Abstract
Background:
Greater quadriceps strength has been associated with lower risk of symptomatic knee osteoarthritis (OA) in older adults. However, factors that confer elevated risk of knee OA (eg, sedentary lifestyle, obesity, and knee injury) also contribute to a reduced tolerance of resistance training programs at ≥60% 1-repetition maximum (1RM). Therefore, the current study assessed whether concurrent application of blood flow restriction (BFR) to low-load resistance training is an efficacious and tolerable means of improving quadriceps strength in men at risk of symptomatic knee OA.
Methods:
Men older than age 45, with a history of knee injury or elevated body mass index (BMI), were randomized to low-load resistance training (30% 1RM) either with or without concurrent BFR. Isotonic double-leg press strength and isokinetic knee extensor strength were assessed before and after 4 weeks of training 3 times/wk. Knee pain (Knee Osteoarthritis Outcome Score) was assessed for tolerance.
Results:
Of the 42 men (mean age 56.1 ± 7.7 years) who were randomized, 41 completed the program. There were no significant intergroup differences in age, BMI, knee pathology, or muscle strength at baseline. Although leg press 1RM improved in both control and BFR groups, there were no significant intergroup differences in primary or secondary measures of muscle strength. The BFR was not associated with worsening of knee pain, but there was a significant improvement in knee pain in the control group.
Conclusions:
In comparison with training without BFR, addition of BFR to 30% 1RM resistance training for 4 weeks did not confer significantly greater increases in leg press or quadriceps strength in older men with risk factors for symptomatic knee OA.
Keywords: muscle strength, resistance training, quadriceps muscle, rehabilitation, blood flow restriction
Introduction
Osteoarthritis (OA) is a leading cause of disability in older adults.1 The knee is the most common weight-bearing joint affected by OA, with an estimated 45% of all adults at risk of developing symptomatic knee OA in their lifetime.2 One biomechanical factor that may significantly contribute to incident symptomatic knee OA and progression of cartilage loss is quadriceps weakness.3–5 Multiple studies have reported that individuals with knee OA have weak quadriceps muscles,4,6–11 and an observational study revealed that higher quadriceps strength protected against the development of incident symptomatic knee OA.5 Since the quadriceps, in addition to other lower limb muscles, are necessary for knee loading and stability during locomotion, increasing quadriceps muscle strength may result in increased physical function of those with or at risk of knee OA and decrease the incidence and/or progression of the disease.
The Osteoarthritis Research Society International recommends strength training as a possible treatment or a method to slow progression of knee OA.12 Strength training, as recommended by the American College of Sports Medicine, must be performed at a minimum resistance training load of 60% to 70% 1-repetition maximum (1RM) for strength gain and 70% to 85% 1RM for muscle hypertrophy.13 Unfortunately, factors that confer elevated risk of symptomatic knee OA (eg, obesity, knee pain, knee injury/surgery)14,15 also contribute to a perceived reduced tolerance of the high-load programs recommended for eliciting strength gains.16 Thus, for reducing disease risk and enhancing physical function, there is a need for a means of effectively strengthening the quadriceps muscles while limiting pain and adverse joint loading in people with or at risk of knee OA.
An alternative to traditional strength training that may be well tolerated by those with or at risk of knee OA is blood flow restriction (BFR) augmented low-load resistance training. Blood flow restriction is attained through administering pressure externally with a pneumatic cuff. The applied pressure occludes venous outflow while maintaining arterial inflow.17 Studies of healthy adults have revealed that strength gains and muscle hypertrophy in the context of BFR low-load training (even 20% 1RM) are similar to those achieved with traditional strength training.18–21 Furthermore, a recent study found that BFR low-load training over the course of 4 weeks was an effective means of stimulating strength gains in older women with risk factors for knee OA, in comparison to low-load training only.22 Additionally, in that study, muscle strength was gained without increasing knee pain or interfering with activities of daily living (ADL) or quality of life (QOL). Therefore, the primary aim of the current study was to assess whether BFR is an effective means of increasing lower limb strength in men at risk of knee OA, through a double-blind, randomized controlled study.
Methods
Participants
Community-dwelling, ambulatory men, aged 45 and older, volunteered to participate in the study at a university-based exercise laboratory. The participants were recruited via advertisements in clinics, businesses, and local newspapers; e-mail; and postal letters. Participants were included if they either had radiographic knee OA without symptoms or had at least 1 of the following risk factors for symptomatic knee OA: knee injury resulting in inability to walk without assistance for at least 2 days; knee surgery (other than bilateral knee arthroplasty); knee pain, aching, or stiffness on most of the prior 30 days; or were overweight or obese (body mass index [BMI] over 25 kg/m2). These specific inclusion criteria were used in order to remain consistent with the inclusion criteria for the Multicenter Osteoarthritis Study,23 in order to maintain generalizability to those participants in whom muscle weakness was found to confer elevated risk of symptomatic knee OA. Phone screens were administered to confirm that those interested had not participated in resistance training in the 3 months prior to their appointment, that they did not anticipate missing more than 2 of the 12 exercise sessions in the following 4 weeks, and that they did not report health issues or conditions that might restrict safe participation in the study (eg, bilateral knee replacements; lower limb surgery in the last 6 months; back, hip, or knee problems that affect walking; diagnosis of inflammatory joint or muscle disease, such as rheumatoid or psoriatic arthritis or polymyalgia rheumatica; neurologic diagnoses, such as multiple sclerosis or peripheral neuropathy; history of cancer, peripheral vascular disease or deep venous thrombosis; history of myocardial infarction or stroke in the last year; chest pain during exercise or at rest; or need for supplemental oxygen).
The investigators’ institutional review board (IRB) approved the study protocol, and it was registered at http://clinicaltrials.gov/show/NCT01487525. Participants provided written informed consent following completion of an IRB-approved consent process. In addition, vital signs were assessed before testing while seated to ensure a blood pressure less than 180/100 and heart rate >40 and <110 beats/min.
Group Assignment
A random number generator (randomization.com) was used for 1:1 assignment to either low-load resistance training (control) or low-load resistance training with BFR (intervention). Exercise trainers not involved in the baseline or outcome assessments sequentially opened each participant’s sealed, opaque envelope containing his group assignment. Participants were unaware of which group was considered the intervention, were instructed not to talk about their exercise experience if they incidentally met another participant, and appointments were staggered to avoid interaction between participants.
To ensure accurate data, all exercise equipment was calibrated before the initiation of the study. In addition, the outcome assessor was uninvolved with both the training protocol and randomization of participants and was trained and certified in the strength testing protocols.
Bilateral Leg Press Isotonic Strength (Primary Outcome Measure)
Isotonic leg press strength of each participant was measured on an instrumented pneumatic leg press with digital output (Keiser A420; Keiser, Fresno, California) in order to determine the proper training load for each participant. The seat was adjusted to ensure that each participant’s knees and hips were flexed at a 90° angle while both feet were on the foot pedals. Participants were familiarized with use of the machine during an orientation session 2 to 4 days before the baseline measurement visit. Prior to strength testing, participants completed a warm-up at lower resistances: 10 repetitions at 25% of their orientation 1RM and then 5 repetitions at 50% of orientation 1RM. Strength testing consisted of performing full range of motion, bilateral leg presses at increasing resistances until they could no longer fully extend their legs. If the load was considered to be too light, the participant was then given a 3- to 5-minute break before attempting a higher resistance. The 1RM was defined as the resistance at which the participant was unable to perform greater than 1 complete leg press. Trainers provided standardized verbal encouragement until the participant was no longer able to perform the leg presses. During final visits, conducted a median of 3 days following completion of the 4-week training, the same assessor repeated these measurements.
Isokinetic Knee Extensor Strength (Secondary Outcome Measure)
Studies that found knee extensor weakness to be a risk factor for incident symptomatic and progressive knee OA were conducted with an isokinetic dynamometer.3–5 Therefore, in addition to the primary outcome measure, leg press strength and peak isokinetic knee extensor torque for each lower limb were measured using a Biodex System 3 Dynamometer (Biodex Medical Systems, Inc, Shirley, New York) during baseline and final visits. Biodex medical system software version 3.30, System 3 PRO, Rev N was used for data acquisition. Participants were familiarized with the machine and were seated in a chair with a hip joint angle of 85°. The center of the lateral femoral condyle was visually aligned with the axis of rotation of the dynamometer. The seat back was positioned such that the participants were comfortably sitting with their back flush against the seat back, and the popliteal fossa overhanging the front edge of the seat by approximately 2 fingerbreadths, leaving the knee free to move. The dynamometer arm length was adjusted to each participant and the shin pad was secured proximal to the medial malleoli with straps. Participants were stabilized with bilateral shoulder straps, a lap belt, and strap over the thigh to be tested, such that participants could not lift their thigh or body off the chair upon extension. Participants performed 4 maximum isokinetic knee extensions and flexions at 60°/s for their maximum range between 90° of knee flexion and full extension. Settings for the chair and dynamometer were recorded upon initial testing and were again used for follow-up testing.
Knee Pain (Tolerance of the Intervention)
The Knee Osteoarthritis Outcome Score (KOOS) was used to assess for exacerbation of knee symptoms or worsening function in participants over the course of the study. The KOOS is a reliable 42-item self-administered questionnaire that covers 5 patient-relevant dimensions: pain, other disease-specific symptoms, ADL, sport and recreation, and knee-related QOL.24 The KOOS is responsive and sensitive to changes in people with knee OA.25
Low-Load Resistance Training Protocol
All participants completed the protocol outlined in Figure 1 using the bilateral leg press. During the training period, the training load remained at 30% of each participant’s 1RM. Those randomized to the control group completed the protocol without the BFR device and those randomized to the intervention group completed the protocol with the BFR device.
Figure 1.
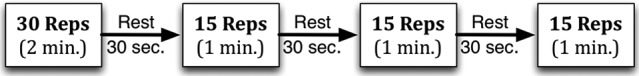
Exercise training protocol.
Intervention—BFR
Femoral blood flow was restricted with the Kaatsu Master BFR device (Sato Sports Plaza, Tokyo, Japan), which includes a control unit, pneumatic pump, and 2 pneumatic cuffs (65 mm width and 650 mm length). The cuffs were applied to the proximal aspect of each thigh. The device causes pooling of blood in capacitance vessels distal to the cuffs by restricting arterial while occluding venous blood flow.20,26,27 Before training, an initial pressure of 30 mm Hg for the first training and 40 mm Hg for all subsequent trainings was applied per the protocol detailed in Table 1. The distance from the upper pole of the patella to the cuff position was measured and recorded, and the cuff was placed at the same position on all subsequent visits. At each training session, the cuff was incrementally pressurized as detailed in Table 1. Following the cuff pressurization, 4 sets of leg press were completed as previously described and depicted in Figure 1. The total time the cuff was inflated was 6.5 minutes: 5 minutes of exercise and 1.5 minutes of rest between sets. Individual contraction duration lasted a total of 4 seconds—2-second shortening and 2-second lengthening contraction duty cycle—as this was previously shown to increase fatigue index.28,29
Table 1.
Partial Blood Flow Restriction Cuff Pressures.
| Week and session # | Initial cuff pressure upon application, mm Hg | One-minute incremental inflation pressures, mm Hga | Final exercise pressure, mm Hg | |||
|---|---|---|---|---|---|---|
| W1§1 | 30 | 100 | 120 | 140 | 160 | |
| W1§2 | 40 | 100 | 120 | 140 | 160 | |
| W1§3 | 40 | 100 | 120 | 140 | 160 | |
| W2§1-W2§3 | 40 | 120 | 140 | 160 | 180 | |
| W3§1-W3§3 | 40 | 120 | 140 | 160 | 180 | 200 |
| W4§1-W4§3 | 40 | 120 | 140 | 160 | 180 | 200 |
aThe cuff was repeatedly inflated for 1 minute at each indicated pressure and then deflated for 10 seconds before continuing to the next pressure level, during week 1. For weeks 2 to 4, the cuff was repeatedly inflated for 30 seconds then deflated for 10 seconds before continuing to the next pressure level.
Statistical Methods
An a priori sample size was estimated based upon prior data collected for the clinically significant difference in isokinetic knee extensor torque5 and appropriate standard deviations (SDs) within and between groups. At a 1-sided significance level of .05, a SD in the knee extensor strength response variable of 56 Nm, and a power of 0.80, detection of an intergroup difference in mean change of 12.2 Nm would require at least 38 participants for this 2-treatment parallel-design study. Therefore, anticipating up to a 10% dropout rate, we recruited 42 participants.
Participant demographics that met the normality assumption were summarized using means ± SDs. Baseline characteristics of each intervention group were compared using 2-sample t tests for continuous data (eg, age, BMI, and outcome assessments) and using Fisher’s exact test for categorical data (ie, presence of knee OA). The number of visits attended was not normally distributed and was summarized with medians and interquartile ranges, and differences were analyzed using the Wilcoxon rank sum test. For within-group analyses, paired t tests were used for each person-based variable of interest (ie, change in leg press 1RM and KOOS). For the main study analyses, the intergroup differences in person-based outcome variables were compared using 2-sample t tests for percentage of change in the outcomes. For the limb-based outcome variable (ie, knee extensor muscle strength), regression models were constructed to control for limb as a repeated factor within participants. As no significant intergroup differences existed in baseline characteristics other than isotonic leg press strength, analyses were not further adjusted. The SAS version 9.3 (SAS Institute Inc, Cary, North Carolina) was used for all analyses.
Results
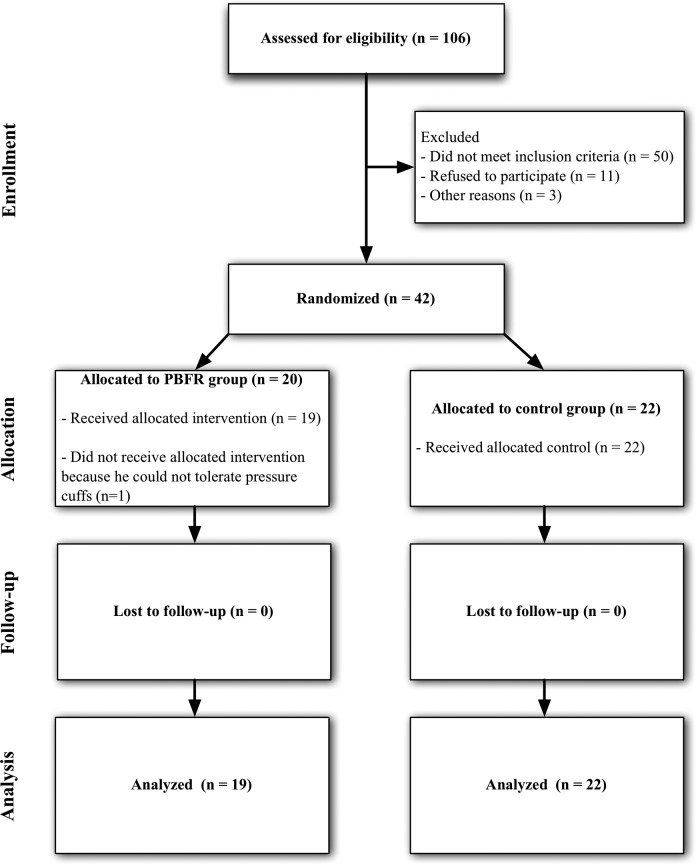
A total of 44 participants between the ages of 45 and 90 years (mean: 56.1 ± 7.7 years) met eligibility criteria and were enrolled in the study (Figure 2). The study was conducted between November 29, 2011, and February 27, 2012. Following enrollment, 3 participants discontinued the study due to lack of time (N = 2) and intolerance to the intervention pressure cuff (N = 1). At baseline, there were no statistically significant differences between patient’s characteristics or baseline measurements (Table 2).
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Table 2.
Participant Characteristics at Baseline.a
| Variable | Group | P value | |
|---|---|---|---|
| Control | BFR | ||
| Age, years | 56.1 ± 7.7 | 58.4 ± 8.7 | .377 |
| BMI, kg/m2 | 30.4 ± 4.2 | 31.3 ± 5.3 | .536 |
| Presence of knee osteoarthritis, % | 9.1 | 26.3 | .219 |
| Isotonic leg press strength, Lbs | 289.0 ± 48.1 | 346.1 ± 95.5 | .020 |
| Isokinetic knee extensor strength, Nm | 151.9 ± 34.8 | 169.7 ± 39.0 | .275 |
| KOOS pain score, points | 76.6 ± 22.1 | 83.3 ± 15.4 | .253 |
Abbreviations: BFR, blood flow restriction; BMI, body mass index; KOOS, Knee Osteoarthritis Outcome Score; SD, standard deviation.
aMean ± SD or frequency.
The median (interquartile range) number of sessions attended by the control group was 12 (12-12) and by the BFR group was 12 (12-12). There were no significant differences in the number of training sessions attended (P = .128).
There were no statistically significant intergroup differences in primary or secondary outcome measures, comparing control and BFR participants (Table 3). However, leg press 1RM increased significantly in both the control (13.5 ± 16.8 kg, P = .001) and the BFR groups (11.3 ± 14.0 kg, P = .003). Significant improvements in isokinetic knee extensor strength (7.0 ± 3.0 Nm, P = .026) and KOOS scores (5.6 ± 11.7 points, P = .042) were observed in the control group, but not in the BFR group (−0.1 ± 3.3, P = .987; and 2.9 ± 10.0, P = .220, respectively).
Table 3.
Percentage of Change in Outcomes (Follow-Up vs Baseline).a
| Variable | Group | P value | |
|---|---|---|---|
| Control | BFR | ||
| Isotonic leg press strength | 4.7 ± 1.3% (P < .002) | 3.1 ± 0.9% (P = .003) | .322 |
| Isokinetic knee extensor strength | 6.7 ± 2.3% (P = .006) | 0.4 ± 2.4% (P = .883) | .066 |
| KOOS pain score | 14.2 ± 7.2% (P = .062) | 4.9 ± 3.3% (P = .155) | .254 |
Abbreviations: BFR, blood flow restriction; KOOS, Knee Osteoarthritis Outcome Score; SE, standard error.
aMean ± SE.
Discussion
The purpose of the current study was to assess whether BFR in combination with low-load resistance training is an effective and well-tolerated means of increasing quadriceps muscle strength in men with or at risk of symptomatic knee OA. We found that after 4 weeks, augmentation of 30% 1RM strength training with BFR did not result in significant differences in thigh muscle strength in men with or at risk of symptomatic knee OA. A secondary finding was that low-load training with or without BFR did not exacerbate knee-specific pain, as determined by KOOS scores (Table 3).
Many variations in BFR exercise have been investigated, and while general suggestions are made, the most effective dose and duration of BFR with low-load strength training remain unclear at this time.17,30 Some studies report that 4 weeks or less of lower limb low-load training with concurrent BFR is sufficient to elicit greater increases in strength than low-load training alone.20,22,31 However, our findings with older men with or at risk of knee OA contribute additional evidence that suggests longer durations of training with BFR may be necessary to elicit greater strength gains than low-load training alone.18,32 The current study was similar to another low-load training study of men, in which a short training duration (5 weeks) was insufficient to elicit greater strength gains in the BFR group versus low-load training only.32 Several studies have trained participants for longer durations and detected a greater increase in muscle strength.19,33 For example, a nonblinded study of double leg press and knee extension training in a similar age population, training at 20% to 30% 1RM in combination with BFR was found to be sufficient to induce significant strength increases of 33.4% in leg press and 26.1% in knee extension strength following 12 weeks of training.19 Not only was the increase in strength greater than that observed in the current study, but the BFR group also had significantly greater strength gains than the low-load only group. Therefore, it is possible that increasing the training duration to greater than 4 weeks may result in greater increases in strength with BFR than with low-load training alone in men with or at risk of symptomatic knee OA.
The exact mechanisms associated with strength gains that have been reported with use of BFR during low-resistance training remain incompletely understood. The role of muscle hypertrophy, rather than neural adaptations, has been suggested to be a primary factor associated with strength gains within the initial several weeks of resistance training with BFR.18 Although hypertrophy was not investigated in the current study, it is possible that hypertrophy that occurred in the BFR group was insufficient to exceed the response of neural adaptions that would have been expected in the control group.34,35 Hence, the lack of difference in strength increase between the BFR and the control groups, despite increases in leg press strength in each group and despite successful randomization resulting in an equal distribution of demographic characteristics, may relate to differences in the mechanisms of action of resistance training exercise with and without BFR.
It is also possible that more fit participants were randomly assigned to the BFR group at a higher rate. This possibility is supported by the greater isotonic leg press strength in the BFR group at baseline. Only participants who confirmed that they had not participated in strength training exercise during the 3 months prior to beginning the study were recruited. However, usual physical activity before and during the study was not assessed. Although many participants reported that they were inactive before the study began, some participants reported leading physically active lifestyles (eg, one participant in the BFR group was a bricklayer, another had US Army Reserve Duty, and another was an avid cyclist before and during the study). Physical activity contributes to overall muscle strength and these activities outside of the study could potentially have influenced the outcomes, as randomization did not necessarily successfully distribute these fitness and physical activity factors. Specifically, gains in lower limb strength observed in the control group could be associated with increased exercise or physical activity outside study participation and the reduced responsiveness to the BFR intervention over the 4-week duration could have been observed if those participants entered the study at a higher level of muscle conditioning.
The difference in results between the current study and a prior study of women with risk factors for symptomatic knee OA22 may also be related to fitness level. The women volunteers for the prior study were less physically active and less fit than the men who were recruited for the current study.22 If the women were less physically fit, then it may have been easier to detect gains in strength over the 4-week study duration. Furthermore, the dose of BFR could also play a role in explaining differences between our findings and prior reports. Specifically, the differences between the men in the current study and the women in the prior study may relate to variability in the percentage of blood flow restricted (%BFR) at the same initial cuff pressure. Although the applied cuff pressure was the same in both studies, given that women have greater adipose deposits in their thighs, the women may have achieved greater %BFR at the same level of cuff tightness as the men. Therefore, the applied BFR dose for women may have been more effective in eliciting strength gains in comparison with low-load training without BFR over a 4-week duration than the dose achieved in men at equivalent cuff tightness levels.
In our study, we followed the manufacturer’s instructions for the initial cuff pressure prior to inflation and the timing, progression, and magnitude of the final cuff pressures. Following completion of the current study, research has clarified that, to attain the optimal dose of BFR, cuff pressures need to be adjusted based on cuff width, limb circumference, and also on composition of muscle and fat in the limb.36 For example, Meek et al reported that more lean muscle leads to less %BFR, while a higher percentage of body fat leads to a higher % BFR.37 Based on this more recent evidence, if the current study were to be repeated, it would be desirable to adjust the initial cuff pressure based on these factors to achieve equipotent dosing among participants. To our knowledge, there have not been reports that have compared the effects of anthropometric and/or sex differences on the degree of strengthening conferred by BFR exercise, although Labarbera et al did study the differential effects of sex on muscle fatigue.38 The lack of clarity regarding the determinants of BFR dosing, in the context of the different outcomes between the current study and the prior study of women,22 indicate a need for further investigations, as this information may be important in understanding appropriate training parameters and interpreting the outcomes of BFR training.
This was one of the first randomized controlled trials of BFR utilizing a clinical population of interest—older men with or at risk of knee OA. This population is clinically important as greater strength may be protective against developing symptomatic knee OA and affected individuals may also be unable to tolerate traditional resistance training exercise programs due to having “at-risk” knees. Strengths of the current study included the strict inclusion criteria, allowing the results to be generalized to men who have identifiable risk factors for developing incident symptomatic knee OA. The randomized controlled design enhances internal validity through allowing valid assessment of the efficacy and potential adverse effects of the BFR training. The blinding of participants and outcome assessors to the treatment allocation allowed reduction in measurement bias that otherwise may have confounded interpretation of the study results.39 In addition, there was excellent participant compliance throughout the study as participants in both the control group and the BFR group attended a median of 12 of the 12 sessions. During each individual session, the exercise trainers closely monitored the participants and equipment to ensure the protocol was meticulously followed.
Despite these strengths, there were several limitations to the current study. Muscle hypertrophy could not be assessed, as there were insufficient funds to allow cross-sectional imaging of the quadriceps muscles prior to and following the exercise training. The lack of morphological measurements limits the interpretation of the outcomes of the current study although less so than if a significant between-group difference in strength gains had been detected. Other limitations included not basing the initial cuff pressure and final cuff tightness on the thigh circumference or composition of muscle and fat in the limb and not measuring femoral blood flow during the exercise training to assess the real-time physiological effects of the intervention and not progressing the training load over the 4 weeks of training. Since the 1RM was not revaluated, the exercise load was not altered, if necessary, to maintain 30% 1RM throughout the entire study. It is possible that periodic adjustments to the resistance may have provided a greater challenge and a more adequate stimulus to produce greater strength gains, although this limitation would have limited strength gains in both BFR and non-BFR participants so may not have altered the primary outcome—the difference in strength gains between the groups. The investigators did not originally anticipate a need for progression prior to conducting the current study, as several prior BFR studies of 1- to 4-week duration did not progress the resistance. In addition, physical activity level should have been assessed and possibly entered as a covariate if there had been significant differences in physical activity between the groups. Finally, the inclusion criteria were selected to be identical to those for the study in which knee extensor muscle weakness was found to elevate risk of symptomatic knee OA. However, there is some heterogeneity among participants, due to recruitment for a variety of known risk factors for symptomatic knee OA.
Knee extensor strength is important for reducing risk of incident symptomatic knee OA,3,5 so determining an alternative to traditional high-load strength training programs that is well tolerated and effective for strengthening the knee extensors is a key long-term clinical aim. While traditional strength training may be difficult for men with or at risk for knee OA, low-load strength training in combination with BFR could hold potential as a clinical intervention, if different exercise protocol parameters are found to be efficacious in future research. Importantly, while the current study did not formally evaluate all aspects of safety, measurement of tolerance did reveal that the exercise program, even augmented with BFR, did not increase knee pain (Table 3). In addition, other studies have reported that BFR low-load training is a safe alternative to traditional, high-load exercises, even in older adults.27,40 Therefore, further research utilizing BFR training is desirable to assess for parameters that enable tolerable strengthening and, if possible, eventually assess for protection against development of incident symptomatic knee OA.
In summary, the current study revealed that, for older men with risk factors for symptomatic knee OA, augmentation of leg press resistance training at an average intensity of 30% with BFR did not result in significant differences in leg press or knee extensor muscle strength gains at 4 weeks in comparison with the same training without BFR. Further studies are necessary to clarify whether a different training dose and duration may elicit muscle strength gains with BFR low-load exercise.
Acknowledgments
The authors appreciate the participants’ time in making this study possible and Natalie Glass for coordinating this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a Beeson Career Development Award in Aging (NIH/NIA 1K23AG030945) and an American College of Sports Medicine Research Foundation Kaatsu Training Research Grant.
References
- 1. Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Segal NA, Glass NA, Felson DT, et al. Effect of quadriceps strength and proprioception on risk for knee osteoarthritis. Med Sci Sports Exerc. 2010;42(11):2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Segal NA, Glass NA, Torner J, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18(6):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Segal NA, Torner JC, Felson D, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61(9):1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker KR, Xu L, Zhang Y, et al. Quadriceps weakness and its relationship to tibiofemoral and patellofemoral knee osteoarthritis in Chinese: the Beijing osteoarthritis study. Arthritis Rheum. 2004;50(6):1815–1821. [DOI] [PubMed] [Google Scholar]
- 7. Brandt KD, Heilman DK, Slemenda C, et al. A comparison of lower extremity muscle strength, obesity, and depression scores in elderly subjects with knee pain with and without radiographic evidence of knee osteoarthritis. J Rheumatol. 2000;27(8):1937–1946. [PubMed] [Google Scholar]
- 8. Fisher NM, Pendergast DR. Reduced muscle function in patients with osteoarthritis. Scand J Rehabil Med. 1997;29(4):213–221. [PubMed] [Google Scholar]
- 9. Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22(1):110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010;89(7):541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. 2008;40(3):422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. [DOI] [PubMed] [Google Scholar]
- 13. American College of Sports M. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. [DOI] [PubMed] [Google Scholar]
- 14. Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133(5):321–328. [DOI] [PubMed] [Google Scholar]
- 15. Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25(5):622–627. [DOI] [PubMed] [Google Scholar]
- 16. Messier SP, Mihalko SL, Beavers DP, et al. Strength Training for Arthritis Trial (START): design and rationale. BMC Musculoskelet Disord. 2013;14:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pope ZK, Willardson JM, Schoenfeld BJ. Exercise and blood flow restriction. J Strength Cond Res. 2013;27(10):2914–2926. [DOI] [PubMed] [Google Scholar]
- 18. Loenneke JP, Wilson JM, Marin PJ, Zourdos MC, Bemben MG. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(5):1849–1859. [DOI] [PubMed] [Google Scholar]
- 19. Yasuda T, Fukumura K, Fukuda T, et al. Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand J Med Sci Sports. 2014;24(5):799–806. [DOI] [PubMed] [Google Scholar]
- 20. Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88(6):2097–2106. [DOI] [PubMed] [Google Scholar]
- 21. Laurentino GC, Ugrinowitsch C, Roschel H, et al. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc. 2012;44(3):406–412. [DOI] [PubMed] [Google Scholar]
- 22. Segal NA, Williams GN, Davis MC, Wallace RB, Mikesky AE. Efficacy of blood flow-restricted, low-load resistance training in women with risk factors for symptomatic knee osteoarthritis. PM R. 2015;7(4):376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Segal NA, Torner JC, Yang M, et al. Muscle mass is more strongly related to hip bone mineral density than is quadriceps strength or lower activity level in adults over age 50 year. J Clin Densitom. 2008;11(4):503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iida H, Kurano M, Takano H, et al. Hemodynamic and neurohumoral responses to the restriction of femoral blood flow by KAATSU in healthy subjects. Eur J Appl Physiol. 2007;100(3):275–285. [DOI] [PubMed] [Google Scholar]
- 27. Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bemben MG. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports. 2011;21(4):510–518. [DOI] [PubMed] [Google Scholar]
- 28. Cook SB, Clark BC, Ploutz-Snyder LL. Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sports Exerc. 2007;39(10):1708–1713. [DOI] [PubMed] [Google Scholar]
- 29. Fujita S, Mikesky AE, Sato Y, Abe T. Fatigue characteristics during maximal concentric leg extension exercise with blood flow restriction. Int J KAATSU Train Res. 2007;3(2):27–31. [Google Scholar]
- 30. Nielsen JL, Aagaard P, Bech RD, et al. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol. 2012;590(pt 17):4351–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujita T, Brechue WF, Kurita Y, Sato Y, Abe T. Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int J KAATSU Train Res. 2008;4(1):1–8. [Google Scholar]
- 32. Martin-Hernandez J, Marin PJ, Menendez H, Ferrero C, Loenneke JP, Herrero AJ. Muscular adaptations after two different volumes of blood flow-restricted training. Scand J Med Sci Sports. 2013;23(2):e114–e120. [DOI] [PubMed] [Google Scholar]
- 33. Karabulut M, Sherk VD, Bemben DA, Bemben MG. Inflammation marker, damage marker and anabolic hormone responses to resistance training with vascular restriction in older males. Clin Physiol Funct Imaging. 2013;33(5):393–399. [DOI] [PubMed] [Google Scholar]
- 34. Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36(2):133–149. [DOI] [PubMed] [Google Scholar]
- 35. Carroll TJ, Riek S, Carson RG. Neural adaptations to resistance training: implications for movement control. Sports Med. 2001;31(12):829–840. [DOI] [PubMed] [Google Scholar]
- 36. Loenneke JP, Fahs CA, Rossow LM, et al. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol. 2012;112(8):2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meek AW, Heavrin AM, Segal NA, Mikesky AE. KAATSU cuff tightness and limb anthropometry: effect on blood flow restriction. Med Sci Sports Exerc. 2014;46(5):822–822. [Google Scholar]
- 38. Labarbera KE, Murphy BG, Laroche DP, Cook SB. Sex differences in blood flow restricted isotonic knee extensions to fatigue. J Sports Med Phys Fitness. 2013;53(4):444–452. [PubMed] [Google Scholar]
- 39. Liu CJ, LaValley M, Latham NK. Do unblinded assessors bias muscle strength outcomes in randomized controlled trials of progressive resistance strength training in older adults? Am J Phys Med Rehabil. 2011;90(3):190–196. [DOI] [PubMed] [Google Scholar]
- 40. Clark BC, Manini TM, Hoffman RL, et al. Relative safety of 4 weeks of blood flow-restricted resistance exercise in young, healthy adults. Scand J Med Sci Sports. 2011;21(5):653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]