Abstract
Spontaneous coronary artery dissection (SCAD) is an infrequent cause of acute coronary syndrome (ACS). Conservative management is typically recommended but revascularization may be necessary if ongoing ischemia or adverse anatomical characteristics are present. Percutaneous coronary intervention (PCI) of SCAD can be fraught with challenges, and intracoronary imaging with optical coherence tomography (OCT) may provide insights on optimizing the acute results and identify long-term stent-related adverse events. We report three cases of SCAD treated with drug-eluting stents (DES) with OCT follow-up showing stent mal-apposition at different stages of follow-up. The clinical significance of these OCT findings and management options are discussed.
Keywords: Angiography, arterial occlusive disease, coronary artery disease, optical coherence tomography (OCT), percutaneous coronary intervention (PCI)
Introduction
Spontaneous coronary artery dissection (SCAD) is an infrequent cause of acute coronary syndrome (ACS), involving separation of the intimal-medial arterial wall. Conservative management is typically recommended but revascularization may be necessary for ongoing ischemia or adverse anatomical characteristics. Percutaneous coronary intervention (PCI) may be challenging with SCAD and have suboptimal outcomes. Furthermore, resorption of the intramural hematoma (IMH) with healing can result in stent mal-apposition. We report three cases of SCAD treated with drug-eluting stents (DES) with optical coherence tomography (OCT) follow-up at different stages showing stent mal-apposition and their management/outcomes.
Case series
Case 1
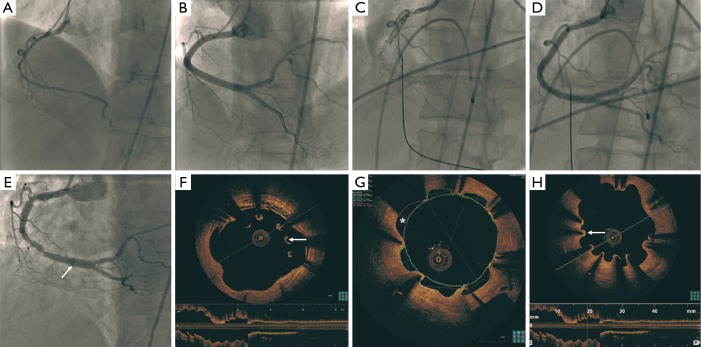
A 60-year-old woman with dyslipidemia and remote smoking was admitted with non-ST-elevation myocardial infarction (NSTEMI) in August 2007. Her coronary angiogram showed diffuse dissection of the proximal to distal right coronary artery (RCA) (Figure 1A). She underwent extensive stenting of the RCA with six cypher stents (total length 128 mm) (Figure 1B). In December 2010, she had very late stent thrombosis (Figure 1C) complicated by cardiogenic shock and heart block. She was treated with balloon angioplasty and discharged on long-term prasugrel and aspirin (Figure 1D). Repeat angiography in August 2013 showed patent RCA stents, but she had diffused peri-stent staining (Figure 1E). OCT showed large variations of the lumen area, with large arcs of mal-apposition (990 microns) (Figure 1F), evaginations (outpouching) reaching 2.5 mm2, and multiple areas of uncovered stent struts (Figures 1G,H,2). The mal-apposition and evagination were presumably related to combination of hypersensitivity response and resorption of IMH. She underwent bypass surgery after consensus agreement of our cardiac team, to avoid life-long dual antiplatelet therapy (DAPT) and recurrent stent thrombosis.
Figure 1.
Diffuse dissection of the proximal to distal RCA (A); stenting of the RCA with six DES (B); very late stent thrombosis of the RCA (C) treated with balloon angioplasty 40 months later (D); patent RCA stents, but diffuse peri-stent staining (arrow) 32 months later (E); OCT showing large arcs of malapposition (arrow) (F); areas of evagination (*) reaching 2.5 mm2 (G); and multiple areas of uncovered stent struts (arrow) (H). RCA, right coronary artery; DES, drug-eluting stents; OCT, optical coherence tomography.
Figure 2.

OCT showing large arcs of malapposition, areas of evagination, and multiple areas of uncovered stent struts (1). OCT, optical coherence tomography. Available online: http://www.asvide.com/articles/619
Case 2
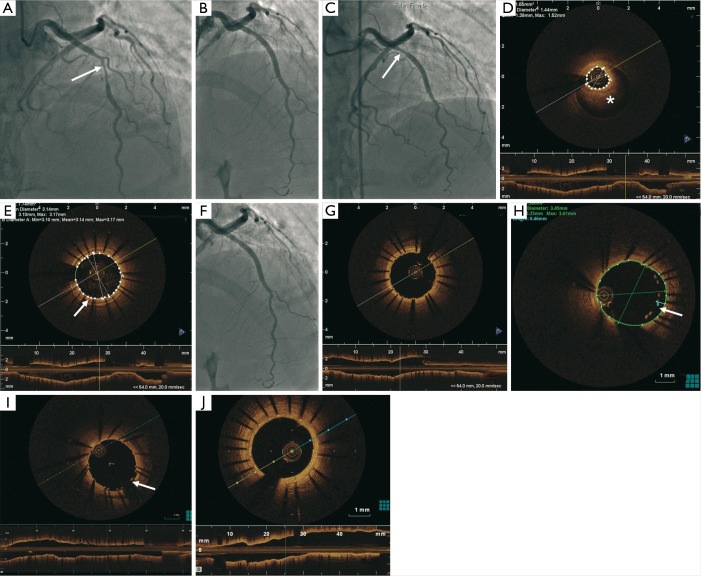
A 48-year-old woman with no cardiovascular risk factor presented with NSTEMI in April 2011 (Figure 3). Her electrocardiogram was unremarkable and the troponin-I peaked at 2.8 mcg/L. Coronary angiography showed a tubular 80% stenosis in the mid left anterior descending artery (LAD) (Figure 3A). She was treated with an Endeavor 3.0 mm × 24 mm DES (Figure 3B), but had recurrent chest pains prompting repeat angiography 9 days later. Her LAD stent was patent but a worsened luminal narrowing was noted proximal to the stent (Figure 3C). OCT showed IMH proximal to the stent (Figure 3D) corresponding to the stenosis observed on angiography, as well as tacked-up IMH in the wall of the stented segment (Figures 3E,4). This was treated with another Endeavor 3.0 mm × 15 mm (Figure 3F) with good stent expansion and apposition on OCT (Figure 3G). Two days later, she developed progressive retrosternal chest pain and she had T-wave inversions in the anterior leads. Repeat SCA showed patent LAD stents, but OCT revealed mal-apposition of the proximal stent at the previous IMH site, with gaps between the struts and intima reaching 460 microns, presumed related to IMH resorption (Figure 3H). To minimize stent mal-apposition, low-pressure balloon angioplasty with a 4.0 mm non-compliant balloon was performed with good final results on OCT, with residual gap of 270 microns (Figure 3I). Repeat angiography 30 months later showed patent LAD stents, with repeat OCT showing complete endothelialization of the stents (Figure 3J).
Figure 3.
Tubular 80% stenosis (arrow) in the mid LAD (A); PCI with a 3.0 mm × 24 mm DES (B); patent LAD stent but severe luminal narrowing proximal to the stent (arrow) 9 days later (C); OCT demonstrating IMH (*) proximal to the stent (D) and tacked-up IMH in the wall of the stented segment (arrow) (E); proximal IMH treated with 3.0 mm × 15 mm DES (F) with good stent expansion and apposition (G); OCT 2 days later revealed malapposition of the proximal LAD stent at the prior site of the IMH with gaps between the struts and the intima reaching 460 µm (arrow) (H); final results on OCT with residual gap of 270 µm (arrow) after balloon angioplasty (I); repeat OCT 30 months later showed complete endothelialization of the stent struts (J). LAD, left anterior descending; PCI, percutaneous coronary intervention; OCT, optical coherence tomography; IMH, intramural hematoma; DES, drug-eluting stents.
Figure 4.

OCT demonstrating IMH proximal to the stent and tacked-up IMH in the wall of the stented segment (2). OCT, optical coherence tomography; IMH, intramural hematoma. Available online: http://www.asvide.com/articles/620
Case 3
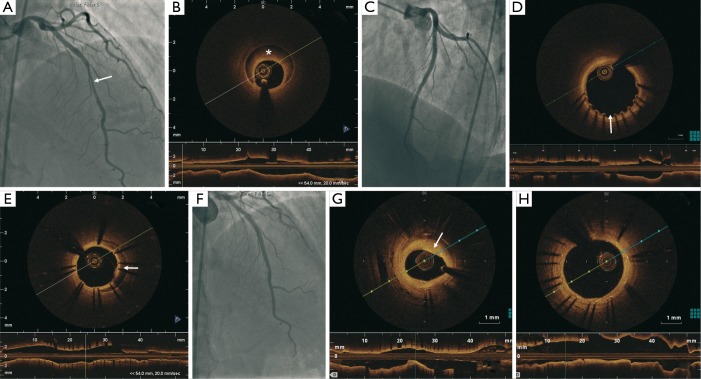
A 45-year-old woman with no cardiovascular risk factor presented with NSTEMI in April 2011 (Figure 5). Her electrocardiogram showed dynamic T-wave inversions and her troponin-I was elevated at 3.4 mcg/L. Coronary angiography demonstrated 50% lesion in the proximal LAD and a 70% tubular lesion in the mid LAD (Figure 5A). FFR of the LAD showed the lesions to be hemodynamically significant (0.78 with maximal hyperemia). OCT revealed a 40 mm coronary dissection extending from the proximal to mid LAD (Figure 5B). This was treated with 2 DES (Promus Element 3.0 mm × 38 mm and Endeavor 3.5 mm × 12 mm) (Figure 5C) using OCT and intravascular ultrasound (IVUS) guidance. She had recurrent atypical chest pains 15 days later, and repeat angiography showed patent stents, but there was mild mal-apposition at the proximal edge of the stents (250 microns) (Figure 5D). There was also persistent IMH covered by the stents (Figures 5E,6). She was managed conservatively since the mal-apposition was not deemed to be severe. Repeat coronary angiography 2 years later showed 50% in-stent restenosis in the distal segment of the stent (Figure 5F) with a minimal lumen area of 2.6 mm2 (Figure 5G). However, she did have complete endothelialization of the stent struts (Figure 5H). She also had mild fibromuscular dysplasia (FMD) of the distal right carotid artery, right vertebral and right external iliac arteries.
Figure 5.
A total of 50% lesion in the proximal LAD and 70% tubular lesion in the mid LAD (arrow) (A); LAD dissection with IMH (*) showed on OCT (B); results after PCI with two DES (C); OCT 15 days later showed patent stents but mild malapposition at the proximal edge of the proximal stent (250 µm) (arrow) (D) and persistent IMH covered by the stents (arrow) (E); 2 years later, 50% in-stent restenosis in the distal segment of the stent (F) with a minimal lumen area of 2.6 mm2 (G) and complete endothelialization of the stent struts (H). LAD, left anterior descending; IMH, intramural hematoma; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; DES, drug-eluting stents.
Figure 6.

OCT 15 days later showed patent stents but mild malapposition at the proximal edge of the proximal stent (250 µm) and persistent IMH covered by the stents (3). OCT, optical coherence tomography; IMH, intramural hematoma. Available online: http://www.asvide.com/articles/621
Discussion
SCAD is an infrequent cause of ACS affecting predominantly women between the ages of 30 to 70 and accounts for up to 4% of all ACS. The true prevalence of SCAD is underestimated and currently unknown (4). Dissection of the coronary intima or media is the hallmark finding and is often associated with IMH, with or without an intimal tear (5). The resulting IMH compresses the arterial lumen, compromising antegrade blood flow to varying degrees, and causes myocardial ischemia and infarction. Several arteriopathies have been reported in the pathogenesis of SCAD (2), and we recently showed that FMD is strongly associated with SCAD and may be a causative factor (6).
SCAD has been under-recognized and a high clinical index of suspicion is important to improve SCAD diagnosis during ACS presentations. Angiographically, SCAD can manifest as smooth-walled luminal compression without staining, especially when SCAD is limited to IMH without intimal disruption, and can mimic atherosclerotic stenosis or spasm. To aid diagnosis, Saw proposed an angiographic classification and diagnostic algorithm for SCAD (7). Type 1 angiographic SCAD represents the pathognomonic angiographic appearance with contrast dye staining of the arterial wall and multiple radiolucent lumen. Type 2 angiographic SCAD represents a diffuse smooth stenosis (typically >20 mm) of varying severity, often with an appreciable abrupt change in arterial calibre. This diffuse stenosis is often missed or misdiagnosed and may require adjunctive intracoronary imaging with IVUS or OCT to make a definitive diagnosis. Type 3 angiographic SCAD has an appearance that mimics atherosclerosis (typically focal or tubular stenosis), and requires intracoronary imaging for diagnosis.
The intracoronary imaging diagnosis of SCAD requires the identification of either IMH and/or double lumen. OCT is usually preferred for its superior spatial resolution of 10-20 microns. OCT can clearly delineate IMH from lipid or calcific lesions, true and false lumens, and even visualize intimal tears. IVUS has a lower spatial resolution (150 microns) and is suboptimal to visualize intimal tears, but can delineate true and false lumens well, identify IMH (homogenous collection behind the intimal-medial membrane), and visualize deeper segments of the vessel wall. Repeat coronary imaging after the initial SCAD presentation is often pursued to reassess the dissected segment, especially if the patient is symptomatic. We commonly repeat coronary angiography or computed tomography angiography 6-8 weeks after the initial event, which allows assessment of arterial healing and stent results. If PCI is performed, adjunctive intracoronary imaging should be considered. Determining the extent of dissection can influence stenting strategies. IVUS and OCT can also confirm guidewire position in the true lumen, and assess optimal stent apposition and expansion (8). At follow-up, OCT can visualize arterial healing and stent apposition well (9). Unfortunately, IVUS does not have optimal resolution to evaluate stent mal-apposition, thus OCT is preferred for such assessment (10). OCT may also be useful to delineate the mechanisms of mal-apposition with SCAD. Resorption of the IMH, vessel enlargement secondary to arterial wall disruption, or hypersensitivity reaction or vessel injury to DES may be responsible for late stent mal-apposition (11).
As shown in our cases, the healing pattern seen on OCT varied and may be related to the type of DES used, the PCI optimization approach, and the timing of imaging follow-up. In case 2, we utilized OCT to optimize stent strut mal-apposition (due to IMH resorption) at subacute follow-up, and long-term repeat OCT showed complete endothelialization at 30 months. In case 3, further angioplasty was not deemed necessary at subacute follow-up as the OCT did not demonstrate severe mal-apposition, and her late follow-up OCT also showed complete endothelialization. Whether the late strut apposition on OCT follow-up of these two cases in comparison to case 1 (severe late mal-apposition) was related to OCT optimization, or simply related to the choice of DES (Endeavor versus Cypher) remains speculative.
Peri-stent contrast staining (PSS) has been described after sirolimus-eluting stent implantation and appeared to be associated with subsequent target-lesion revascularization and very late stent thrombosis (12). PSS is due to evaginations, which are defined as outward bulges in the luminal contour between struts. They seem to be a specific morphological footprint of early-generation sirolimus-eluting stent. They appear to be related to vessel injury (e.g., intra-stent dissection, tissue prolapse) at baseline. As SCAD is associated with severe vessel wall injury by nature, this could represent a risk factor for evaginations. In terms of prognosis, evaginations correlated with mal-apposition and thrombus at follow-up, which are features associated with very late stent thrombosis. In our case, the resorption of IMH was thought to accentuate the degree of PSS observed (case 1), in addition to hypersensitivity to Cypher as causative factors.
The optimal management of SCAD remains relatively controversial and inadequately studied. In our experience, the decision to revascularize should take into account the patient’s clinical status and coronary anatomy. In most cases, conservative management is preferred for stable patients without ongoing pain/ischemia. In the presence of high-risk features (e.g., ongoing pain, ischemia, hemodynamic compromise, arrhythmia) and/or in cases with adverse anatomical characteristics (e.g., left main or proximal lesions, large vessel, abnormal flow), revascularization may be considered (13). With left main dissection, emergency CABG is generally preferred.
Unfortunately, PCI of dissected arteries can be challenging and may be associated with poor results and adverse outcomes. Tweet et al. reported a technical success rate of only 65% among 43 retrospective SCAD patients who underwent PCI (14). Our group also reported long-term durable success with PCI in only ~30% of our 168 patient SCAD cohort (13). The first challenge is to be able to advance the coronary guidewire into the distal true lumen. With angioplasty, the IMH can propagate antegradely or retrogradely, further compromising arterial blood flow and extending the dissection. Some authors advocated the use of cutting balloon to purposely disrupt the intima further (15). Nevertheless, the length of stents required is typically long due to extensive dissections. Furthermore, there is the potential risk of stent mal-apposition with resorption of the IMH resulting in increased stent thrombosis risk. The longer stents required also increase the risk of restenosis. Bioabsorbable vascular scaffold (BVS) has characteristics that could offer theoretical advantages over conventional metallic stents in SCAD cases. However, the use of BVS for SCAD is currently limited to case reports (16). Vessel scaffolding and drug-elution are limited in time (depending on BVS type), allowing vessel healing and eventual vasomotion. The temporary scaffold is absorbed months later, after the initial phase where the scaffolding is necessary to tack the intimo-medial disruption. In theory, even if there were non-endothelialized stent struts or exposed drug polymers, these would resorb over time. Furthermore, using BVS in this usually young population keeps future revascularization options open. The use of a self-expendable stent in a case of traumatic dissection has also been described in a case report (17). Theoretically, self-expendable stents may lower the risk of stent mal-apposition as they may further expand after IMH resorption.
Given the challenges with PCI of SCAD lesions, there are a few general recommendations to improve outcomes. First, given the risk of mal-apposition following IMH resorption, we recommend the use of OCT to guide stent placement. This allows appropriate sizing and reduces the risk of mal-apposition. Second, repeat late OCT imaging should be considered before cessation of DAPT, to exclude significant mal-apposition and to optimize stent expansion if necessary. Even though controversial, long-term DAPT is often recommended in cases of severe late-acquired stent mal-apposition (LASM) observed at follow-up (18). Further studies are needed to define the indications of long-term DAPT based on OCT findings. Prior IVUS studies demonstrated an association of severe LASM (gap ≥4 mm2) with late stent thrombosis (19). Third, the older 1st generation DES should probably be avoided to minimize the risk of adverse hypersensitivity reaction, which may exacerbate the mal-apposition associated with IMH resorption. Finally, the use of cutting balloon, BVS or self-expanding stents may be considered.
Conclusions
PCI of SCAD can be fraught with challenges, and intracoronary imaging especially with high-resolution OCT may provide insights on optimizing the acute stent results and identify long-term stent-related adverse events. We described three cases of long-term DES follow-up with OCT for SCAD PCI, showing differences in healing response of IMH resorption, which may be related to DES choice and procedural optimization with OCT. In addition, as many late stent thrombotic events are associated with DAPT discontinuation, identification of mal-apposition and exposed stent struts on follow-up OCT after PCI can influence the duration of DAPT in SCAD patients.
Acknowledgements
None.
Footnotes
Conflicts of Interest: J Saw has received unrestricted research grant supports (from the Canadian Institutes of Health Research, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, St Jude Medical, Boston Scientific, and Servier), speaker honoraria (AstraZeneca, St Jude Medical, Boston Scientific, Bayer and Sunovion), consultancy and advisory board honoraria (AstraZeneca, St Jude Medical, Boston Scientific, and Abbott Vascular), and proctorship honoraria (St Jude Medical and Boston Scientific). A Fung has received proctorship honoraria from St Jude Medical.
References
- 1.Lempereur M, Fung A, Saw J. OCT showing large arcs of malapposition, areas of evagination, and multiple areas of uncovered stent struts. Asvide 2015;2:072. Available online: http://www.asvide.com/articles/619
- 2.Lempereur M, Fung A, Saw J. OCT demonstrating IMH proximal to the stent and tacked-up IMH in the wall of the stented segment. Asvide 2015;2:073. Available online: http://www.asvide.com/articles/620
- 3.Lempereur M, Fung A, Saw J. OCT 15 days later showed patent stents but mild malapposition at the proximal edge of the proximal stent (250 µm) and persistent IMH covered by the stents. Asvide 2015;2:074. Available online: http://www.asvide.com/articles/621
- 4.Saw J, Sedlak T, Ganesh SK, et al. Cardiology patient page. Spontaneous coronary artery dissection (SCAD). Circulation 2015;131:e3-5. [DOI] [PubMed] [Google Scholar]
- 5.Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013;29:1027-33. [DOI] [PubMed] [Google Scholar]
- 6.Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44-52. [DOI] [PubMed] [Google Scholar]
- 7.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84:1115-22. [DOI] [PubMed] [Google Scholar]
- 8.Poon K, Bell B, Raffel OC, et al. Spontaneous coronary artery dissection: utility of intravascular ultrasound and optical coherence tomography during percutaneous coronary intervention. Circ Cardiovasc Interv 2011;4:e5-7. [DOI] [PubMed] [Google Scholar]
- 9.Porto I, Aurigemma C, Pennestrì F, et al. Intravascular ultrasound-documented healing of spontaneous coronary artery dissection. Circ Cardiovasc Interv 2010;3:519-22. [DOI] [PubMed] [Google Scholar]
- 10.Poon K, Incani A, Small A, et al. Drug eluting stents trapping intramural hematoma in spontaneous coronary artery dissection and healing pattern at six months: optical coherence tomography findings. Cardiovasc Revasc Med 2013;14:183-6. [DOI] [PubMed] [Google Scholar]
- 11.Sawada T, Shite J, Shinke T, et al. Persistent malapposition after implantation of sirolimus-eluting stent into intramural coronary hematoma: optical coherence tomography observations. Circ J 2006;70:1515-9. [DOI] [PubMed] [Google Scholar]
- 12.Imai M, Kadota K, Goto T, et al. Incidence, risk factors, and clinical sequelae of angiographic peri-stent contrast staining after sirolimus-eluting stent implantation. Circulation 2011;123:2382-91. [DOI] [PubMed] [Google Scholar]
- 13.Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645-55. [DOI] [PubMed] [Google Scholar]
- 14.Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579-88. [DOI] [PubMed] [Google Scholar]
- 15.Yumoto K, Sasaki H, Aoki H, et al. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty as evaluated with optical coherence tomography. JACC Cardiovasc Interv 2014;7:817-9. [DOI] [PubMed] [Google Scholar]
- 16.Sengottuvelu G, Rajendran R. Full polymer jacketing for long-segment spontaneous coronary artery dissection using bioresorbable vascular scaffolds. JACC Cardiovasc Interv 2014;7:820-1. [DOI] [PubMed] [Google Scholar]
- 17.Van Mieghem NM, van Weenen S, Nollen G, et al. Traumatic coronary artery dissection: potential cause of sudden death in soccer. Circulation 2013;127:e280-2. [DOI] [PubMed] [Google Scholar]
- 18.Karalis I, Ahmed TA, Jukema JW. Late acquired stent malapposition: why, when and how to handle? Heart 2012;98:1529-36. [DOI] [PubMed] [Google Scholar]
- 19.Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 2007;115:2426-34. [DOI] [PubMed] [Google Scholar]