. 289, 20345–20358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pawlosky R. J., Hibbeln J. R., Herion D., Kleiner D. E., Salem N., Jr. (2009) Compartmental analysis of plasma and liver n-3 essential fatty acids in alcohol-dependent men during withdrawal. J. Lipid Res. 50, 154–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Attar-Bashi N. M., Weisinger R. S., Begg D. P., Li D., Sinclair A. J. (2007) Failure of conjugated linoleic acid supplementation to enhance biosynthesis of docosahexaenoic acid from alpha-linolenic acid in healthy human volunteers. Prostaglandins Leukot. Essent. Fatty Acids. 76, 121–130 [DOI] [PubMed] [Google Scholar]
- 36. Grotemeier A., Alers S., Pfisterer S. G., Paasch F., Daubrawa M., Dieterle A., Viollet B., Wesselborg S., Proikas-Cezanne T., Stork B. (2010) AMPK-independent induction of autophagy by cytosolic Ca2+ increase. Cell Signal. 22, 914–925 [DOI] [PubMed] [Google Scholar]
- 37. Madaro L., Marrocco V., Carnio S., Sandri M., Bouché M. (2013) Intracellular signaling in ER stress-induced autophagy in skeletal muscle cells. FASEB J. 27, 1990–2000 [DOI] [PubMed] [Google Scholar]
- 38. Raney M. A., Turcotte L. P. (2008) Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J. Appl. Physiol. 104, 1366–1373 [DOI] [PubMed] [Google Scholar]
- 39. Gutierrez-Martin Y., Martin-Romero F. J., Henao F. (2005) Store-operated calcium entry in differentiated C2C12 skeletal muscle cells. Biochim. Biophys. Acta 1711, 33–40 [DOI] [PubMed] [Google Scholar]
- 40. Lim G. P., Calon F., Morihara T., Yang F., Teter B., Ubeda O., Salem N., Jr., Frautschy S. A., Cole G. M. (2005) A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 25, 3032–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kato T., Hancock R. L., Mohammadpour H., McGregor B., Manalo P., Khaiboullina S., Hall M. R., Pardini L., Pardini R. S. (2002) Influence of omega-3 fatty acids on the growth of human colon carcinoma in nude mice. Cancer Lett. 187, 169–177 [DOI] [PubMed] [Google Scholar]
- 42. Schønberg S. A., Lundemo A. G., Fladvad T., Holmgren K., Bremseth H., Nilsen A., Gederaas O., Tvedt K. E., Egeberg K. W., Krokan H. E. (2006) Closely related colon cancer cell lines display different sensitivity to polyunsaturated fatty acids, accumulate different lipid classes and downregulate sterol regulatory element-binding protein 1. FEBS J. 273, 2749–2765 [DOI] [PubMed] [Google Scholar]
- 43. Pauwels E. K., Kostkiewicz M. (2008) Fatty acid facts: III. cardiovascular disease, or, a fish diet is not fishy. Drug News Perspect. 21, 552–561 [DOI] [PubMed] [Google Scholar]