Abstract
In the post combination antiretroviral therapy (cART) era, the prevalence of mild forms of HIV-associated neurocognitive disorders (HAND) in individuals with HIV-infection remains high. There is a pressing need to find biomarkers that can aid clinical assessment of HAND, especially in those with mild or no neurocognitive symptoms. Here we hypothesized that a reduction in neural specificity, or the specificity of neuronal tuning, could serve as a potential biomarker of asymptomatic HAND. To directly test this hypothesis, we applied two advanced fMRI techniques to examine the difference in neural specificity between middle-aged HIV+ women and age-matched negative controls, with a focus on the fusiform face area (FFA), a critical region in face processing. Face discrimination performance was assessed outside of the scanner. While the behavioral performance of face discrimination was comparable between the two groups, a reduced neural specificity in the FFA of HIV-positive women was revealed by a novel fMRI analysis technique, local regional heterogeneity analysis, or Hcorr, as well as an established technique, fMRI-rapid adaptation. In contrast, conventional fMRI techniques were insensitive to these early changes. These results suggest that, prior to the onset of detectable behavioral deficits, significant neuronal dysfunctions are already present in HIV+ individuals, and these early neuronal dysfunctions can be detected and assessed via neural specificity, which, in combining with the novel Hcorr technique, has a strong potential to serve as a biomarker of asymptomatic HAND and other neurodegenerative diseases.
Keywords: fMRI-RA, Hcorr, HIV-associated neurocognitive disorders, Neural specificity
Highlights
-
•
We investigate early neuronal dysfunctions in cognitively normal HIV+ women.
-
•
Conventional fMRI technique reveals normal neural activity in the FFA of HIV+ women.
-
•
fMRI-adaptation reveals a decrease in neural specificity in the FFA of HIV+ women.
-
•
Hcorr, a novel fMRI technique, confirms the fMRI-adaptation results
-
•
Hcorr-estimated neural specificity might serve as a biomarker of asymptomatic HAND
1. Introduction
While the introduction of combination antiretroviral therapy (cART) has significantly reduced the incidence of HIV-associated dementia (HAD) — the most severe form of HIV-associated neurocognitive disorders (HAND) (McArthur et al., 1999; McArthur, 2004), the overall prevalence of HAND has remained high (Harezlak et al., 2011; Heaton et al., 2011, 2010; Schouten et al., 2011; Simioni et al., 2010). It has been suggested that about 30–60% of HIV-positive adults are currently living with HAND (Grant, 2008), and more than half of these individuals have the mildest form of HAND referred to as asymptomatic neurocognitive impairment (ANI) (Heaton et al., 2010). This new spectrum poses a significant challenge in clinical settings to identify those rather mild cognitive impairments (Moore et al., 2012; Valcour, 2011). Currently the diagnosis of HAND is based on neuropsychological testing. While these tests are well developed, many of them are neither sensitive nor specific to HAND (Haddow et al., 2013; Hasbun et al., 2012; Sakamoto et al., 2013; Skinner et al., 2009; Valcour et al., 2011), or are too time-consuming to be used in routine clinical assessment (Spudich and Ances, 2012). In addition to the development of new neuropsychological testing that is more specific and more sensitive to HAND, biomarkers that correlate with disease progression could be extremely helpful in assisting clinical diagnosis and evaluating therapeutic effects as they provide a more objective measurement and avoid the practice effects associated with repetitive behavioral testing (Ciborowski, 2009; Fox, 2013; Marcotte et al., 2013). However, despite significant progress in research, the reliable detection of HAND (especially mild forms of HAND) with biomarkers remains a major challenge. For instance, recent studies have suggested biomarkers of HIV-disease might not closely correlate with cognitive impairments in the post-cART era (Gelman et al., 2013).
In contrast, recent advances in neuroimaging techniques like magnetic resonance imaging (MRI) have offered a noninvasive way to detect and assess pathological changes due to varying neurodegenerative diseases, including HAND (Descamps et al., 2008; Holt et al., 2012). For instance, structural MRI has revealed volume reduction in varying brain regions that can be linked to cognitive impairments (Ances et al., 2012; Chang et al., 2011; Towgood et al., 2012). However, brain atrophy usually happens at rather late stages of disease with significant and usually irreversible brain damage. Given that HIV-infection is hypothesized to affect neuronal function long before changes in anatomy can be detected, functional MRI (fMRI), with its ability to directly image brain function, has the potential to be a critical tool in detecting early signs of HAND particularly in clinically asymptomatic patients. However, the effectiveness of recent efforts to use fMRI to study HIV has been limited by the absence of a clear model of how HIV affects the neuronal processing that gives rise to behavioral deficits, and how these changes in neuronal processing could be detected with fMRI at high sensitivity. Indeed, recent fMRI studies of HIV have generated some “conflicting” findings: both increased and decreased neural activity have been reported in HIV-positive patients (Ances et al., 2008; Castelo et al., 2006; Chang et al., 2001; Maki et al., 2009; Melrose et al., 2008). These contradictions might reflect the technical limitations of conventional fMRI techniques: lower activation levels could reflect neuronal loss in HIV-infected patients or highly specific neuronal tuning in healthy controls (which would also lead to a decreased number of neurons responding). In contrast, higher activation levels could result either from HIV-induced decreases in neural specificity (which would lead to an increase in number of neurons responding) or from fully functional neuronal populations in healthy controls. While these two scenario have very different implications for behavioral performance, they cannot be dissociated using conventional fMRI techniques that rely on comparing average fMRI response amplitude and cannot measure neural specificity (Grill-Spector et al., 2006).
In contrast, a more advanced fMRI technique, fMRI-Rapid Adaptation (fMRI-RA) (Grill-Spector et al., 2006), has proven to be able to measure neural specificity more directly than conventional fMRI techniques, with a direct link to behavioral performance. In fMRI-RA, the response to a pair of stimuli presented in rapid succession is measured for pairs similar or different in a specific perceptual aspect (e.g., viewpoint or shape), and the contrast between the combined response level when the property is the same or different is interpreted as an index of stimulus representational dissimilarity at the neural level (Jiang et al., 2007; Kourtzi and Kanwisher, 2001). For instance, using fMRI-RA and morphed face, we (Jiang et al., 2006) and others (Gilaie-Dotan and Malach, 2007) have provided evidence suggesting that the neural tuning of FFA neurons in healthy young adults are highly selective/specific, in line with the high performance in discriminating different faces. In contrast, individuals with autism spectrum disorders (ASD) often have difficulties in discriminating face shapes (Snow et al., 2011). We have shown that the impaired face discrimination performance can be quantitatively linked to a decrease in neural specificity in the FFA, estimated via both fMRI-RA as well as a novel fMRI analysis technique, local regional heterogeneity analysis, or Hcorr. These results suggest that neural specificity may serve as a sensitive measure of neuronal dysfunction. In contrast to fMRI-RA that estimates neural specificity via the profile of fMRI response associated with stimuli similarity, local regional heterogeneity analysis, or Hcorr, which we recently developed, estimates neural specificity via voxel-wise correlations. Briefly, Hcorr calculates the variance of local voxel-wise correlations in a region of interest (ROI), resulting in a measure of heterogeneity of neural responses in the ROI, also termed Hcorr, as an indirect measure of neural specificity (Jiang et al., 2013). We have recently validated this technique in several independent data sets, and provided evidence that varying cognitive performance can be quantitatively linked to Hcorr at corresponding brain regions (Jiang et al., 2013).
Here we hypothesized that, in HIV-infected adults who have suppressed viral load and are cognitively normal, subtle changes of neuronal functions are already present, even in the absence of behavioral impairments, and these early neuronal dysfunctions can be detected and assessed via a decrease in neural specificity. To directly test this hypothesis, we examined the difference in neural specificity between middle-aged HIV-positive women who were on cART with suppressed viral load and matched HIV-negative controls, using the well-established fMRI-RA, as well as the novel Hcorr technique. Because the temporal cortex is one of the most affected regions due to HIV-infection (Kallianpur et al., 2012; Küper et al., 2011; Sarma et al., 2014; Wiley et al., 1991) and the neural bases of face processing have been well studied (Kanwisher and Yovel, 2006), we investigated the neural specificity in the fusiform face area (FFA), a critical face processing region in the ventral temporal lobe (Kanwisher and Yovel, 2006).
2. Material and methods
2.1. Women's interagency HIV study (WIHS)
The WIHS was designed to examine the natural and treated history of HIV disease among women in response to its rising epidemic in 1994–1995. Its study design is detailed elsewhere (Bacon et al., 2005; Barkan et al., 1998). The original enrollment recruited 2054 HIV-infected and 569 HIV-uninfected women from six study sites (Chicago, Los Angeles, San Francisco, Washington, DC, Brooklyn, and the Bronx) in the United States. Women visit a study site every 6 months; during each visit, structured interviews are performed to collect data on socio-demographic characteristics, substance use and sexual behaviors, health care utilization, antiretroviral therapy, other treatments, and disease outcomes. In addition, physical and obstetric/gynecologic examinations are performed by the medical staff and biological specimens are collected. The local Institutional Review Board at each site approved the study protocol, and all women gave their written informed consent. As of January 2013, about 90% of HIV+ participants in the WIHS were receiving cART, and among those on cART, about 70% achieved a viral load level of less than 80 copies/ml.
2.2. Participants
Twenty-eight middle-aged (43–56 years old, mean 50.3 ± 0.8) women with no major psychiatric disorders or other confounding health problems participated in this cross-sectional study. There were 15 HIV-positive receiving cART and 13 HIV-negatives. All of them were recruited from the Washington, DC site of the Women's Interagency HIV Study (WIHS) from visit 36–38 (from year 2012 to year 2013). The two groups were matched on age, education, and socioeconomic status and other factors that might affect their brain functions (see Table 1). HIV+ women with neurologic complications were excluded from the study. Data from one additional HIV+ woman was excluded as she was 60 years old (while the oldest HIV− control was 56 years old), though including her data yielded nearly identical results. Experimental procedures for this study were approved by Georgetown University's Institutional Review Board and written informed consent was obtained from all subjects prior to the experiment.
Table 1.
Participant characteristics by HIV status.
| Variables | HIV+ women (N = 15) |
HIV− women (N = 13) |
p value |
|---|---|---|---|
| Age at visit (years), mean (sd) | 50.1 (4.4) | 50.2 (4.1) | 0.953 |
| Years of education, mean (sd) | 13.5 (2.7) | 15.5 (4.2) | 0.147 |
| MMSEa total score, mean (sd) | 60.1 (7.4) | 59.7 (6.7) | 0.871 |
| CESDb score at visit, mean (sd) | 6.5 (9.2) | 6.8 (10.2) | 0.919 |
| Race/ethnicity, n (%) | 0.311 | ||
| White, non-Hispanic | 1 (6.7) | 0 (0.0) | |
| Black. non-Hispanic | 13 (86.7) | 10 (76.9) | |
| Hispanic | 1 (6.7) | 3 (23.1) | |
| Menopause, n (%) | 9 (60.0) | 5 (38.4) | 0.256 |
| Hepatitis C virus antibody positive, n (%) | 2 (13.3) | 2 (15.4) | 1.00 |
| Recent marijuana use, n (%) | 1 (6.7) | 2 (15.4) | 0.583 |
| Recent crack use, n (%) | 1 (6.7) | 1 (7.7) | 1.00 |
| Current smoking, n (%) | 4 (26.7) | 4 (30.8) | 1.00 |
| Current drink, n (%) | 0.0143 | ||
| Abstainer | 11 (73.3) | 3 (23.1) | |
| Light (<3 drinks/week) | 2 (13.3) | 8 (61.5) | |
| Moderate (3–13 drinks/week) | 2 (13.3) | 2 (15.4) | |
| Heavy (>=14 drinks/week) | 0 (0.0) | 0 (0.0) | |
| CD4+ cell counts at visit, mean (sd) | 702.5 (301.9) | ||
| Viral load at visit, median (25%–75% IQRc) | 20 (20.0, 186) | ||
| Antiretroviral medication use, n (%) | |||
| HAART | 13 (86.7) | ||
| No therapy | 2 (13.3) | ||
| Antiretroviral therapy adherence, n (%) | |||
| 100% | 10 (76.9) | ||
| 95%–99% | 2 (15.4) | ||
| <75% | 1 (7.7) | ||
| History of opportunistic infections, n (%) | 5 (33.3) | 0 (0.0) | 0.044 |
The bold fonts indicate the significance as the numbers are p values.
MMSE: Mini-mental state examination.
CESD: Center for Epidemiologic Studies Depression Scale.
IQR: Interquartile range.
2.3. Face discrimination experiment
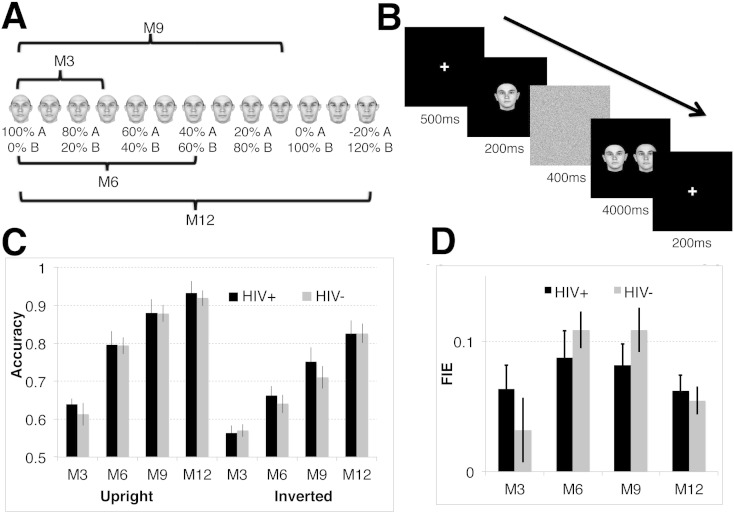
Using face stimuli generated by a photorealistic face morphing system (Blanz and Vetter, 1999) along twenty-five within-gender morph lines based on fifty individual prototype faces (200 by 256 pixels, twenty-six females, see Fig. 1A), we tested subjects' face discrimination abilities using a two-alternative forced choice (2AFC) paradigm (Fig. 1B). For each trial, after a 500 ms fixation, the target face was presented for 200 ms, followed by a mask image for 400 ms, followed by two choice faces presented side-by-side for 4000 ms or when participants responded (whichever came first), and participants were asked to judge which one of the two faces was the same as the target face. The next trial would automatically start 1000 ms after subjects made a response. If subjects failed to respond within 4000 ms, an auditory alarm (“beep”) would be presented, and the next trial would start 1000 ms after the beep.
Fig. 1.
Face discrimination performance. (A) Shows an example “morph line” between a pair of face prototypes (shown at the far left and second from the right), created using the photorealistic morphing system (42). The relative contribution of each face prototype changes smoothly along the line. For example, the fourth face from the left (the third morph) is a mixture of 70% of the face on the far left and 30% of the face on the far right. Four conditions, M3/6/9/12, which correspond to shape differences of 30%, 60%, 90%, and 120%, were examined in the present study. The difference between two prototype faces was defined as 100%; 120% difference was achieved by extrapolation along the morph line beyond face prototypes (42). (B) Design of the 2AFC face discrimination experiment. Subjects viewed a target face, followed by a mask, followed by two test faces, presented side-by-side, and had to indicate which of the test faces was identical to the previously presented target face. There were four levels of shape difference, M3/6/9/12, between the two test faces as explained in (A). (C) The averaged performance from fifteen HIV-positive women and thirteen controls. Error bars indicate SEM.
One of the choice faces was always the same as the sample face, while the other choice face differed from the first one by one of four possible different levels of similarity. This was done by creating “morph lines” interpolated between two prototype faces, and then choosing face images separated by a specified distance along this continuum (Fig. 1A). We tested four different levels of intra-pair similarity, with shape differences of 30%, 60%, 90%, and 120% (conditions M3/6/9/12, respectively, see Fig. 1A), with 100% corresponding to the distance between two prototype faces, and 120% difference created by extrapolation (Blanz and Vetter, 1999). Within each trial, the three faces were either all upright or all inverted. Stimuli were presented to participants on an LCD monitor on a dark background, 1024 × 768 resolution, 60 Hz refresh rate, at a distance of 60 cm. An in-house software package was used to present the stimuli and to record the responses. Participants completed a total of 800 trials (100 per condition) in sixteen blocks.
Face discrimination experiment was conducted outside of the MRI scanner and after the participants finished the MRI scanning (with a short break in between). The face discrimination data from three subjects was discarded due to failure to follow instructions.
2.4. Functional localizer scans
To locate the FFA regions, a block design was used to collect MRI images from two localizer scans for each subject (Haxby et al., 1999; Jiang et al., 2013, 2006; Kanwisher et al., 1997). Briefly, during each run, following an initial 10.2 s fixation period, 50 grayscale images of faces, houses, and scrambled faces were presented to participants in blocks of 30.6 s (each image was displayed for 512 ms and followed by a 100 ms blank screen), and were separated by a 20.4 s fixation block (Fig. 2). Each block was repeated twice in each run, which lasted for 316.2 s, and participants were asked to passively view these images while keeping their fixation at the center of the screen. The face and house images used in the localizer scans were downloaded from Internet and post-processed using programs written in MATLAB (The Mathworks, MA) to eliminate background variations, and to adjust image size, luminance, and contrast. The final size of all images was scaled to 200 by 200 pixels, and half of the faces were scrambled using a grid of 20 by 20 pixel elements while the outlines of the faces were kept intact. The data from the localizer scans were also used in the local regional heterogeneity analysis (see below) to probe the sparsity of FFA neuronal activations.
Fig. 2.
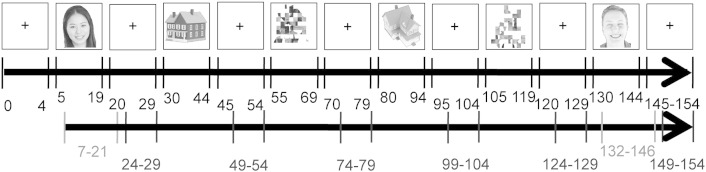
Functional localizer scans. Participants were asked to passively view blocks of face, house, and scrambled face images that were separated by fixation blocks. The numbers (in black font) indicate the first and last MRI acquisition of each block. MRI images that were chosen for the local regional heterogeneity analysis are shown in red (face blocks) and blue (fixation blocks). Due to the lag of hemodynamic responses, for face blocks, the images from n0 + 2 to nm + 2 were used (n0 is the time point when face blocks started, and nm is the time point when face blocks ended), and the images from n0 + 4 to nm were used for fixation blocks to avoid signal overlap from the blocks before and after it (n0 is the time point when a fixation block started, and nm is the time point when it ended).
2.5. Rapid event-related (ER) scans
MRI images from three (n = 22, ten HIV-negative) or four (n = 6, three HIV-negative) ER scans were collected for each participant. Each run lasted 538.56 s and had two 10.2 s fixation periods, one at the beginning and the other at the end. Between the two fixation periods, a total of 127 trials were presented to participants at a rate of one every 4.08 s. During each trial (except null trials), two faces were displayed sequentially (300 ms each with a 400 ms blank screen in-between), and followed by a 3080 ms blank screen (Jiang et al., 2013). For each run, the data from the first two trials were discarded, and analyses were performed on the data of the other 125 trials — 25 each of the five different conditions: three conditions of interest of varying intra-pair stimulus similarity (M3/M6/M9, see Fig. 1A above) (Jiang et al., 2013), task trials, in which an ‘oddball’ target face, which participants needed to identify, could appear as either the first or the second one of the pair of faces, and null trials (Jiang et al., 2013). Performance inside the scanner was nearly perfect and did not differ between the two groups of participants. Trial order was randomized and counterbalanced using M-sequences (Buracas and Boynton, 2002). While inside the scanner, participants were asked to watch all the faces but only respond to instances of the target face by pressing a button with the right hand. Morphed faces (200 by 200 pixels) along ten within-gender morph lines of twenty individual prototype faces (ten females) were used, along with one additional oddball target face, different from the face prototypes used to generate the morphed stimuli. The stimuli of both localizer and ER scans were presented on black background using E-Prime software (http://www.pstnet.com/products/e-prime/), back-projected on a translucent screen located at the rear of the scanner, and viewed by participants through a mirror mounted on the head coil. The fMRI data from the ER scans of one participants were discarded due to technical problems.
2.6. MRI data acquisition
MRI data were acquired at the Georgetown University's Center for Functional and Molecular Imaging using an echo-planar imaging (EPI) sequence on a 3.0 Tesla Siemens Trio scanner (Flip angle = 90°, TR = 2.04 s, TE = 29 ms, FOV = 205, 64 × 64 matrix) with a twelve-channel head coil. Thirty-five interleaved axial slices (thickness = 4.0 mm, no gap; in-plane resolution = 3.2 × 3.2 mm2) were acquired for the two functional localizer and all functional runs. At the end, three-dimensional T1-weighted MPRAGE images (resolution 1 × 1 × 1 mm3) were sagittally-acquired from each subject (TR = 1.9 s, TE = 2.52 ms, FOV = 250, 1 mm thickness with 18% oversampling, creating 1 mm cubic voxels).
3. Data analysis
3.1. MRI data preprocessing
After discarding the images acquired during the first three acquisitions of each run, the EPI images were temporally corrected to the middle slice, spatially realigned and unwrapped together with the images from the localizer scans using the SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), then all images were re-sliced to 2 × 2 × 2 mm3, normalized to a standard MNI reference brain in Talairach space, and smoothed with 6 mm Gaussian kernel using SPM8.
The FFA regions were identified for each individual participant independently with the data from the localizer scans. We first modeled the hemodynamic activity for each condition (face, scrambled face, and house) in the localizer scans with the standard canonical hemodynamic response function, then identified the FFA ROI with the contrast of face versus house masked by the contrast of face versus baseline (p < 0.0001). To obtain comparably-sized FFAs across participants, we defined the FFA as the ROI consisting of the 45 contiguous voxels with the highest statistical threshold for each subject (Jiang et al., 2013).
After removing low frequency temporal noise from the EPI runs with a high pass filter (1/128 Hz), fMRI responses were modeled with a design matrix comprising the onset of each non-null trial and movement parameters as regressors using SPM8. We extracted the hemodynamic response for each subject in the right FFA, using a standard canonical hemodynamic response function plus temporal derivative, with the MarsBar toolbox (http://marsbar.sourceforge.net) and in-house software written in Matlab (http://www.mathworks.com/products/matlab/).
3.2. Local regional heterogeneity analysis
Recently we introduced a novel technique to probe the sparseness of neuronal activation patterns and provide an indication of neuronal selectivity. The technique, called local regional heterogeneity analysis, or Hcorr, is calculated as the standard error of voxel-wise correlations of fMRI activation patterns in a pre-defined functional or anatomical defined brain region (Jiang et al., 2013). This novel technique is motivated by findings from monkey training studies showing that learning produces sparser codes, with neurons after training responding to fewer stimuli, along with improved discrimination performance (Freedman et al., 2006, 2006; Kobatake et al., 1998). In other words, the sparseness of activation patterns is related to the specificity of neuronal tuning: the high specific neurons produces a sparse neural code, as each neuron only responds to a small set of stimuli highly similar to its preferred stimulus/stimuli. In contrast, less specific neurons respond to a greater number of dissimilar stimuli, leading to greater overlap in responses among neurons and less sparse representations. This then predicts that the sharply tuned population should have a higher degree of heterogeneity in neural correlations (as small groups of neurons (e.g., FFA neurons) preferring similar stimuli (e.g., faces) should show high correlation with each other but low correlation with other neurons) compared to the population with more broadly tuned neurons (in which more neurons respond in a similar way, increasing the uniformity of correlations). This hypothesis is supported by single-unit studies that have found that neurons with similar tuning tend to show more correlated firing than neurons with dissimilar tuning (Bair et al., 2001; Jermakowicz et al., 2009; Lin et al., 2014). Thus, when measured with fMRI, neural tuning specificity should correlate with the degree of sparseness, which in turn should correlate with the heterogeneity of correlations, i.e., the standard deviation or standard error of correlations between voxels. In other words, across subjects, a lower local regional heterogeneity of correlations should be associated with a lower neural specificity, and vice versa.
For the local regional heterogeneity analysis at FFA, we first extracted the raw time series data in the FFA from the first localizer scan (see Fig. 2) for each participant (with normalization but without additional smoothing), followed by removal of the mean, any linear trends, and low frequency variations. General linear model was used to regress out variance due to head movements and global signal change (Friston et al., 1995), then the fMRI data from every time point in the face blocks and fixation blocks (after compensating for delays caused by the slow hemodynamic response) were used in a pair-wise correlation analysis between each voxel, which resulted in a set of pairwise correlation coefficients (for n voxels), rij.
| (1) |
We then calculated a measure of local heterogeneity, Hcorr, as the standard error of the mean (SEM) of those correlation coefficients (rij, i < j, because rij = rji, and rii = 1).
| (2) |
4. Results
4.1. Demographic, neuropsychological, medical, and other profiles of HIV-positive patients and HIV-negative controls
As shown in Table 1, the HIV-positive group and HIV-negative group were matched in terms of age, years of education, race/ethnicity, menopause status, hepatitis C virus status, recent use of marijuana, crack, and tobacco, alcohol use disorders, general neurocognitive function as measured by mini-mental status examination (MMSE), and depression symptom as measured by Center for Epidemiological Studies Depression (CESD). The study participants had an average age of 50 years and an average 14 years of education, with the vast majority of women being Black, non-Hispanic. Among these HIV+ women, the average CD4+ T cell count was 702 cells/mm3 and the median HIV RNA level was 20 copies/mm3. About 87% of the HIV+ women were on antiretroviral therapy, with about 93% of those on therapy having a ≥ 95% self-reported adherence levels.
4.2. Comparable face discrimination performance between middle-aged HIV+ women and age-matched HIV− controls
A mixed-design ANOVA on response accuracy, with two within-subjects factors, face shape differences (M3/6/9/12) and face orientations (upright vs. inverted), and one between-subject factor, HIV status (HIV-positive vs. HIV-negative), revealed a significant difference between upright and inverted face conditions (F(1,25) = 80.258, p < 0.001) and between the four morph steps of face dissimilarities (F(3,75) = 245.139, p < 0.001), and a significant interaction between face orientation and morph steps (F(3,75) = 15.516, p < 0.001), suggesting participants were more accurate in discriminating upright faces than inverted faces, with an increase in accuracy with increased shape difference in face pairs and even more so with upright faces. However, there were no significant difference between the two groups (p > 0.64), nor any significant interactions between HIV status and other within-subject factors (at least p > 0.14), suggesting that the HIV-positive and HIV-negative subjects had comparable behavioral performance in discriminating the morphed faces.
Furthermore, a same mixed-design ANOVA on reaction time also revealed a significant difference between upright and inverted face conditions (F(1,25) = 4.423, p < 0.047) and between the four morph steps of face dissimilarities (F(3,75) = 34.182, p < 0.001), and a significant interaction between face orientation and morph steps (F(3,75) = 21.945, p < 0.001), suggesting participants were faster in discriminating upright faces than inverted faces, with a decrease in reaction time with increased shape difference in face pairs and even more so with upright face. However, there were no significant difference between the two groups (p > 0.58), nor any significant interactions between HIV status and other within-subject factors (at least p > 0.40). These results provided further support that the behavioral performance between the two groups is comparable.
4.3. No significant differences in fMRI response amplitude to faces in the FFA measured via conventional fMRI techniques
To investigate whether potential early change in neuronal function can be detected with conventional fMRI techniques that rely on comparing blood oxygen level dependent (BOLD) signal, all subjects participated in an fMRI experiment that included two functional localizer runs in which subjects viewed blocks of faces, scrambled faces, and houses, separated by blocks of fixation (Fig. 2). After identifying the right FFA using the contrast of faces versus houses masked by faces versus baseline in each individual subject (Jiang et al., 2013, 2006), we then extracted the fMRI responses to faces in the right FFA from localizer scans. There was no difference in fMRI responses to face between the HIV-positive participants and HIV-negative controls (p > 0.43) (Fig. 3A).
Fig. 3.
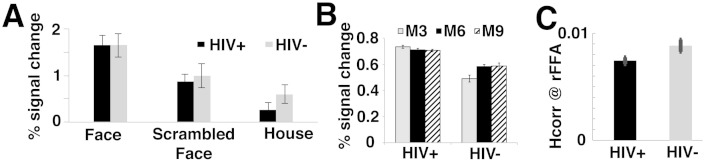
FMRI data. (A) Conventional fMRI techniques revealed no difference between the middle-aged HIV-positive women and age-matched controls, with compatible mean fMRI responses to face, house, and scrambled face from the two functional localizer scans. In contrast, both fMRI-RA (B) or Hcorr (C) revealed a reduced neural specificity in the FFA of HIV-positive women than that of HIV-negative controls. (B) Three conditions of interests, M3/6/9, were included in the fMRI-RA experiment. In contrast to a full recovery from adaptation at M6 condition in controls (similar to what we have found in healthy younger adults (Jiang et al., 2006)), there was no recover from adaptation (even at M9 condition) in HIV-positive individuals, suggesting a significant decrease in neural specificity (reflecting as a broadened neuronal tuning) in the FFA of HIV+ women. (C) Local voxel-wise correlations also revealed a lower Hcorr value in the FFA of HIV-positive individuals than HIV-negative controls, confirming a reduced neural specificity in FFA, even in the absence of behavioral deficits. Error bars show within-subject SEM.
4.4. FMRI-RA revealed a significant decrease in neural specificity in the FFA of HIV-positive individuals, in the absence of behavioral deficits
Next, we applied fMRI-RA to examine the selectivity of face representations in both HIV-positive participants and HIV-negative controls, as a measure of neural specificity — that is, an earlier recovery from adaptation suggests a sharper tuning to the morphed face, which in turn is correlated with higher neural specificity, and vice versa.
The fMRI responses to pairs of faces in the M3/6/9 conditions were extracted from the independently defined right FFA ROI of each individual (same ROI as above). A mixed-design ANOVA, with a within-subject factor (shape similarity between pairs of faces, M3/6/9), and a between-subject factor (HIV status, HIV-positive vs. HIV-negative), revealed a significant interaction between the two factors (F(2,50) = 5.051, p < 0.05, Bonferroni corrected for multiple comparisons of three main fMRI data analyses as shown in Fig. 3), but no significant effects of face shapes (F(2,50) = 1.550, p = 0.226), nor of HIV status (F(1,25) = 1.310, p = 0.263). Post-hoc analyses revealed a trend of release from adaptation from M3 to M6/9 conditions in HIV-negative controls (p < 0.037, one-tailed t-test), but not in HIV-positive individuals (p > 0.621, one-tailed t-test). These results suggest a broadened neuronal tuning to morphed face in the FFA of HIV-positive middle-aged women, which in turn suggests a significant decrease in neural specificity, despite a comparable face discrimination performance between the HIV-positive and HIV-negative participants (Fig. 3B).
4.5. Local heterogeneity of voxel-wise correlations (Hcorr) confirmed the reduction in neural specificity in the FFA of HIV-positive patients
The data from the fMRI-RA experiment revealed a significant decrease in neural specificity in the FFA of middle-aged HIV-positive participants, in the absence of behavioral impairments. Here we sought to independently confirm this finding using the novel fMRI data analysis technique, local regional heterogeneity analysis, or Hcorr, which we recently developed to estimate neural specificity. Indeed, this novel analysis revealed a trend of difference in Hcorr between the two groups (p < 0.126, two tailed t-test, Bonferroni corrected for multiple comparisons of three main fMRI data analyses as shown in Fig. 3). However, removing one outlier HIV+ subject whose Hcorr is more than 4 standard deviation from the mean of HIV+ group revealed a highly significant difference between the two groups (p < 0.05, corrected), confirming an early decrease in neural specificity among middle-aged HIV-positive women, despite being on HAART with well-controlled disease (see Table 1). While the two independent techniques with independent fMRI data sets revealed converging results, the differences between the two groups are relatively small, i.e., the group difference in Hcorr was non-significant with a Bonferroni correction for multiple comparisons. Such a relatively weak difference suggests that neuronal dysfunction in these HIV-positive individuals are subtle and might be at early stage of disease, in line with their “normal” behavioral performance in discriminating face shapes (Fig. 1). Therefore, future studies are needed to validate and advance this novel Hcorr technique and its application in studying HAND with behaviorally impaired HIV-positive patients, with whom a larger difference in Hcorr and fMRI-adaptation is expected.
5. Discussion
There is a high prevalence of mild forms of HAND in the cART era (Heaton et al., 2010), that has been associated with increased risk of virologic failure, symptomatic cognitive impairments, and mortality (Ellis and Deutsch, 1997; Grant et al., 2014). Therefore there is pressing need to find biomarkers that can identify neural targets for early and more effective treatments (especially in those without a behavioral deficit yet), in order to prevent progression to symptomatic HAND and to preserve and improve neurocognitive functions. Using two different fMRI techniques, fMRI-RA and Hcorr, we provided converging evidence that neural specificity (or the specificity of neuronal tuning) is able to detect neuronal dysfunction in HIV-positive women, prior to the onset of behavioral deficits. These results suggest that neural specificity could have a potential to serve as a non-invasive biomarker of HAND disease progression, especially when behavioral assays cannot detect the changes.
Previous fMRI studies have found that neural specificity is sensitive to training/learning (with an increase in neural specificity along with an increase in behavioral performance) (Jiang et al., 2007), disease (with a decrease in neural specificity along with a decrease in behavioral performance) (Jiang et al., 2013), and aging (Goh et al., 2010; Lee et al., 2011). In particular, neural specificity has been shown to be a sensitive measure of cognitive aging being lower in older compared to younger adults (Goh et al., 2010; Lee et al., 2011). It has been proposed that the reduction in neural specificity in the aged brain might reflect an age-related neural dedifferentiation (Li et al., 2001), that is, neurons in the aged brain become less selective thus less able to support the processing of stimuli with subtle differences, which eventually lead to a decrease in cognitive performance. Data from single-unit recording studies of animals (Leventhal et al., 2003) and neuroimaging studies of human subjects (Goh et al., 2010; Lee et al., 2011) have provided direct experimental support to the age-related neural dedifferentiation theory. In particular, using a paradigm similar to the present study, a recent cognitive aging study (Goh et al., 2010) has found that, despite comparable behavioral performance in discriminating faces with parametric shape changes between young and older adults, the neural specificity in the FFA of older adults was reduced. These earlier findings suggest that, in healthy older adults, a reduction in neural specificity might precede the onset of detectable behavioral impairments. Interestingly, we also found a reduced neural specificity in the FFA of HIV+ women in the absence of behavioral deficits, suggesting that the early pathological changes among these middle-aged HIV+ individuals might resemble those often observed in the elderly adults, consistent with the HIV-induced accelerated/premature aging hypothesis (Deeks, 2011; Ernst and Chang, 2004; Guaraldi et al., 2011; High et al., 2012; Martin and Volberding, 2010; Meir-Shafrir and Pollack, 2012; Önen and Overton, 2011; Smith et al., 2012). These early neuronal dysfunctions — reduced neural specificity, when combined with additional aging-related neural dedifferentiation, might be responsible for the lower cognitive reserve (van Gorp et al., 1994) and higher risk of cognitive impairments (Deeks, 2011; Martin and Volberding, 2010; Meir-Shafrir and Pollack, 2012) among older HIV+ adults.
While fMRI-RA is currently the standard fMRI technique to estimate neural specificity, it requires a lengthy scanning time (as about 30–40 min in the present study), which significantly limits the feasibility of using it in a typical clinical setting. In contrast, we recently developed a novel fMRI data analysis technique, local regional heterogeneity analysis, or Hcorr, to estimate neural specificity (Jiang et al., 2013). This novel technique is motivated by findings from monkey training studies showing that learning produces sparser codes, with neurons after training responding to fewer stimuli, along with improved discrimination performance (Freedman et al., 2006, 2006; Kobatake et al., 1998). We have shown that this novel Hcorr technique is able to estimate neural specificity with fMRI data from a merely 5-minute scan, as in the present and our previous studies (Jiang et al., 2013), making it a more feasible and convenient clinical diagnostic tool to estimate neural specificity than fMRI-RA in routine clinical practice. Another unique advantage that Hcorr has over fMRI-RA is that Hcorr has the potential to probe neural specificity at any functionally or anatomically defined brain region using a single data set, including resting state scans. More specifically, because previous single unit recording studies (Bair et al., 2001; Jermakowicz et al., 2009; Lin et al., 2014) found that pair-wise correlations between two neurons in the presence of visual stimuli can be detected in the absence of stimuli, thus the voxel-wise correlations and Hcorr within a given region should be similar with or without a task. Similar neural mechanisms might also underlie the findings from fMRI studies of resting state (Fox and Raichle, 2007; Smith et al., 2009). This unique feature makes Hcorr an ideal tool to examine widespread pathological changes in diseases like HAND (Clifford and Ances, 2013; Lindl et al., 2010). However, future studies are needed to verify and validate this novel Hcorr technique in studying HAND as the difference in Hcorr in the present study did not survive correction for multiple comparisons. The relatively weak effect might be due to the following factors: i) FFA might not be the mostly affected brain regions due to HIV-infection, therefore it would be interesting to examine neural specificity in other more clinically relevant brain regions in future studies, such as hippocampus (our preliminary results indeed reveal a decrease in neural specificity in the hippocampus of HIV-positive women); ii) the neuronal dysfunction at FFA is still subtle in these HIV-positive women, as indicated by their “normal” behavioral performance in face discrimination, therefore future studies are needed to examine changes in neural specificity in HIV-positive individuals with behavioral symptoms, with whom we predict a more significant decrease in neural specificity; and iii) the HIV-positive population is rather heterogeneous, i.e., removing one outlier HIV+ subject whose Hcorr is more than 4 standard deviation from the mean of HIV+ group dramatically improved the significance in group comparison, therefore a future study with a larger sample size should be conducted to validate and advance the novel Hcorr technique and its application in HAND. Nevertheless, our results thus suggest that Hcorr can be an excellent choice of clinical tool to estimate the decrease in neural specificity in individuals with HIV-infection, especially in those with no or mild clinical symptoms of HAND.
However, one key question remains to be addressed by future studies: what is/are the driving force(s) of the decrease in neural specificity among HIV+ adults? Computational simulation and theoretical works of cognitive aging have proposed that increased neuronal noise due to reduced dopamine activity (Li et al., 2001) and decreased neuronal selectivity due to age-related synaptic injury (Morrison and Baxter, 2012) might be responsible for age-related reduction in neural specificity. Coincidently, HIV infection is known to impair dopamine transporter functions (Purohit et al., 2011) and synaptic functions (Atluri et al., 2013; Ellis et al., 2007; Ellis, 2010; Everall et al., 1999; Masliah et al., 1992; Nath and Steiner, 2014). For instance, dopamine reduction has been found in the HIV-infected brain (Wang et al., 2004) and is related to the concentration of HIV-1 RNA (Kumar et al., 2009) and cognitive impairments (Nath et al., 2000). The synaptodendritic injury and its manifestation have been proposed as the key contributor to the neurocognitive impairments among HIV-infected adults in the cART era (Ellis et al., 2007). Based on data from the present and earlier studies, we propose that, similar to HIV-negative older adults, the reduced neural specificity among HIV+ individuals might be due to increased neuronal noise resulting from impaired dopamine system and decreased neuronal selectivity due to synaptodendritic injury; and the reduction in neural specificity might serve as a biomarker to identify brain regions that are most vulnerable to HIV infection (i.e., regions with highest reduction in neural specificity) and individuals at risk of symptomatic neurocognitive impairments. However, we acknowledge that there is no experimental proof support of our novel hypothesis. In addition, the relationship between dopamine activity, synaptodendritic injury, neural specificity, and neurocognitive functions is not clear nor straightforward. For instance, in contrast to the unidirectional decease of dopamine activity due to aging, HIV infection could also lead to an elevated levels of dopamine in the dopaminergic synapses in the early asymptomatic stage of HIV infection (Scheller et al., 2010). Therefore, it remains to be tested whether there is a change in neural specificity right after seroconversion, as previous studies have suggested the presence of neuronal dysfunction in the acute and early period of infection (Doyle et al., 2013), thus future interdisciplinary studies are needed to relate fMRI (especially Hcorr) measured neural specificity to neuronal dysfunction such as dopamine activity, synaptodendritic injury, and ultimately to neurocognitive functions, in order to build a strong foundation for the clinical applications of this novel and non-invasive biomarker using the Hcorr technique. Furthermore, future studies are also needed to confirm whether reduced neural specificity, as well as the novel Hcorr technique, can be used to examine early neuronal dysfunction in other HIV+ patients, including middle-aged and older men with HIV-infection, and in other brain regions such as hippocampus. In addition, we predict that the reduction in neural specificity might be more significant in HIV+ individuals with detectable behavioral impairments. However, such a prediction needs to be verified with at least three groups of participants, HIV+ individuals with or without measurable behavioral impairments, and negative controls.
In summary, our study revealed a lower neural specificity in cognitively normal HIV+ women compared to matched HIV− women, in the absence of behavioral deficits, and this change in neural specificity can be detected via both Hcorr and fMRI-RA techniques. Furthermore, the novel Hcorr technique could be used as a clinical tool to assess the risk/severity of HAND, due to its simplicity, robustness, and high sensitivity.
Conflict of interest
Georgetown University has filed a patent application on the technology described in this paper, and Xiong Jiang, PhD, is a co-inventor on the patent.
Acknowledgments
We thank Pilar Hamilton for assistance in data collection, Lakshmi Goparaju for assistance with subject recruitment, Volker Blanz for the morphed face stimuli, Maximilian Riesenhuber for the stimuli used in the functional localizer scans, and Michael Plankey and Seble Kassaye for helpful comments and suggestions. We thank all the participants in this study for generously helping us in this research. This study was supported by a NEW Investigator Award from the District of Columbia Developmental Center for AIDS Research Award Number P30AI087714, a Transitional Award from the Department of Medicine, Georgetown University, and the Women's Interagency HIV Study (WIHS), Georgetown University (NIAID award 5U01AI069494). We also appreciate the support of the Intellectual and Development Disorders Research Center (IDDRC) grant: 5P30HD040677-13.
References
- Ances B.M., Ortega M., Vaida F., Heaps J., Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J. Acquir. Immune Defic. Syndr. 2012;59(5):469–477. doi: 10.1097/QAI.0b013e318249db17. 22269799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances B.M., Roc A.C., Korczykowski M., Wolf R.L., Kolson D.L. Combination antiretroviral therapy modulates the blood oxygen level-dependent amplitude in human immunodeficiency virus-seropositive patients. J. Neurovirol. 2008;14(5):418–424. doi: 10.1080/13550280802298112. 19040188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri V.S.R., Kanthikeel S.P., Reddy P.V.B., Yndart A., Nair M.P.N. Human synaptic plasticity gene expression profile and dendritic spine density changes in HIV-infected human CNS cells: role in HIV-associated neurocognitive disorders (HAND) PLOS One. 2013;8(4) doi: 10.1371/journal.pone.0061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon M.C., von Wyl V., Alden C., Sharp G., Robison E., Hessol N., Gange S., Barranday Y., Holman S., Weber K., Young M.A. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin. Diagn. Lab. Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. 16148165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair W., Zohary E., Newsome W.T. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J. Neurosci. 2001;21(5):1676–1697. doi: 10.1523/JNEUROSCI.21-05-01676.2001. 11222658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan S.E., Melnick S.L., Preston-Martin S., Weber K., Kalish L.A., Miotti P., Young M., Greenblatt R., Sacks H., Feldman J. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. 9504278 [PubMed] [Google Scholar]
- Blanz V., Vetter T. A Morphable Model for the Synthesis of 3D Faces. ACM Press/Addison-Wesley Publishing Co.; 1999. pp. 187–194. [Google Scholar]
- Buracas G.T., Boynton G.M. Efficient design of event-related fMRI experiments using M-sequences. Neuroimage. 2002;16(3 1):801–813. doi: 10.1006/nimg.2002.1116. 12169264 [DOI] [PubMed] [Google Scholar]
- Castelo J.M., Sherman S.J., Courtney M.G., Melrose R.J., Stern C.E. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. 16769942 [DOI] [PubMed] [Google Scholar]
- Chang L., Andres M., Sadino J., Jiang C.S., Nakama H., Miller E., Ernst T. Impact of apolipoprotein E ε4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage. 2011;58(4):1017–1027. doi: 10.1016/j.neuroimage.2011.07.010. 21803164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Speck O., Miller E.N., Braun J., Jovicich J., Koch C., Itti L., Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57(6):1001–1007. doi: 10.1212/wnl.57.6.1001. 11571324 [DOI] [PubMed] [Google Scholar]
- Ciborowski P. Biomarkers of HIV-1-associated neurocognitive disorders: challenges of proteomic approaches. Biomark. Med. 2009;3(6):771–785. doi: 10.2217/bmm.09.63. 20477714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford D.B., Ances B.M. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013;13(11):976–986. doi: 10.1016/S1473-3099(13)70269-X. 24156898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. 21090961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps M., Hyare H., Stebbing J., Winston A. Magnetic resonance imaging and spectroscopy of the brain in HIV disease. J. HIV Ther. 2008;13(3):55–58. 19039299 [PubMed] [Google Scholar]
- Doyle K.L., Morgan E.E., Morris S., Smith D.M., Little S., Iudicello J.E., Blackstone K., Moore D.J., Grant I., Letendre S.L., Woods S.P., Translational Methamphetamine AIDS Research Center (TMARC) Group Real-world impact of neurocognitive deficits in acute and early HIV infection. J. Neurovirol. 2013;19(6):565–573. doi: 10.1007/s13365-013-0218-2. 24277439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. HIV and antiretroviral therapy: impact on the central nervous system. Prog. Neurobiol. 2010;91(2):185–187. doi: 10.1016/j.pneurobio.2009.10.016. 19857545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R., Langford D., Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 2007;8(1):33–44. doi: 10.1038/nrn2040. 17180161 [DOI] [PubMed] [Google Scholar]
- Ellis R.J., Deutsch R., Heaton R.K., Marcotte T.D., McCutchan J.A., Nelson J.A., Abramson I., Thal L.J., Atkinson J.H., Wallace M.R., Grant I. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch. Neurol. 1997;54(4):416–424. doi: 10.1001/archneur.1997.00550160054016. 9109743 [DOI] [PubMed] [Google Scholar]
- Ernst T., Chang L. Effect of aging on brain metabolism in antiretroviral-naive HIV patients. AIDS. 2004;18(Suppl. 1):S61–S67. 15075499 [PubMed] [Google Scholar]
- Everall I.P., Heaton R.K., Marcotte T.D., Ellis R.J., McCutchan J.A., Atkinson J.H., Grant I., Mallory M., Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9(2):209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. 10219738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.S. Biomarkers for NeuroAIDS: recent progress in the field. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2013;8(5):1055–1058. doi: 10.1007/s11481-013-9515-z. 24292958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. 17704812 [DOI] [PubMed] [Google Scholar]
- Freedman D.J., Riesenhuber M., Poggio T., Miller E.K. Experience-dependent sharpening of visual shape selectivity in inferior temporal cortex. Cereb. Cortex. 2006;16(11):1631–1644. doi: 10.1093/cercor/bhj100. 16400159 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D., Turner R., Frackowiak R.S. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2(2):157–165. doi: 10.1006/nimg.1995.1018. 9343598 [DOI] [PubMed] [Google Scholar]
- Gelman B.B., Lisinicchia J.G., Morgello S., Masliah E., Commins D., Achim C.L., Fox H.S., Kolson D.L., Grant I., Singer E., Yiannoutsos C.T., Sherman S., Gensler G., Moore D.J., Chen T., Soukup V.M. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J. Acquir. Immune Defic. Syndr. 2013;62(5):487–495. doi: 10.1097/QAI.0b013e31827f1bdb. 23242157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilaie-Dotan S., Malach R. Sub-exemplar shape tuning in human face-related areas. Cereb. Cortex. 2007;17(2):325–338. doi: 10.1093/cercor/bhj150. 16525131 [DOI] [PubMed] [Google Scholar]
- Goh J.O., Suzuki A., Park D.C. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 2010;51(1):336–344. doi: 10.1016/j.neuroimage.2010.01.107. 20139012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I. Neurocognitive disturbances in HIV. Int. Rev. Psychiatry. 2008;20(1):33–47. doi: 10.1080/09540260701877894. 18240061 [DOI] [PubMed] [Google Scholar]
- Grant I., Franklin D.R., Jr, Deutsch R., Woods S.P., Vaida F., Ellis R.J., Letendre S.L., Marcotte T.D., Atkinson J.H., Collier A.C., Marra C.M., Clifford D.B., Gelman B.B., McArthur J.C., Morgello S., Simpson D.M., McCutchan J.A., Abramson I., Gamst A., Fennema-Notestine C., Smith D.M., Heaton R.K. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82:2055–2062. doi: 10.1212/WNL.0000000000000492. 24814848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. 16321563 [DOI] [PubMed] [Google Scholar]
- Guaraldi G., Orlando G., Zona S., Menozzi M., Carli F., Garlassi E., Berti A., Rossi E., Roverato A., Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clinical Infectious Diseases. 2011;53(11):1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- Haddow L.J., Floyd S., Copas A., Gilson R.J.C. A systematic review of the screening accuracy of the HIV Dementia Scale and International HIV Dementia Scale. PLOS One. 2013;8(4) doi: 10.1371/journal.pone.0061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J., Buchthal S., Taylor M., Schifitto G., Zhong J., Daar E., Alger J., Singer E., Campbell T., Yiannoutsos C., Cohen R., Navia B., HIV Neuroimaging Consortium Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. 21297425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbun R., Eraso J., Ramireddy S., Wainwright D.A., Salazar L., Grimes R., York M., Strutt A. Screening for neurocognitive impairment in HIV individuals: the utility of the Montreal cognitive assessment test. J. Aids Clin. Res. 2012;3(10):186. doi: 10.4172/2155-6113.1000186. 23956944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V., Ungerleider L.G., Clark V.P., Schouten J.L., Hoffman E.A., Martin A. The effect of face inversion on activity in human neural systems for face and object perception. Neuron. 1999;22(1):189–199. doi: 10.1016/s0896-6273(00)80690-x. 10027301 [DOI] [PubMed] [Google Scholar]
- Heaton R.K., Franklin D.R., Ellis R.J., McCutchan J.A., Letendre S.L., LeBlanc S., Corkran S.H., Duarte N.A., Clifford D.B., Woods S.P., Collier A.C., Marra C.M., Morgello S., Mindt M.R., Taylor M.J., Marcotte T.D., Atkinson J.H., Wolfson T., Gelman B.B., McArthur J.C., Simpson D.M., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T.L., Wong J., Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. 21174240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Clifford D.B., Franklin D.R., Woods S.P., Ake C., Vaida F., Ellis R.J., Letendre S.L., Marcotte T.D., Atkinson J.H., Rivera-Mindt M., Vigil O.R., Taylor M.J., Collier A.C., Marra C.M., Gelman B.B., McArthur J.C., Morgello S., Simpson D.M., McCutchan J.A., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T.L.L., Wong J., Grant I., For the CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. 21135382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- High K.P., Brennan-Ing M., Clifford D.B., Cohen M.H., Currier J., Deeks S.G., Deren S., Effros R.B., Gebo K., Goronzy J.J., Justice A.C., Landay A., Levin J., Miotti P.G., Munk R.J., Nass H., Rinaldo C.R., Shlipak M.G., Tracy R., Valcour V., Vance D.E., Walston J.D., Volberding P. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J. Acquir. Immune Defic. Syndr. 2012;60(Suppl. 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. 22688010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J.L., Kraft-Terry S.D., Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J. Neurovirol. 2012;18(4):291–302. doi: 10.1007/s13365-012-0114-1. 22653528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermakowicz W.J., Chen X., Khaytin I., Bonds A.B., Casagrande V.A. Relationship between spontaneous and evoked spike-time correlations in primate visual cortex. J. Neurophysiol. 2009;101(5):2279–2289. doi: 10.1152/jn.91207.2008. 19211656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Bollich A., Cox P., Hyder E., James J., Gowani S.A., Hadjikhani N., Blanz V., Manoach D.S., Barton J.J., Gaillard W.D., Riesenhuber M. A quantitative link between face discrimination deficits and neuronal selectivity for faces in autism. Neuroimage Clin. 2013;2:320–331. doi: 10.1016/j.nicl.2013.02.002. 24179786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Bradley E., Rini R.A., Zeffiro T., Vanmeter J., Riesenhuber M. Categorization training results in shape- and category-selective human neural plasticity. Neuron. 2007;53(6):891–903. doi: 10.1016/j.neuron.2007.02.015. 17359923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Rosen E., Zeffiro T., VanMeter J., Blanz V., Riesenhuber M. Evaluation of a shape-based model of human face discrimination using fMRI and behavioral techniques. Neuron. 2006;50(1):159–172. doi: 10.1016/j.neuron.2006.03.012. 16600863 [DOI] [PubMed] [Google Scholar]
- Kallianpur K.J., Kirk G.R., Sailasuta N., Valcour V., Shiramizu B., Nakamoto B.K., Shikuma C. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb. Cortex. 2012;22(9):2065–2075. doi: 10.1093/cercor/bhr285. 22016479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. 9151747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361(1476):2109–2128. doi: 10.1098/rstb.2006.1934. 17118927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobatake E., Wang G., Tanaka K. Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. J. Neurophysiol. 1998;80(1):324–330. doi: 10.1152/jn.1998.80.1.324. 9658053 [DOI] [PubMed] [Google Scholar]
- Kourtzi Z., Kanwisher N. Representation of perceived object shape by the human lateral occipital complex. Science. 2001;293(5534):1506–1509. doi: 10.1126/science.1061133. 11520991 [DOI] [PubMed] [Google Scholar]
- Kumar A.M., Fernandez J.B., Singer E.J., Commins D., Waldrop-Valverde D., Ownby R.L., Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J. Neurovirol. 2009;15(3):257–274. doi: 10.1080/13550280902973952. 19499455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küper M., Rabe K., Esser S., Gizewski E.R., Husstedt I.W., Maschke M., Obermann M. Structural gray and white matter changes in patients with HIV. J. Neurol. 2011;258(6):1066–1075. doi: 10.1007/s00415-010-5883-y. 21207051 [DOI] [PubMed] [Google Scholar]
- Lee Y., Grady C.L., Habak C., Wilson H.R., Moscovitch M. Face processing changes in normal aging revealed by fMRI adaptation. J. Cogn. Neurosci. 2011;23(11):3433–3447. doi: 10.1162/jocn_a_00026. 21452937 [DOI] [PubMed] [Google Scholar]
- Leventhal A.G., Wang Y., Pu M., Zhou Y., Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300(5620):812–815. doi: 10.1126/science.1082874. 12730605 [DOI] [PubMed] [Google Scholar]
- Li S.C., Lindenberger U., Sikström S. Aging cognition: from neuromodulation to representation. Trends Cogn. Sci. 2001;5(11):479–486. doi: 10.1016/s1364-6613(00)01769-1. 11684480 [DOI] [PubMed] [Google Scholar]
- Lin C.-P., Chen Y.-P., Hung C.P. Tuning and spontaneous spike time synchrony share a common structure in macaque inferior temporal cortex. J. Neurophysiol. 2014;112:856–869. doi: 10.1152/jn.00485.2013. 24848472 [DOI] [PubMed] [Google Scholar]
- Lindl K.A., Marks D.R., Kolson D.L., Jordan-Sciutto K.L. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2010;5(3):294–309. doi: 10.1007/s11481-010-9205-z. 20396973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki P.M., Cohen M.H., Weber K., Little D.M., Fornelli D., Rubin L.H., Perschler P., Gould F., Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72(19):1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. 19433739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte T.D., Deutsch R., Michael B.D., Franklin D., Cookson D.R., Bharti A.R., Grant I., Letendre S.L. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J. Neuroimmune Pharmacol. 2013;8(5):1123–1135. doi: 10.1007/s11481-013-9504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Volberding P. HIV and premature aging: a field still in its infancy. Ann. Intern. Med. 2010;153(7):477–479. doi: 10.7326/0003-4819-153-7-201010050-00013. 20921548 [DOI] [PubMed] [Google Scholar]
- Masliah E., Achim C.L., Ge N., DeTeresa R., Terry R.D., Wiley C.A. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann. Neurol. 1992;32(3):321–329. doi: 10.1002/ana.410320304. 1416802 [DOI] [PubMed] [Google Scholar]
- McArthur J.C. HIV dementia: an evolving disease. J. Neuroimmunol. 2004;157(1–2):3–10. doi: 10.1016/j.jneuroim.2004.08.042. 15579274 [DOI] [PubMed] [Google Scholar]
- McArthur J.C., Sacktor N., Selnes O. Human immunodeficiency virus-associated dementia. Semin. Neurol. 1999;19(2):129–150. doi: 10.1055/s-2008-1040831. 10718534 [DOI] [PubMed] [Google Scholar]
- Meir-Shafrir K., Pollack S. Accelerated aging in HIV patients. Rambam Maimonides Med. J. 2012;3(4):e0025. doi: 10.5041/RMMJ.10089. 23908849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose R.J., Tinaz S., Castelo J.M., Courtney M.G., Stern C.E. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav. Brain Res. 2008;188(2):337–347. doi: 10.1016/j.bbr.2007.11.021. 18242723 [DOI] [PubMed] [Google Scholar]
- Moore D.J., Roediger M.J., Eberly L.E., Blackstone K., Hale B., Weintrob A., Ganesan A., Agan B.K., Letendre S.L., Crum-Cianflone N.F. Identification of an abbreviated test battery for detection of HIV-associated neurocognitive impairment in an early-managed HIV-infected cohort. PLOS One. 2012;7(11):e47310. doi: 10.1371/journal.pone.0047310. 23144815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J.H., Baxter M.G. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012;13(4):240–250. doi: 10.1038/nrn3200. 22395804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A., Anderson C., Jones M., Maragos W., Booze R., Mactutus C., Bell J., Hauser K.F., Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J. Psychopharmacol. 2000;14(3):222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A., Steiner J. Synaptodendritic injury with HIV-Tat protein: what is the therapeutic target? Exp. Neurol. 2014;251:112–114. doi: 10.1016/j.expneurol.2013.11.004. 24246278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önen N.F., Overton E.T. A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Curr. Aging Sci. 2011;4(1):33–41. 21204781 [PubMed] [Google Scholar]
- Purohit V., Rapaka R., Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol. Neurobiol. 2011;44(1):102–110. doi: 10.1007/s12035-011-8195-z. 21717292 [DOI] [PubMed] [Google Scholar]
- Sakamoto M., Marcotte T.D., Umlauf A., Franklin D. Jr, Heaton R.K., Ellis R.J., Letendre S., Alexander T., McCutchan J.A., Morgan E.E., Woods S.P., Collier A.C., Marra C.M., Clifford D.B., Gelman B.B., McArthur J.C., Morgello S., Simpson D., Grant I., CHARTER Group Concurrent classification accuracy of the HIV dementia scale for HIV-associated neurocognitive disorders in the CHARTER cohort. J. Acquir. Immune Defic. Syndr. 2013;62(1):36–42. doi: 10.1097/QAI.0b013e318278ffa4. 23111573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma M.K., Nagarajan R., Keller M.A., Kumar R., Nielsen-Saines K., Michalik D.E., Deville J., Church J.A., Thomas M.A. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. Neuroimage. Clin. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. 24380059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C., Arendt G., Nolting T., Antke C., Sopper S., Maschke M., Obermann M., Angerer A., Husstedt I.W., Meisner F., Neuen-Jacob E., Müller H.W., Carey P., Ter Meulen V., Riederer P., Koutsilieri E. Increased dopaminergic neurotransmission in therapy-naïve asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J. Neural Transm. 2010;117(6):699–705. doi: 10.1007/s00702-010-0415-6. 20454983 [DOI] [PubMed] [Google Scholar]
- Schouten J., Cinque P., Gisslen M., Reiss P., Portegies P. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS. 2011;25(5):561–575. doi: 10.1097/QAD.0b013e3283437f9a. 21160410 [DOI] [PubMed] [Google Scholar]
- Simioni S., Cavassini M., Annoni J.-M., Rimbault Abraham A., Bourquin I., Schiffer V., Calmy A., Chave J.-P., Giacobini E., Hirschel B., Du Pasquier R.A. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. 19996937 [DOI] [PubMed] [Google Scholar]
- Skinner S., Adewale A.J., DeBlock L., Gill M.J., Power C. Neurocognitive screening tools in HIV/AIDS: comparative performance among patients exposed to antiretroviral therapy. HIV Med. 2009;10(4):246–252. doi: 10.1111/j.1468-1293.2008.00679.x. 19187172 [DOI] [PubMed] [Google Scholar]
- Smith R.L., de Boer R., Brul S., Budovskaya Y., van Spek H. Premature and accelerated aging: HIV or HAART? Front. Genet. 2012;3:328. doi: 10.3389/fgene.2012.00328. 23372574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. 19620724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow J., Ingeholm J.E., Levy I.F., Caravella R.A., Case L.K., Wallace G.L., Martin A. Impaired visual scanning and memory for faces in high-functioning autism spectrum disorders: it's not just the eyes. J. Int. Neuropsychol. Soc. JINS. 2011;17(6):1021–1029. doi: 10.1017/S1355617711000981. 21892988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S.S., Ances B.M. Neurologic complications of HIV infection. Top. Antivir. Med. 2012;20(2):41–47. 22710906 [PMC free article] [PubMed] [Google Scholar]
- Towgood K.J., Pitkanen M., Kulasegaram R., Fradera A., Kumar A., Soni S., Sibtain N.A., Reed L., Bradbeer C., Barker G.J., Kopelman M.D. Mapping the brain in younger and older asymptomatic HIV-1 men: frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex. 2012;48(2):230–241. doi: 10.1016/j.cortex.2011.03.006. 21481856 [DOI] [PubMed] [Google Scholar]
- Valcour V., Paul R., Chiao S., Wendelken L.A., Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clin. Infect. Dis. 2011;53(8):836–842. doi: 10.1093/cid/cir524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V.G. Evaluating cognitive impairment in the clinical setting: practical screening and assessment tools. Top. Antivir. Med. 2011;19(5):175–180. 22298886 [PMC free article] [PubMed] [Google Scholar]
- Van Gorp W.G., Miller E.N., Marcotte T.D., Dixon W., Paz D., Selnes O., Wesch J., Becker J.T., Hinkin C.H., Mitrushina M. The relationship between age and cognitive impairment in HIV-1 infection: findings from the Multicenter AIDS Cohort Study and a clinical cohort. Neurology. 1994;44(5):929–935. doi: 10.1212/wnl.44.5.929. 8190299 [DOI] [PubMed] [Google Scholar]
- Wang G.-J., Chang L., Volkow N.D., Telang F., Logan J., Ernst T., Fowler J.S. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain J. Neurol. 2004;127(11):2452–2458. doi: 10.1093/brain/awh269. 15319273 [DOI] [PubMed] [Google Scholar]
- Wiley C.A., Masliah E., Morey M., Lemere C., DeTeresa R., Grafe M., Hansen L., Terry R. Neocortical damage during HIV infection. Ann. Neurol. 1991;29(6):651–657. doi: 10.1002/ana.410290613. 1909852 [DOI] [PubMed] [Google Scholar]