Abstract
Sherman et al. commented on the precedence of enantiodivergence, listing a number of congeneric natural products with opposite chirality. However, these “congeners” are not derived from enantiodivergent biosyntheses. Instead, they are antipodes arising from separate enantiomeric biosyntheses. A distinct feature of the biosynthesis of the cyclic pyrrole-imidazole dimers is the production of antipodal congeners without the corresponding enantiomers.
We found that cyclic pyrrole-imidazole dimers exist in nature as two sets of enantiomers (1). Unlike other natural products, all the [3+2] dimers exist in one enantiomeric form, whereas all the [2+2] and [4+2] dimers have the opposite chirality. Although the biosynthetic pathways of these alkaloids have not been genetically characterized, emerging evidence suggests that they are assembled from two molecules of pyrrole-imidazole monomers through single-electron transfer (SET)–promoted cycloaddition reactions (2). The [2+2] and [4+2] cycloaddition reactions of 1 give 2 and 3 that are antipodal to the [3+2] cycloadduct 4 (Fig. 1A). This “enantiodivergent” biosynthesis should not be confused with the “enantiomeric” biosynthesis that generates enantiomers—for example, (+)- and (−)-linalyl diphosphate (6) from geranyl diphosphate (5) (Fig. 1B) (3). The two enantiomers of limonene (7) and carvone (8) can further be produced from 6 through stereoselective cyclization and oxidation reactions.
Fig. 1. Enantiodivergent versus enantiomeric biosyntheses.
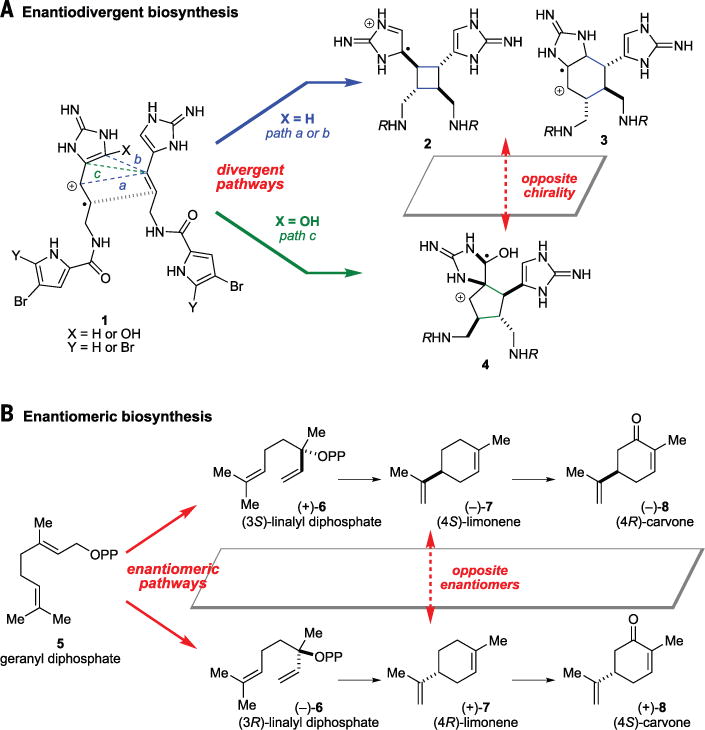
Enantiodivergent biosynthesis (A) leads to the formation of antipodal congeners, whereas enantiomeric biosynthesis (B) produces enantiomers.
We agree with Sherman et al. (4) that the world of natural products is full of enantiomeric diversity, although most natural products are produced in only one enantiomeric form. The terpene class of molecules, in particular, has long been recognized to exist with a wide variety of optical purities. Sherman and Williams, together with Finefield and Kreitman, have reviewed this topic in (3), which we cited in (1). We note that enantiomeric biosynthesis has also been referred to as enantiodivergent biosynthesis at times, and there are no clear definitions for these terms. Within the scope of this discussion, we use “enantiodivergent” biosynthesis only when the enantiodetermining step (eds) bifurcates in both absolute stereochemistry and framework connectivity. For an eds that generates enantiomers, we apply the term “enantiomeric” biosynthesis.
Sherman et al. commented that numerous examples of enantiodivergent biosynthesis exist. However, their list of antipodal congeners represents additional cases of enantiomeric biosynthesis. For example, (+)-secoisolariciresinol and (−)-matairesinol are presented as antipodal congeners, but the opposite enantiomers (−)-secoisolariciresinol and (+)-matairesinol also exist in nature (3). Matairesinol actually comes from secoisolariciresinol through enantiospecific oxidation by NAD-dependent secoisolariciresinol dehydrogenase (5). Likewise, additional “antipodal congeners” in fig. 2 in (4) are mostly antipodes arising from two separate enantiomeric biosyntheses instead of divergent bond formation with opposite facial selectivity. The enantiomers of (+)-sesamin, (+)-syringaresinol, (−)-lariciresinol, ent-kaurene, ent-pimara-8(14),15-diene, isopimara-7,15-diene, (−)-abietic acid, (−)- stephacidin A, and (+)-versicolamide B can all be found in nature (3, 6–8). As to nonactin, the biosynthetic eds is the formation of (+)- and (−)-nonactic acid (3).
Fig. 2. Biosynthetic pathways for the reverse prenylated indole alkaloids.
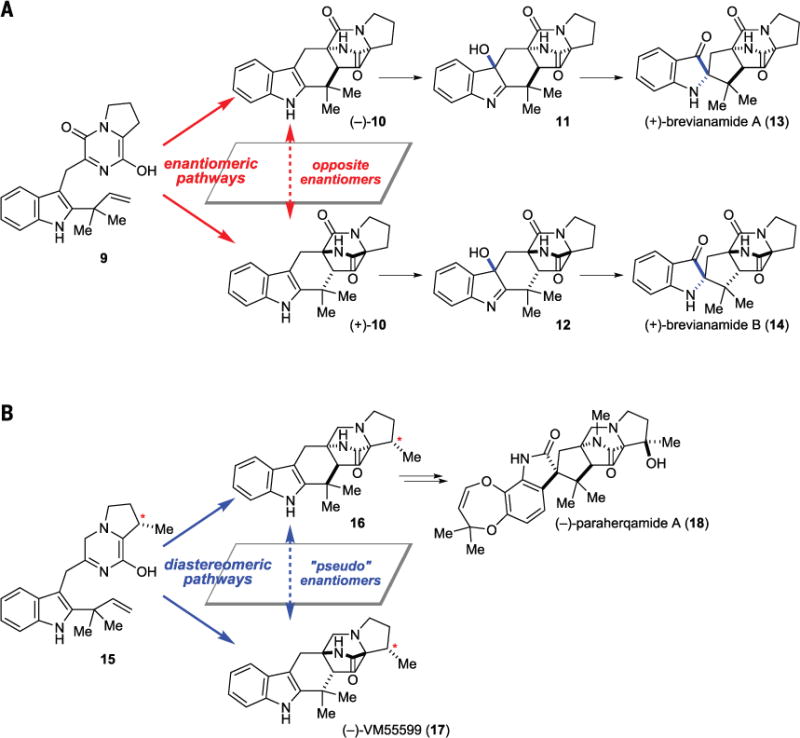
(A) Brevianamides A and B arise from enantiomeric biosynthesis. (B) Paraherquamide A and VM55599 arise from diastereomeric biosynthesis.
The reverse prenylated indole alkaloids are intriguing fungal metabolites that nicely showcase the molecular complexity and diversity of natural products. Their syntheses and biosyntheses have been studied by Williams and others extensively (9). Brevianamides A and B should also arise from enantiomeric biosynthesis because the eds is the Diels-Alder reaction of the achiral precursor 9 to give (−)- and (+)-10 (Fig. 2A) (10). These biosynthetic intermediates are then oxidized from the same face of the indole to give diastereomers 11 and 12. Subsequent stereospecific rearrangement gives brevianamides A (13) and B (14), correspondingly. Similarly, VM55599 (17) and paraherquamide A (18) arise from diastereomeric Diels-Alder reactions of the chiral precursor 15, which is assembled from L-tryptophan, L-isoleucine, and isoprenyl diphosphate (Fig. 2B) (11). Feeding experiments support the notion that 16 is a biosynthetic intermediate of 18.
In closing, it is fascinating that biosynthetic eds can generate opposite enantiomers of natural products. It is even more perplexing that the biosynthetic eds of the cyclic pyrrole-imidazole dimers generates antipodal congeners. We have examined all the reports of the isolation of cyclic pyrrole-imidazole dimers. To date, all optically characterized [3+2] dimers are uniformly antipodal to the [2+2] and [4+2] dimers, regardless of the species and geographic location of the producing sponges. This mismatched chiral relationship is different from the enantiomeric relationship previously described.
Acknowledgments
Financial support was provided by NIH (NIGMS R01-GM079554 and R01-GM073949), the Welch Foundation (I-1596), and The University of Texas Southwestern Medical Center.
REFERENCES AND NOTES
- 1.Ma Z, et al. Science. 2014;346:219–224. doi: 10.1126/science.1255677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stout EP, Wang YG, Romo D, Molinski TF. Angew Chem Int Ed. 2012;51:4877–4881. doi: 10.1002/anie.201108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finefield JM, Sherman DH, Kreitman M, Williams RM. Angew Chem Int Ed. 2012;51:4802–4836. doi: 10.1002/anie.201107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman DH, Tsukamoto S, Williams RM. Science. 2015;349:149. doi: 10.1126/science.aaa9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia ZQ, Costa MA, Pélissier HC, Davin LB, Lewis NG. J Biol Chem. 2001;276:12614–12623. doi: 10.1074/jbc.M008622200. [DOI] [PubMed] [Google Scholar]
- 6.Kenmoku H, Tanaka M, Ogiyama K, Kato N, Sassa T. Biosci Biotechnol Biochem. 2004;68:1574–1577. doi: 10.1271/bbb.68.1574. [DOI] [PubMed] [Google Scholar]
- 7.Aiyar VN, Seshadri TR. Curr Sci. 1972;41:839–840. [Google Scholar]
- 8.Lu T, Vargas D, Franzblau SG, Fischer NH. Phytochemistry. 1995;38:451–456. doi: 10.1016/0031-9422(94)00625-4. [DOI] [PubMed] [Google Scholar]
- 9.Williams RM, Cox RJ. Acc Chem Res. 2003;36:127–139. doi: 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]
- 10.Baldas J, Birch AJ, Russell RA. J Chem Soc Perkin Trans. 1974;1:50–52. [Google Scholar]
- 11.Sanz-Cervera JF, Williams RM. J Am Chem Soc. 2002;124:2556–2559. doi: 10.1021/ja017425l. [DOI] [PubMed] [Google Scholar]


