Abstract
The innate immune system of multicellular animals is regulated by the nervous system.
The most evolutionarily ancient type of immunity, called “innate,” exists in all living multicellular species. When exposed to pathogens or cellular damage, cells of an organism’s innate immune system activate responses that coordinate defense against the insult, and enhance the repair of tissue injury. There is a modern-day cost associated with these processes, however, because innate mechanisms can damage normal tissue and organs, potentially killing the host. Human life is a balance between dual threats of insufficient innate immune responses—which would allow pathogens to prevail—and overabundant innate immune responses—which would kill or impair directly. What has been the key to maintaining this balance throughout years of mammalian evolution? On page 729 of this issue, Sun et al. (1) report that neurons in a nematode worm can regulate innate immunity, a mechanism dating back to the early origins of the nervous system itself.
Research on the pathophysiology of infection in the late 20th century revealed that molecules produced by the innate immune system, not pathogens, account for the major physiological, metabolic, and pathological responses to infection in mammals. Cytokines and other molecules were associated with the signs and symptoms of infection, ranging from fever, anorexia, and fatigue, to lethal shock and tissue injury. By understanding the “cytokine theory of disease,” it became possible to develop highly selective drugs that neutralize cytokines and experimentally modify the pathophysiology of infection (2). This same approach subsequently revolutionized the treatment of inflammatory disease in humans with other, noninfectious, but inflammatory conditions. Today, millions of patients with arthritis, colitis, and other inflammatory syndromes have benefited from therapy with cytokine-blocking agents.
These advances also underscored the importance in understanding mechanisms that control innate immunity and restrain it from injuring the host. Early work focused on soluble factors that control innate immune responses by inhibiting the synthesis or action of cytokines. This “protective mediator” list grew to include glucocorticoid hormones, soluble cytokine receptor fragments, and other anti-inflammatory factors (3). More unexpected, however, were later findings that information propagated in neurons controls the magnitude of the mammalian innate immune response. Action potentials traveling in the vagus nerve to the spleen and other organs culminate in the release of acetylcholine, an evolutionarily ancient molecule that effectively inhibits cytokine production by innate immune cells. The cytokine-blocking mechanism requires signal transduction through α7 nicotinic acetylcholine receptors expressed on macrophages and other cytokine-producing immune cells (4). Signals generated via this neural circuit tonically suppress innate immunity, because lesions in this pathway enhance the innate immune response to pathogens and injury (5). It may be possible to use this inhibitory pathway to therapeutic advantage, because selective electrical stimulation of the vagus nerve inhibits the production of tumor necrosis factor, interleukin-1 and other cytokines, and prevents the pathology associated with arthritis, colitis, ischemic tissue injury, and other syndromes in experimental models.
Sun et al. reveal that neural control of innate immunity in mammals is present in Caenorhabditis elegans, one of the simplest organisms with a nervous system. This indicates that the regulatory mechanism dates back to the origins of the nervous system. C. elegans is a soil nematode, ~1 mm in length, that feeds on bacteria in decaying vegetable matter. Its nervous system consists of only 302 sensory neurons, motor neurons, and interneurons. The neurotransmitters include acetylcholine and γ-aminobutyric acid, which facilitate locomotion and pathogen avoidance. The authors found that worms lacking a cell surface receptor called OCTR-1 in two types of neurons exhibit substantially improved survival against the bacterial pathogen Pseudomonas aeruginosa. These neurons—the “ASH” and “ASI” sensory neurons—extend processes into an opening at the anterior end of the worm, where they are exposed to the environment. The protection was observed in the presence of living, but not dead bacteria, indicating that these neurons inhibit the innate immune response to bacterial pathogens. The protection was not attributed to enhanced pathogen avoidance, or pathogen accumulation. Rather, the neurons targeted, in nonneuronal cells, the molecular basis of the unfolded protein response, a mechanism that regulates organellar (endoplasmic reticulum) accumulation of unfolded proteins during periods of heightened protein synthesis, as in infection. Together, these results show that the tonic output of innate immunity is negatively regulated by signals originating in ASH and ASI sensory neurons in the worm (see the figure). Hence, in worms, as in mammals, the innate immune system is not autonomous; it is innervated. Additional work is needed in the worm model of Sun et al. to determine the nature of the signals that activate OCTR-1, a putative catecholamine receptor, in sensory neurons. Further work should also reveal how the sensory neurons regulate the unfolded protein response pathway in nonneuronal cells.
Figure. Innate innervation.
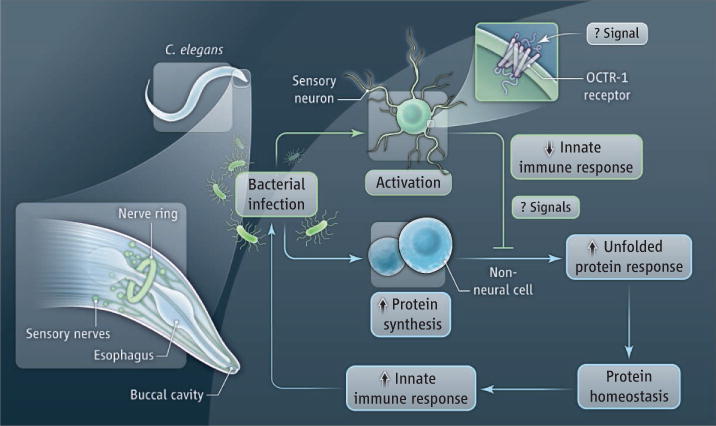
Infection of C. elegans with a pathogen stimulates the innate immune response and activates the synthesis of new proteins, potentially causing the accumulation of unfolded proteins in host cells. To restore protein homeostasis, the unfolded protein response is activated. ASH and ASI sensory neurons negatively regulate the innate immune response to infection by blocking the unfolded protein response in nonneuronal cells. The OCTR-1 receptor in the sensory neurons is required for this effect.
How might such a neural controlling system operate, and what are the possible therapeutic implications? A general principle in physiology is that innervation of a system enables reflex control mechanisms to provide a regulatory framework that can fine tune responses over time and space. The inflammatory reflex, a prototypical circuit in mammals, is activated by exposure to pathogens or injury and modulates the innate immune response (5). Neurons express Toll-like receptors and cytokine receptors, which detect the presence of pathogens (and perhaps damaged tissue as well) and cytokines, respectively (6, 7). This activates action potentials that ascend to the central nervous system, which in turn sends efferent signals out to the immune system to dampen the responses. Such circuits provide control precision and integration not possible with diffuse, humoral control systems. Thus, the innate immune system is both the origin of signals that are converted to action potentials in the sensory arc, and it is the target of signals descending in the motor arc (5).
We have learned much about the efferent pathways controlling innate immunity, and there is now great interest in understanding more about how inflammatory and injurious factors activate the afferent input into this immunological and neurological system. Understanding these mechanisms as a function of “top-down” neurological regulatory processes—that is, as immune responses that are the result of action potentials originating in neurons—should stimulate the identification of new molecular targets and the development of therapeutic alternatives. From worms to humans, there is a consistent evolutionary theme that the immune system detects changes in the environment, and its actions modify the behavior of the animal. The loop is closed by reflex signals originating in the nervous system that modify innate immune responses to maintain homeostasis.
References
- 1.Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Science. 2011;332:729. doi: 10.1126/science.1203411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tracey KJ. J Clin Invest. 2007;117:289. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C. Nature. 2002;420:846. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, et al. Nature. 2003;421:384. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ. Nat Rev Immunol. 2009;9:418. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanga FY, Nutile-McMenemy N, DeLeo JA. Proc Natl Acad Sci USA. 2005;102:5856. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boettger MK, et al. Arthritis Rheum. 2008;58:2368. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]


