Abstract
A key question in immunology concerns how sterile injury activates innate immunity to mediate damaging inflammation in the absence of foreign invaders. The discovery that HMGB1, a ubiquitous nuclear protein, mediates the activation of innate immune responses led directly to the understanding that HMGB1 plays a critical role at the intersection of the host inflammatory response to sterile and infectious threat. HMGB1 is actively released by stimulation of the innate immune system with exogenous pathogen-derived molecules and is passively released by ischemia or cell injury in the absence of invasion. Established molecular mechanisms of HMGB1 binding and signaling through TLR4 reveal signaling pathways that mediate cytokine release and tissue damage. Experimental strategies that selectively target HMGB1 and TLR4 effectively reverse and prevent activation of innate immunity and significantly attenuate damage in diverse models of sterile and infection-induced threat.
Keywords: cytokine, host defense, cell death, ischemia, autoimmunity
INTRODUCTION
Injury and infection have challenged the survival of species throughout evolution. Innate immunity emerged as the defensive frontline that coordinates the responses to these dual threats. This early detection system uses primitive receptors encoded in the genome that respond to products of pathogenic origin and to host molecules that accumulate during cell injury and death. Activation of innate immunity wards off invasion and calls into action other organ systems that contribute appropriate metabolic, hemodynamic, and immunological responses to eradicate pathogens, facilitate healing, and initiate the transition to adaptive immunity. The discovery a decade ago that HMGB1, a highly conserved protein secreted by innate immune cells in response to pathogenic products and released by injured or dying cells, occupies a central role in the pathogenesis of both sterile and infectious inflammation, filled a critical niche in understanding immunity. The principle established is that the products of cellular injury, produced by the host, activate fundamental defensive responses (innate immunity) that are indistinguishable from the responses activated by molecules produced by microbes and pathogens.
Although wholly unexpected until some 25 years ago, advances in the biology of inflammation revealed that specific cytokines are necessary and sufficient pathogenic mediators of disease and that selectively targeting such individual mediators attenuates the major clinical signs and symptoms of inflammation. Tumor necrosis factor (TNF), IL-1, and IL-6 have become mainstays of cytokine-based treatment of inflammation in the clinic. They were originally identified as therapeutic targets from studies into the pathophysiology of endotoxemia, fever, sepsis, and later, autoimmune disease. These agents have indeed had an impact on the human condition, improving the quality of life for many patients and paving the way for new methods of developing pharmaceuticals for clinical use. But they are not effective in all patients, and additional therapeutic options are needed.
During the past decade, the widespread use of antagonists that neutralize HMGB1 in preclinical models of disease has directly implicated this molecule in regulating innate and adaptive immunity in health and during arthritis, colitis, sterile ischemia, traumatic injury, cancer, and infection. For decades, investigators argued that HMGB1 was merely a structural protein that resided in the nucleus where it functions to stabilize DNA structure and modulate transcriptional activity (1). The major structural features of HMGB1 are its two DNA-binding domains, homologous regions approximately 80 amino acids long, termed the A and B boxes, and a C-terminal domain composed of aspartic and glutamic acids (Figure 1). The search for therapeutically exploitable cytokines that mediate the damaging aftereffects of infection and injury revealed that HMGB1 is an actively secreted cytokine, produced by macrophages and other inflammatory cells during the innate immune response to invasion (2). Like other members of the proinflammatory cytokine family, biologically active HMGB1 can be expressed on the plasma membrane or released by activated inflammatory cells to accumulate in vivo during infection and injury; it alters the metabolic and immunological activities of hematopoietic, epithelial, and neuronal cells; it mediates fever, anorexia, acute-phase responses, and vascular leakage syndrome, activities that are synergistically modulated by cytokines and pathogen-derived molecules; and administration of agents that specifically inhibit HMGB1 activity (antibodies, antagonist proteins, release inhibitors) to animals with ischemia and inflammatory diseases interrupts the progression of tissue injury and suppresses inflammatory responses. Selectively targeting HMGB1 with neutralizing antibodies provided key pathogenic insights and elucidated important biological activities. This positions HMGB1 at the intersection between sterile and infectious inflammation. Here, we review the progress in the field of HMGB1 biology and the status of HMGB1 as a therapeutic target for inflammatory diseases caused by injury, ischemia, or infection.
Figure 1.
Structure of human HMGB1, a 25-kDa protein of 215 amino acids. Note that 20% of the residues are lysines and that the protein is organized in three domains made up by two positively charged DNA-binding structures (A and B box) and a negatively charged acidic tail composed of 30 glutamic and aspartic acids, exclusively. The A and B boxes are helical structures, partly covered by the tail, which is folded over the protein. There are two nuclear emigration signals in the proximal part of the A and B boxes, respectively, that can bind to the nuclear exportin CRM1. There are also two nuclear-localization signals, as indicated in the figure. The primary HMGB1 sequence is 98.5% identical in all mammals, and two of the three substitutions occur in the repetitive carboxyl terminus with switches of aspartic and glutamic acids. Truncation of the full-length HMGB1 demonstrates that the extracellular cytokine activity resides within the B box. This activity can be competitively inhibited by truncated A box protein. The cysteine in position 106 in the B box is indispensable for its cytokine role, given that oxidation or selective mutation of this residue abolishes the activity of HMGB1 signaling to activate cytokine release.
FROM HMG1 TO HMGB1
HMGB1 was rediscovered at least twice since it was originally identified by Johns and colleagues more than 30 years ago as a member of a group of nonhistone chromosomal proteins termed high mobility group proteins (3). HMGB1 was rediscovered in 1991 by Heikki Rauvala and colleagues, who isolated a membrane-bound protein from the advancing plasma membrane of neurites during embryonic brain development (4). During this period, we had embarked on an unrelated course of study with the objective of isolating cytokines that could be therapeutically modulated to prevent the damaging effects of innate immunity. The chosen approach was to identify mediators of inflammation based on purifying proteins released by macrophages activated by exposure to bacterial lipopolysaccharide (LPS). In 1999, we reported that HMG1 is secreted as a cytokine mediator of inflammation and organ damage and that inhibiting its previously unrecognized inflammatory activities conferred therapeutic advantage during the response to infection and injury (2). As interest in the high mobility group proteins expanded from the field of molecular biology and into immunology and as additional family members were isolated, revision of the nomenclature became necessary. In 2001, the high mobility group protein superfamily was established based on similarities in member chemical-physical properties. As part of this process, HMG1 was renamed HMGB1 (5).
HMGB1 RELEASE DURING STERILE INJURY AND INVASIVE THREAT
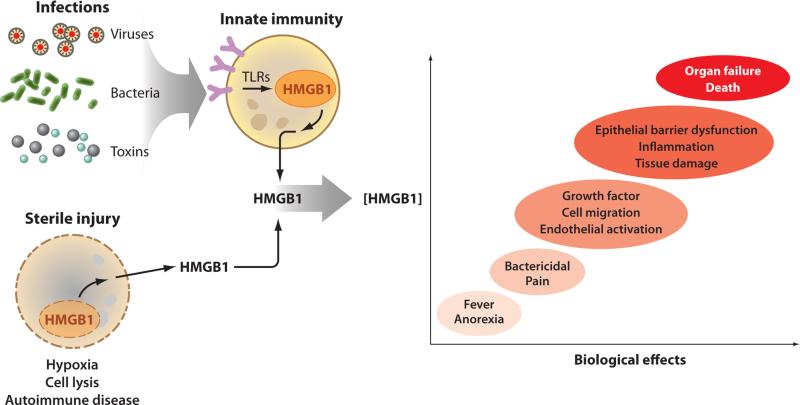
The discovery that HMGB1 occupies a pivotal role in mediating inflammation focused investigation into delineating the mechanistic answers to the important question of how HMGB1 is secreted. Two major pathways of HMGB1 release occur during invasion or injury, one active and the other passive. These are differentiated on the basis of molecular mechanisms, release kinetics, and downstream signaling responses (Figure 2). Passive release, initiated by damage of cellular integrity, is nearly instantaneous (6, 7). Active secretion of HMGB1, initiated by cellular signal transduction through plasma membrane receptor interaction with extracellular products, occurs more slowly (2, 8). Active secretion of HMGB1 occurs when monocytes, macrophages, natural killer cells, dendritic cells, endothelial cells, platelets, and other immunologically competent cells are exposed to microbe-associated molecular patterns (MAMPs), pathogen-associated molecular patterns (PAMPs), and endogenously derived inflammatory mediators including TNF, IL-1, and IFN-γ. Like other proinflammatory mediators that participate in feed-forward regulation, HMGB1 induces its own release in vivo and in vitro (Figure 3). Other cells that can be stimulated to actively secrete HMGB1 include neurons, astrocytes, erythroleukemia cells, neuroblastoma cells, and other tumor cells. Most cells, including monocytes and macrophages, constitutively express HMGB1 protein and mRNA under basal conditions. Following activation of macrophages with LPS, HMGB1 mRNA levels rise over the course of several hours and remain elevated for 24–48 h. The active secretion of HMGB1 into the extracellular milieu begins 8–12 h after ligation of Toll-like receptors (TLRs) and continues to increase for 18–36 h, a time frame that is significantly delayed compared with TNF and IL-1, the prototypical early proinflammatory cytokines (2).
Figure 2.
HMGB1 is released during infection by activating innate immunity and during sterile injury as a passive phenomenon. Exposure to pathogens activates the highly conserved innate immune response, which triggers the release of HMGB1 from monocytes, macrophages, and other cells at the frontline of host defense. HMGB1 shuttles from the nucleus to the cytosol, where it accumulates in intracellular vesicles prior to secretion. This process can take up to 8 h to complete. The passive release of HMGB1 in cells undergoing necrotic cell death is much faster because HMGB1 is loosely attached to nuclear DNA in living cells. HMGB1 binding to chromatin increases during programmed cell death, causing some of it to be retained, but ingestion of apoptotic bodies by macrophages stimulates significant release of HMGB1 by the macrophages (not illustrated). Thus, infection, necrosis, and apoptosis can all lead to elevated HMGB1 levels. In low levels, HMGB1 mediates sickness behavior and antibacterial activities that contribute to inflammatory responses that can resolve. The release of larger amounts of HMGB1, however, is associated with the development of epithelial barrier failure, organ dysfunction, and even death.
Figure 3.
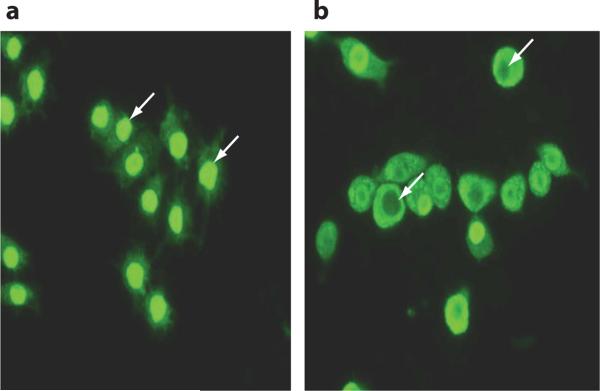
The images illustrate intracellular HMGB1 localization in macrophages (a) before and (b) after 24-h LPS activation. Intracellular HMGB1, visualized with green immunofluorescent FITC staining, is predominately localized in the nucleus in resting macrophages (exemplified with white arrows in a). Following activation by exposure to LPS for 24 h, nuclear HMGB1 is phosphorylated, acetylated, and actively transported from the nucleus to the cytoplasm. Some nuclei even empty their HMGB1 contents (white arrows in b).
HMGB1 release occurs during programmed cell death from at least two sources: (a) directly from the apoptotic cells and (b) by monocytes activated to secrete HMGB1 following exposure to apoptotic cell bodies (9, 10). Initially, investigators thought that cells undergoing apoptosis do not release significant quantities of HMGB1 as assayed by the immunogenic capacity to induce macrophage TNF release. Later evidence revealed that cells undergoing apoptosis indeed release considerable quantities of immunodetectable HMGB1 but that it is immunologically inactive. That is, it fails to significantly stimulate TNF release from responding macrophages compared with HMGB1 released passively during cell necrosis, which is a potent stimulator of TNF production in responding macrophages. The important answer to this dichotomy lies within the reactive oxygen species generated by mitochondria in apoptotic cells that suppresses the inflammatory activity of HMGB1 by oxidizing the cysteine at position 106, a critical residue positioned in the immunostimulatory B box domain of the full-length protein (11). This mechanism provides a critical understanding of why apoptosis fails to activate significant inflammatory responses because experimentally blocking the HMGB1 oxidation step (in order to prevent its immunogenic deactivation) converts the apoptotic events from a typical, immunologically tolerizing event into an immunologically stimulating, proinflammatory event. Prior results that endogenous HMGB1 (derived from necrotic cells) is required for the stimulation of monocyte TNF release (7) and that subjecting recombinant HMGB1 (rHMGB1) to mildly oxidizing conditions renders it immunologically inactive (12) confirm the importance of C106 in the molecular mechanism of HMGB1-mediated inflammation. Thus, endogenous HMGB1 occupies a crucial functional role as a signaling molecule that informs other cells that damage or invasion has occurred.
INFLAMMATORY CELLULAR RESPONSES AND HMGB1 RECEPTORS
HMGB1 has been implicated as a classical proinflammatory mediator because (a) HMGB1 release is stimulated by injury and invasion; (b) it activates immunocompetent cells to produce TNF, IL-1, and other proinflammatory responses; (c) it mediates fever, anorexia, and the sickness syndrome in vivo; (d ) these activities are synergistically enhanced in the presence of exogenous TLR agonists and other proinflammatory cytokines; and (e) it can be specifically targeted to therapeutic advantage in sterile and infectious disease syndromes associated with elevated HMGB1 levels. The inflammatory cellular responses to HMGB1 are listed in Table 1.
Table 1.
Biological activities of extracellular HMGB1
| Target cells | Cellular responses to HMGB1 (references) |
|---|---|
| Macrophages/monocytes | ■ Induction of cytokine, chemokine, and metalloproteinase synthesis; transendothelial migration of monocytes (7, 15, 25, 127) |
| Dendritic cells | ■ Maturation and migration to lymph nodes; increased immunogenicity of handled antigens; secretion of proinflammatory mediators (14, 16, 120) |
| Neutrophils | ■ Activation; chemotaxis (18, 23, 128) |
| Platelets | ■ Procoagulant activity by membrane-HMGB1 expressed on the cell surface of activated platelets (99, 111) |
| T lymphocytes | ■ Proliferation of naive T lymphocytes; Th1 polarization (16, 120) |
| B lymphocytes | ■ Nuclear assistance in VDJ recombination; potentiated activation by HMGB1-DNA-IgG complexes (129, 130) |
| Epithelial cells | ■ Hyperpermeability causing barrier dysfunction in gastrointestinal and respiratory tracts; bactericidal effects (51, 60, 61, 126, 131, 132) |
| Endothelial cells | ■ Proangiogenic; upregulation of adhesion molecules (112, 113, 133, 134) |
| Smooth muscle cells | ■ Migration; proliferation; cytoskeleton reorganization (108, 135) |
| Vessel-associated stem cells (mesoangioblasts) | ■ Proliferation; transendothelial migration (136, 137) |
| Cardiomyocytes | ■ Recruitment and activation of precursor cells used for repair (107) ■ Negative inotropic effects (138, 139) |
| Osteoclasts | ■ Migration; enhanced osteoclastogenesis and TNF synthesis via HMGB1 interaction with the TNF promoter (80, 140) |
| Neurons | ■ Neurite outgrowth during embryogenesis (4, 13) |
| Astrocytes | ■ Proinflammatory activation; glutamate release (97) |
| Microglial cells | ■ Proinflammatory activation; glutamate release (92, 95, 96) |
| Tumor cells | ■ Proliferation; induction of proteolytic enzymes enabling invasiveness; facilitation of metastasis formation (121, 141) |
One distinction from the canonical proinflammatory cytokines (e.g., TNF and IL-1) is that HMGB1 elicits cellular and biological inflammatory responses by signal transduction through receptors that were previously identified for interaction with foreign molecules. Unlike TNF and IL-1, whose cognate plasma membrane receptor families are clearly defined, HMGB1 interacts with several seemingly unrelated receptors that had been previously identified for their capacity to transduce activation signals from exogenous (TLR2, TLR4, and TLR9) and endogenous (RAGE) ligands. Immunologists who had presumed that functional cytokine receptor families were of necessity biochemically restricted to a limited repertoire of cognate cytokines were surprised by the realization that HMGB1 specifically modulates cellular responses via the receptors that can be activated by exogenous, foreign ligands. This has revealed an elegant and simple system, because HMGB1 is a highly conserved and evolutionarily ancient protein capable of activating a uniform cassette of inflammatory responses to infectious or sterile damage. As discussed in detail below, the central role of HMGB1 in gating the magnitude of the inflammatory response to clinical syndromes associated with sterile injury and infection has been revealed by observing the loss of proinflammatory activity following administration of HMGB1 antagonists and by removing either HMGB1 or its receptors through genetic knockout techniques.
The first receptor implicated as a binding partner for HMGB1 is the receptor for advanced glycation end products (RAGE), a transmembrane, cell surface, multiligand member of the immunoglobulin superfamily (13). HMGB1 signaling through RAGE mediates chemotaxis and stimulation of cell growth, differentiation of immune cells, the migration of immune and smooth muscle cells, and the up-regulation of cell surface receptors, including RAGE and TLR4 (14, 15, 16, 17, 18). HMGB1 physically interacts with RAGE, but interaction with TLR4 is required for HMGB1 activation of cytokine release in macrophages because RAGE knockout macrophages and TLR2 knockout macrophages produce TNF when exposed to HMGB1, but TLR4 knockout macrophages do not (Figure 4) (19). HMGB1 binds to TLR4-MD2 as measured by surface plasmon resonance and transduces a signal that stimulates macrophage release of TNF. The binding and signaling both require the redox-sensitive cysteine in position 106, and substitution at this position prevents HMGB1 from binding to TLR4 (19). TLR4 is the primary receptor of endogenous extracellular HMGB1 in mediating macrophage activation, cytokine release, and tissue injury (6, 20–23). This signaling activates IKB kinase (IKK)-β and IKK-α (endotoxin activates only IKK-β) and the nuclear translocation of activated NF-κB (24). There are significant differences in HMGB1- and endotoxin-mediated signaling because HMGB1 binds to TLR4 with much less affinity compared with LPS, and it activates gene expression patterns that are distinct compared with the expression pattern mediated by endotoxin (18, 19, 24). HMGB1 and LPS both significantly increase the nuclear translocation of NF-κB and the phosphorylation of Akt and p38 MAPK, but LPS causes a significantly higher magnitude of NF-κB activation and TNF release compared with HMGB1 (18). Moreover, the induction of TNF release by HMGB1 exhibits a biphasic kinetic profile, whereas endotoxin induces a single, monophasic stimulation of TNF release (25).
Figure 4.
HMGB1 signals by binding to TLR4 to activate macrophage/monocyte cytokine release (left) and by binding to RAGE to modulate endothelial and tumor cell function (right). In monocytes/macrophages, HMGB1 binds TLR4 in the context of MD2 through a mechanism that requires cysteine in position 106. The addition of dithiothreitol (DTT) to HMGB1 denatures this interaction. HMGB1-TLR4 signaling activates MyD88-dependent nuclear translocation of NF-κB, which upregulates the expression of cytokine and other inflammatory mediators. In endothelial cells and other somatic cells, e.g., tumors and smooth muscle, HMGB1 interacts with RAGE. This signaling, which is not sensitive to DTT, is transduced by mechanisms that are incompletely known but culminate on Cdc42 and Rac. Other binding partners may be required, but RAGE signaling has been implicated in cell growth, differentiation, migration, and expression of cell surface proteins. HMGB1 interaction with CD24 and Siglec-10 can mediate a signal that inhibits activation of NF-κB and prevents cytokine release mediated by HMGB1-TLR4 signaling.
Studies of animal models reveal that HMGB1 levels are significantly increased during ischemia-reperfusion injury, increasing within 1 h after reperfusion and remaining elevated for up to 24 h (26) (Figure 5). Treatment of wild-type (C3H/HeOuj) mice with anti-HMGB1 antibodies significantly protects against liver damage, but antibody administration fails to protect TLR4-defective (C3H/Hej) mice, which also develop less damage compared with the wild-type (C3H/HeOuj) mice. HMGB1 signaling through TLR4 is required for cross-presentation of antigens in solid tumors subjected to radiation or chemotherapy (20, 21). TLR4 expression in renal tubules from deceased-donor kidneys stains positively for HMGB1, which directly implicates HMGB1-TLR4 signaling in the development of kidney graft inflammation and sterile injury in humans (27). Moreover, HMGB1 binding to TLR4 in human synovial fibroblasts from rheumatoid arthritis (RA) patients can be revealed by proximity ligation assay, indicating that these molecules interact in the inflammatory cellular milieu (19).
Figure 5.
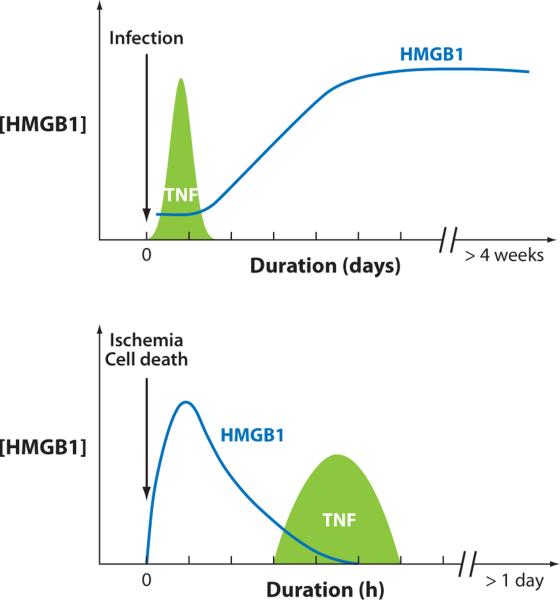
HMGB1 is an early mediator in sterile injury and a late mediator in infection. Infection activates innate immune cells to produce HMGB1, which occurs after a significant lag, placing it downstream of an early TNF response. During ischemia and other forms of sterile cell injury, HMGB1 is released as an early mediator that in turn activates the later release of TNF and other cytokines.
It remains speculative whether other HMGB1-binding proteins participate in cytokine release and the pathogenesis of infection and sterile injury, including TLR2, TLR9, CXCL12, thrombospondin, syndecan, TREM1, and MAC1. It has been known for decades that HMGB1 can facilitate cellular uptake of DNA, and recent evidence has placed this mechanism into the context of inflammation (28). The principle established is that HMGB1 modulates the inflammatory responses to sterile and infectious threat by TLR4 receptor signaling (19). HMGB1 also binds to CD24, a membrane protein expressed by immunocytes, which in turn associates with Siglec-10 to selectively suppress the nuclear translocation of NF-κB induced by HMGB1-mediated TLR4 activation, but not pathogen-mediated TLR activation (29). Together, these results indicate that HMGB1 signaling through TLR4, in the context of sterile injury or infection, can be differentially modulated by cross-talk from HMGB1 signaling through CD24-Siglec-10.
PHYSIOLOGICAL AND PATHOPHYSIOLOGICAL RESPONSES TO HMGB1
Space limitations prevent the presentation of all available data addressing the biology of HMGB1 in sterile and infectious inflammation. An abridged summary of the physiological activities of HMGB1 in organ systems is given in Table 2, and the results from preclinical models of selectively inhibiting HMGB1 action in experimental disease are summarized in Table 3. Here we discuss experimental therapeutic modalities that target HMGB1 release, biological activity, and receptor signal transduction, with a focus on mechanisms for attenuating inflammation and damage in conditions associated with increased extracellular HMGB1 levels, morbidity, and mortality.
Table 2.
Physiological and pathophysiological effects of HMGB1
| Organ system | Response to HMGB1 (references) |
|---|---|
| Central nervous | ■ Anorexia, fever, weight loss, sickness syndrome (98, 142) |
| Cardiovascular | ■ Vascular leakage syndrome, suppression of cardiac output (131, 139) |
| Pulmonary | ■ Hypoxia, inflammation, neutrophil recruitment, ARDS-like syndrome, degradation of lung matrix (60, 64) |
| Gastrointestinal | ■ Inflammation, bacterial translocation, loss of epithelial barrier function (48, 51) |
| Renal-hepatic | ■ Loss of epithelial barrier function, renal tubular injury, hepatic ischemia-reperfusion injury (114, 143) |
| Hematological | ■ Modulation of plasminogen activation (144) |
Table 3.
Experimental disease models responding to therapy targeting HMGB1
| Experimental model | Mode of therapeutic HMGB1 targeting (references) |
|---|---|
| Endotoxemia | ■ Neutralizing polyclonal anti-HMGB1 antibodies (2, 31) |
| ■ Thrombomodulin-mediated inactivation (31, 32) | |
| ■ Decreased release due to nuclear retention (35, 36) | |
| Sepsis induced by lethal peritonitis | ■ Neutralizing monoclonal anti-HMGB1 antibodies (9) |
| ■ Neutralizing polyclonal anti-HMGB1 antibodies (37, 38) | |
| ■ The antagonist HMGB1 A box peptide (37) | |
| ■ Blockade of HMGB1-RAGE signaling (39) | |
| ■ Neutralization by polyspecific intravenous IgG (40) | |
| ■ Polymyxin B filter therapy (42) | |
| ■ Decreased release due to nuclear retention (35, 36, 43–47) | |
| Gastrointestinal disorders | ■ Neutralizing polyclonal anti-HMGB1 antibodies (49, 50) |
| ■ Decreased release due to nuclear retention (52, 53) | |
| Pancreatitis | ■ Neutralizing polyclonal anti-HMGB1 antibodies (58) |
| ■ The antagonist HMGB1 A box peptide (57) | |
| ■ Decreased release due to nuclear retention (56) | |
| Respiratory disorders | ■ Neutralizing polyclonal anti-HMGB1 antibodies (60, 61, 64, 67, 132) |
| ■ The antagonist HMGB1 A box peptide (62) | |
| ■ Thrombomodulin-mediated inactivation (145) | |
| ■ Polymyxin B filter therapy (59) | |
| Arthritis | ■ Neutralizing polyclonal anti-HMGB1 antibodies (67, 74) |
| ■ The antagonist HMGB1 A box peptide (74) | |
| ■ Neutralization by thrombomodulin (75) | |
| ■ Blockade of HMGB1-RAGE signaling (76) | |
| ■ Decreased release due to nuclear retention (46, 77, 78) | |
| Hemorrhagic shock | ■ Neutralization by anti-HMGB1 monoclonal antibody (51, 61) |
| ■ Decreased release due to nuclear retention (89, 146) | |
| Stroke | ■ Neutralizing monoclonal anti-HMGB1 antibodies (100) |
| ■ Neutralizing polyclonal anti-HMGB1 antibodies (101) | |
| ■ The antagonist HMGB1 A box peptide (101) | |
| Myocardial infarction | ■ The antagonist HMGB1 A box peptide (103) |
| Transplantation | ■ The antagonist HMGB1 A box peptide (119) |
| Ischemia-reperfusion injury | ■ Neutralizing polyclonal anti-HMGB1 antibodies (26) |
| ■ The antagonist HMGB1 A box peptide (103) | |
| ■ Decreased release due to nuclear retention (114, 115, 116) |
Endotoxemia
HMGB1 released during endotoxemia mediates lethality downstream of the early proinflammatory cytokines (2, 30–33). Administration of lethal doses of endotoxin to mammals activates a biphasic cytokine response that can be divided into early and late kinetic profiles. The classical proinflammatory cytokine response occurs relatively early, with peak levels of TNF or IL-1 occurring within hours. HMGB1 release takes place significantly later, reaching a plateau 16 to 32 h after the onset of endotoxemia (2). This late occurrence of HMGB1 is required for the full expression of lethal inflammation in endotoxemia. Administration of nontoxic quantities of HMGB1 together with harmless doses of LPS is synergistically toxic or lethal (2). Administration of anti-HMGB1 antibodies to endotoxemic animals several hours after the early peak of TNF confers significant protection from lethality. This delayed administration of anti-HMGB1 antibodies demonstrates that endotoxemia can be therapeutically modulated through a much wider window than previously described for early proinflammatory cytokines (2, 31). Another strategy to neutralize extracellular HMGB1 activity is to administer recombinant soluble thrombomodulin, which binds to HMGB1 via thrombomodulin's N-terminal lectin domain and significantly increases survival of mice after lethal endotoxemia (31, 32). Pharmacological agents that prevent the export of HMGB1 from the nucleus of activated monocytes and block its cellular release have also been used to therapeutic advantage in models of lethal endotoxemia. Efferent vagus nerve signaling prevents the nuclear export of HMGB1 through alpha7-nicotinic acetylcholine receptor-mediated signaling, a mechanism mediated by the cholinergic anti-inflammatory pathway (30, 33–36). Electrical vagus nerve stimulation and administration of selective alpha7-nicotinic acetylcholine receptor agonists decrease extra-cellular HMGB1 levels and prevent mortality in lethal endotoxemia (35, 36).
Sepsis Induced by Live Bacterial Infection
The key findings of HMGB1 biology derived from studies of endotoxin poisoning have been recapitulated in studies of genuine sepsis caused by infection with replicating bacteria. HMGB1 serum levels increase over a 24- to 48-h period after initiation of infection in a standard animal model of polymicrobial gram-negative sepsis caused by surgical cecal ligation and perforation (CLP sepsis model) in rodents. The administration of neutralizing polyclonal and monoclonal anti-HMGB1 antibodies significantly improves survival from sepsis even when the first dose of antibodies is applied 24 h after the onset of infection (9, 37). This is a wide therapeutic window relative to other agents that selectively target cytokine mediators of sepsis. Importantly, anti-TNF antibodies worsen survival from sepsis in this model, highlighting the important differences between TNF and HMGB1: Acute shock and tissue injury are mediated by TNF, whereas lethal organ failure and epithelial barrier failure without shock are mediated by HMGB1. Therapeutic intervention with anti-HMGB1 antibodies is also an effective strategy to limit systemic inflammatory response syndrome following major traumatic injury (38). These results emphasize a generalized role for HMGB1 in the initiation and propagation of inflammation and organ injury in settings of infectious as well as sterile systemic inflammation.
Another successful strategy to inhibit the activity of HMGB1 in the CLP model is based on administering recombinant HMGB1 A box (37). The truncated protein is a competitive antagonist of HMGB1 that displaces HMGB1 binding to cells. Repeated injections of HMGB1 A box significantly improves survival in animals with established sepsis. The response to A box is time and dose dependent, with maximal efficacy seen when A box is first administered 12 or 24 h after cecal perforation, placing these results in line with those observed with anti-HMGB1 antibody administration. Delayed administration of monoclonal anti-RAGE antibodies to wild-type mice for up to 24 h after CLP significantly improves survival (39). Therapy with polyspecific IgG for intravenous use (IVIG) has been successfully applied in CLP with resultant decreases in serum and lung tissue levels of HMGB1 that have been attributed to HMGB1-specific antibodies present in pooled IVIG (40, 41). Hemoperfusion with polymyxin B–immobilized fiber columns effectively removes HMGB1 from the circulation of piglets subjected to CLP and significantly decreases serum HMGB1 levels in patients with shock (42).
Covalent DNA adducts generated by the platinating agent cisplatin sequesters nuclear HMGB1, and administration of nontoxic doses of cisplatin significantly attenuates mortality following CLP (43). Treatment also results in decreased systemic release of HMGB1 and protection from end organ injury. Administration of rHMGB1 to cisplatin-treated septic mice recapitulates the lethality of CLP (43). Nuclear retention of HMGB1 has been observed following activation of the cholinergic anti-inflammatory pathway, which also significantly improves survival in the CLP model. Transcutaneous vagus nerve stimulation and administration of selective alpha7-nicotinic acetylcholine receptor subunit agonists reduce HMGB1 serum levels and significantly increase survival from CLP (44). In agreement with the delayed kinetic profile of HMGB1 release in this model, these therapeutic interventions can be delayed for up to 24 h after the onset of cecal perforation and still confer protection. Ethyl pyruvate, an aliphatic ester derived from pyruvic acid, decreases the nuclear translocation of NF-κB, significantly inhibits HMGB1 release, ameliorates organ dysfunction, and improves survival in CLP, even when therapy is delayed for 24 h after the onset of infection (45). The endogenous orexigenic peptide ghrelin and the neuropeptides urocortin and vasoactive intestinal peptide (VIP) have been identified as inhibitors of HMGB1 secretion. Therapy with each of these peptides rescues mice from mortality in the CLP model even when injected 24 h after induction of disease, and administration of recombinant HMGB1 completely reverses the protective effect of the peptides (46, 47). Together, the preponderance of evidence for neutralizing or inhibiting HMGB1 in lethal sepsis reveals that HMGB1 occupies a major pathogenic role on the final common pathway to death that can be targeted to therapeutic advantage.
Gastrointestinal Inflammation
A fundamental mechanistic aspect of HMGB1-mediated toxicity is epithelial dysfunction (48). Models of inflammatory bowel disease based on chemically induced colitis respond favorably to anti-HMGB1 antibody therapy (49). Moreover, anti-HMGB1 reduces tumor incidence in this colitis-associated cancer model. Intraperitoneal LPS injection, cecal perforation, and hemorrhagic shock each cause increased HMGB1 levels in serum and bile, and these increased levels mediate the development of intestinal hyperpermeability and bacterial translocation. Gut barrier dysfunction can be significantly restored by treatment with either anti-HMGB1 antibodies, ethyl pyruvate, or ghrelin (50–53).
Pancreatitis
Serum HMGB1 levels are significantly elevated in patients with acute pancreatitis, and levels correlate with the severity of the clinical course and survival (54, 55). Similar results have been observed in preclinical animal models of acute pancreatitis (54). HMGB1 blocking therapy by polyclonal anti-HMGB1 antibodies, A box protein, or ethyl pyruvate administered in five models of acute pancreatitis reduces the incidence of multiple organ failure syndrome and significantly increases survival (56–58).
Respiratory Disorders
Hemoperfusion with polymyxin B–immobilized fiber columns in patients with sepsis and inflammatory lung injury reduces serum HMGB levels (59). HMGB1 blocking therapies ameliorate disease manifestations in acute lung injury, including anti-HMGB1 antibodies and A box (60–62). Patients subjected to long-term ventilator therapy develop significantly increased HMGB1 levels in bronchoalveolar lavage fluid (63). Analogous observations have been made in ventilator-induced lung injury in rabbits, where intratracheal installation of anti-HMGB1 antibodies attenuates the damage (64). Anti-HMGB1 antibodies as well as ethyl pyruvate suppress the development of pulmonary fibrosis in a mouse model based on bleomycin-induced lung injury (65). HMGB1 levels are increased in the sputum of patients with cystic fibrosis and have been implicated in mediating neutrophil chemotaxis via a CXC chemokine receptor–dependent mechanism; the addition of anti-HMGB1 antibodies to sputum significantly reduces chemotaxis (66).
Arthritis and Other Autoimmune Diseases
Serum and synovial fluid HMGB1 levels are increased in patients with RA (67–70). Biopsy specimens from rheumatoid synovitis reveal aberrant HMGB1 expression, particularly in areas where the synovial tissue (pannus) invades intra-articular cartilage and bone, which causes joint destruction (67–69). Intra-articular corticosteroid injections, which ameliorate joint inflammation, reduce extracellular synovial HMGB1 expression in RA patients (71). Administration of gold salts, a traditional therapy for RA, inhibits HMGB1 release from activated macrophages (72). Injection of recombinant HMGB1 into the knee joints induces long-lasting, destructive arthritis (73). The prototypical experimental model used to study novel therapeutic approaches for RA is collagen type II–induced arthritis (CIA) in rodents. Activated synovial macrophages and fibroblasts in CIA actively secrete HMGB1 within the arthritis lesions; there is a distinct colocalization of aberrant HMGB1 expression with tissue hypoxia (67). Arthritis and inflammation in CIA are significantly attenuated by administration of monoclonal anti-HMGB1 antibodies, polyclonal anti-HMGB1 antibodies, A box protein, recombinant thrombomodulin, soluble RAGE, alpha7 nicotinic acetylcholine receptor subunits, or the HMGB1-release inhibitors oxaliplatin, PACAP, or ghrelin (46, 67, 74–78). Importantly, neutralization of HMGB1 confers significant protection against cartilage and bone destruction that is the hallmark of permanent disability in RA. The mechanism of damage may be mediated by HMGB1 acting as a transcription factor that upregulates TNF expression in osteoclasts and as a coactivator for the transcription of TNF and IL-1 (76–80). Treatment of CIA animals with a single injection of the DNA-platinating compound oxaliplatin confers strong beneficial, transient clinical improvement coinciding with intranuclear HMGB1 entrapment. Upon cessation of oxaliplatin, a clinical rebound effect follows that coincided with extracellular HMGB1 release, presumably because DNA platination is reversed (78). In an important new model of spontaneous arthritis, DNase II−/−x IFN-IR−/− mice develop symmetric polyarthritis with significantly enhanced synovial tissue expression of cytosolic and extracellular HMGB1 (81). Administration of either neutralizing anti-HMGB1 monoclonal antibodies or A box ameliorates the severity of arthritis and confers significant protection against joint destruction (81).
Pathological HMGB1 expression has also been implicated in polymyositis and dermatomyositis (in muscle tissue) and in systemic lupus erythematosus (SLE) (in serum and dermal tissue) (82–84). Glucocorticoid treatment of patients with chronic myositides causes reduced expression of HMGB1 in muscle tissue in association with clinical improvement (82). Exposure of murine skeletal muscle to HMGB1 decreases calcium release from sarcoplasmic reticulum during repeated tetanic contractions and upregulates expression of MHC class I, suggesting a pathogenic role in the development of muscle fatigue (85).
Serum HMGB1 levels are significantly increased in patients with active lupus and correlate to disease activity (86). Accumulation of dead cells also has been implicated in the pathogenesis of SLE, and recent evidence indicates that HMGB1-nucleosome complexes from apoptotic cells injected into normal mice are capable of breaking immunological tolerance, leading to the production of antibodies against double-stranded DNA (87). The overexpression of HMGB1 may be critical to pathogenesis because neither HMGB1-free nucleosomes from viable cells nor apoptotic bodies from HMGB1 gene–deficient animals manage to break tolerance or activate dendritic cells or macrophages (87).
Hemorrhagic Shock/Trauma
Early clinical observations revealed that hemorrhagic shock stimulates an increase in serum HMGB1 levels within hours after aortic aneurysm rupture (88). In animal models, HMGB1 release also occurs early in the course of hemorrhagic shock, and it occupies an important role in pathogenesis (23, 51, 89, 90). Necrotic somatic cells and activated platelets rapidly deliver the extracellular- and cell membrane–presented HMGB1. Treatment with neutralizing anti-HMGB1 antibodies improves survival and ameliorates hemorrhage-induced acute lung injury and gut barrier dysfunction (51). Hemorrhagic shock–induced lung injury and NADPH oxidase activation in neutrophils are HMGB1-TLR4-mediated events (23, 61, 90). Additional HMGB1-targeted therapeutic interventions that have improved the outcome in experimental hemorrhagic shock include the use of soluble RAGE protein, which binds HMGB1, or activation of the cholinergic anti-inflammatory pathway, which inhibits HMGB1 release (89).
Cerebral Ischemia and Injury
HMGB1 contributes to the molecular mechanisms that mediate tissue damage during ischemic injury in the nervous system. There are two major pathways of HMGB1-dependent cell neuronal death: (a) glutamate excitotoxicity-induced neuronal death in the ischemic core and (b) delayed inflammatory damage in the penumbra. Patients with cerebral ischemia have elevated circulating HMGB1 levels within hours after the onset of symptoms (91). Tissue ischemia in rodent models of vascular occlusion demonstrates that HMGB1 is immediately released into the extracellular space (92–94). Nuclear HMGB1 translocates from the neuronal cell nucleus into the cytoplasm within 1 h after the onset middle cerebral artery occlusion and is then exported from the cell (92). During reperfusion, HMGB1 is aberrantly expressed in the penumbra by activated microglia cells, astrocytes, macrophages, and endothelial cells (92). Short, hairpin RNA-mediated HMGB1 downregulation significantly reduces tissue damage during ischemia (93). Increased levels of extracellular HMGB1 stimulate glutamate release, which in turn mediates neuronal cytotoxicity (95, 96). Elevated HMGB1 levels lead to recruitment of monocytes/macrophages and activation of astrocytes and microglia and induce production of TNF and IL-6 (96–98). HMGB1 enhances procoagulant activity, which contributes to damage propagation by clotting microvasculature and worsening ischemia (99). Administration of neutralizing monoclonal anti-HMGB1 antibodies to animals subjected to middle cerebra artery occlusion confers significant neuroprotection and reduces the magnitude of cell death (100). Intracerebroventricular injection of rHMGB1 significantly worsens the severity of tissue loss during ischemia. Therapy based on administration of polyclonal anti-HMGB1 antibodies, A box, and soluble RAGE confers significant benefit during occlusion (101). Direct injection of HMGB1 into the hippocampus prior to injection of kainic acid significantly enhances the severity of seizures (102). TLR4-defective C3H/HeJ mice were resistant to seizures, and administration of A box to wild-type mice significantly attenuated seizure severity. Together with other results, HMGB1-TLR4 signaling is implicated in the generation and perpetuation of trauma-induced seizures (102).
Myocardial Infarction
Serum HMGB1 levels are significantly increased in patients with acute myocardial infarction (MI), and elevated levels are associated with adverse clinical outcomes including pump failure, cardiac rupture, and in-hospital deaths (91). To date, studies addressing the question of whether HMGB1 exerts salutary or detrimental effects in acute MI have generated conflicting results (103–106). Administration of the HMGB1 A box in a mouse model of transient coronary vessel occlusion was associated with significant attenuation of tissue damage. In agreement with this mechanism, systemically administered rHMGB1 significantly worsened the severity of damage (103). Conflicting results were observed from a therapeutic study of neutralizing monoclonal anti-HMGB1 antibodies administered during acute transient coronary occlusion: Antibody-treated animals developed significantly increased infarcts (105). Cardiac-specific overexpression of HMGB1 conferred significant protection against tissue damage during MI and was associated with improved cardiac function compared with controls (106). Administration of rHMGB1 directly into the myocardium during acute MI enhances cardiac tissue regeneration by activating resident cardiac c-kit+ cells to form new myocytes during healing (106). Direct intramyocardial injection of rHMGB1 has been proposed as a method to facilitate tissue repair following MI (106, 107).
Atherosclerosis
Smooth muscle cells isolated from atherosclerotic plaques, but not normal arteries, secrete extracellular HMGB1 when loaded with cholesterol, and when exposed to HMGB1, they proliferate, migrate, and are activated to secrete more HMGB1 (108). Several cell types in atherosclerotic plaque contribute to HMGB1 release, including endothelial cells, smooth muscle cells, neointimal foam cells, macrophages, and activated platelets (108–111). Recombinant HMGB1 stimulates inflammatory responses in endothelial cells, including enhanced expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), RAGE, TNF, CXCL8, CCL2, plasminogen activator inhibitor 1 (PAI-1), and tissue plasminogen activator (tPA) (112). These inflammatory responses in endothelial cells can be recapitulated by B box, which significantly enhances expression of ICAM-1, VCAM-1, E-selectin, CXCL8, and G-CSF (113). Exposure of HUVECs to HMGB1 significantly increases phosphorylation of ELK-1 signal transduction proteins and the nuclear translocation of NF-κB (113).
Ischemia-Reperfusion Injury and Transplantation
Ischemia-reperfusion activates innate immunity, and the resultant cytokine release directly mediates the development of systemic inflammatory responses and local tissue damage. HMGB1 is released as an early mediator of ischemia-reperfusion injury (26, 27, 88, 103, 114–116). Administration of neutralizing HMGB1 antibodies mitigates hepatic injury during liver ischemia-reperfusion (26). Ethyl pyruvate inhibits HMGB1 release and attenuates tissue damage after spinal cord or renal ischemia-reperfusion (114, 115). Administration of cisplatin and glycyrrhizin confers significant protection against tissue injury and prevents extracellular HMGB1 release in hepatic ischemia-reperfusion models (116). The preponderance of data from animal models of ischemia and HMGB1 directly implicate TLR4 as the receptor that mediates cytokine release and resultant tissue damage (6, 26, 117).
HMGB1 is released passively from damaged cells during organ transplantation, contributing to elevated plasma HMGB1 levels that correlate to hepatocellular injury in the human liver (118). Reperfusion after cold ischemia in explanted liver is associated with immediate and extensive HMGB1 efflux from the graft; similar results have been observed in murine cardiac transplantation (119). Therapeutic administration of the HMGB1 A box significantly enhances cardiac allograft survival (119). In the mixed lymphocyte reaction, HMGB1 is required for maturation and migration of immature dendritic cells during the priming of naive T lymphocytes and Th1 polarization (16, 120). The mechanism by which A box prolongs graft survival depends on retarded allorecognition responses that result in depressed alloimmune reactions and delayed allograft rejection.
Tumors
As with TNF and other cytokines that possess both growth factor and cytotoxic activities, HMGB1 has been implicated in diverse roles in tumor biology. It can mediate tumor cell growth and immune cell–dependent cytotoxicity (21, 121–123). Undifferentiated cells and tumors express high levels of HMGB1. Rapidly growing tumors can outstrip their blood supplies, and the resultant intratumoral ischemia can contribute to HMGB1 release. Extracellular HMGB1 in the tumor microenvironment stimulates tumor cell proliferation, promotes neoangiogenesis, recruits macrophages, enhances the formation of plasmin and metalloproteinases to facilitate tumor invasiveness, and enhances the formation of metastases (121–123). Administration of HMGB1 antagonists is efficacious in slowing tumor growth in some settings (121–123). However, HMGB1 is required for recruiting and activating dendritic cells and for enabling cytotoxic T cell responses against tumor cells during radiation therapy (20, 21). During chemotherapy or radiotherapy, dendritic cell signaling through TLR4 and MyD88 is activated by HMGB1, which results in cross-presentation of tumor antigen. The clinical importance of this HMGB1-TLR4 signaling cascade was recently revealed by studies of breast cancer patients because individuals with a TLR4 loss-of-function allele relapsed significantly faster compared with carriers of the normal TLR4 allele (21). Together, these results indicate that HMGB1 release and signal transduction through TLR4 confers significant protection against cancer, and more study is under way to address potential therapeutic advantage.
HMGB1 AT THE INTERSECTION OF STERILE AND INFECTIOUS THREAT: FUTURE ISSUES
The HMGB1 paradigm has enabled an understanding of how sterile injury elicits innate immune responses that are qualitatively indistinguishable from the responses activated by foreign, microbial-derived molecules. Major clinical signs and symptoms resulting from cytokine release cannot determine whether the inciting event is sterile or infectious. As we have reviewed here, HMGB1 is a soluble factor released into the extracellular milieu that activates inflammatory responses in responding cells. Another argument, however, should be made: Intracellular and extracellular HMGB1 have critical roles in the early detection of invasion and injury that culminates in activating innate immunity. HMGB1 is ubiquitous in the extracellular milieu and in tissue culture conditions (serum levels in healthy mammals are on the order of 10 ng/ml, rising to as high as 200 ng/ml during infection and injury). Extracellular HMGB1 binds endotoxin, bacterial DNA, viral RNA, and other pathogenic molecules at the earliest stages of invasion, and the formation of these complexes synergistically increases the capacity for activation of innate immunity (2, 124). IL-1β released by either infectious or sterile injury also forms complexes with HMGB1, which synergistically enhances the innate immune response to both factors (124, 125).
Whereas cell biologists tend to focus on the plasma membrane–bound member of a receptor-ligand pair as being the receptor, immunologists have long studied numerous examples of cytokine receptors that function as soluble factors in the extracellular milieu. In this context, these soluble receptors interact with binding partners to form complexes that in turn activate or suppress biological responses in responding cells. Depending on the nature of other factors in the extracellular milieu, the soluble receptors are capable of either increasing or decreasing the activities of their binding partners and mediating pleiotropic immune and metabolic responses in responding cells. HMGB1 in the extracellular milieu forms complexes with pathogenic molecules that can synergistically increase the magnitude of innate immune responses. In the dozens of animal models of sterile and infection-induced inflammation cited here, and in others that could not be included because of space limitations, the overwhelming evidence indicates that removing or neutralizing HMGB1 significantly inhibits activation of innate immune responses and reduces tissue damage.
Advances in gene knockout technology, the availability of neutralizing anti-HMGB1 monoclonal antibodies and reagents that suppress HMGB1 release, knowledge of the importance of the post-translational modification of cysteine 106, and evidence that HMGB1 binds and signals via TLR4 to mediate tissue damage all reveal that endogenous HMGB1 occupies a central role in modulating innate immunity and mediating injury. Additional knowledge should enable the development of therapeutics for clinical studies of HMGB1.
As was true for other cytokine targets that have already achieved clinical approval, it will be important to generate antibodies that suppress injury and prevent TLR4-dependent signaling without significantly interfering with beneficial responses attributed to HMGB1. These include CD24-mediated counter-regulation of inflammation, cell migration, antimicrobial actions, potential antitumor activities, and enhanced tissue repair (20, 29, 126). Technical considerations for producing neutralizing monoclonal antibodies and A box should incorporate knowledge based on selectively modulating HMGB1 bioactivity in inflammatory responses mediated by TLR4, as distinguished from HMGB1 growth and migration properties mediated by RAGE. Development of neutralizing antibodies will likely incorporate additional knowledge about the molecular structure of endogenous HMGB1 released by stressed, hypoxic, or necrotic cells and potential post-translational modifications. Clearly, the time has arrived to translate the physiologically relevant results from experimental models into therapeutics for clinical trials.
SUMMARY POINTS.
HMGB1 resides at the intersection of foreign and endogenous molecules that activate innate immunity: It is released by injured cells in the absence of exogenous agents and by cells exposed to pathogen-derived molecules.
HMGB1 is a proinflammatory cytokine that mediates cytokine release, inflammation, maturation of dendritic cells, epithelial barrier failure, and endothelial activation.
The two DNA-binding domains of HMGB1 have distinct biological activities: The B box recapitulates the inflammatory activities of the full-length protein, whereas the A box antagonizes it.
HMGB1 binding and signaling through TLR4 mediates cytokine release and tissue injury in animal models of infection, ischemia, and injury.
Anti-HMGB1 antibodies confer significant protection against damage in diverse acute and chronic diseases caused by infection, autoimmunity, and ischemia.
HMGB1: High mobility grop box 1
MAMP: microbe-associated molecular pattern
PAMP: pathogen-associated molecule pattern
TLR: Toll-like receptor
RAGE: receptor for advanced glycation end products
ACKNOWLEDGMENTS
HMGB1 research in the authors’ laboratories is supported by the Karolinska University Hospital, the Karolinska Institutet, and the Swedish Research Council (U. Andersson) and by the National Institutes of General Medical Sciences (K.J. Tracey).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim. Biophys. Acta. 2010;1799:101–13. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, et al. HbMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [Discovery of the role of HMGB1 as a necessary and sufficient mediator of inflammation.] [DOI] [PubMed] [Google Scholar]
- 3.Johns EW, Goodwin CHM, Walker JM, Sanders C. Chromosomal proteins related to histones. Ciba Found. Symp. 1975;28:95–112. [Google Scholar]
- 4.Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J. Biol. Chem. 1991;266:16722–29. [PubMed] [Google Scholar]
- 5.Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem. Sci. 2001;26:152–53. doi: 10.1016/s0968-0004(00)01777-1. [DOI] [PubMed] [Google Scholar]
- 6.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4–dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 2007;204:2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–95. doi: 10.1038/nature00858. [Cell injury and necrosis require HMGB1 to induce inflammation.] [DOI] [PubMed] [Google Scholar]
- 8.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, et al. The nuclear protein HMGB1 is secreted by monocytes via a nonclassical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin S, Wang H, Yuan R, Li H, Ochani M, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006;203:1637–42. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauley J, Pisetsky DS. The translocation of HMGB1 during cell activation and cell death. Autoimmunity. 2009;42:299–301. doi: 10.1080/08916930902831522. [DOI] [PubMed] [Google Scholar]
- 11.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [Blocking oxidation of HMGB1 prevents tolerance induction by apoptotic cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Wang H, Mason JM, Levine J, Yu M, et al. Recombinant HMGB1 with cytokine-stimulating activity. J. Immunol. Methods. 2004;289:211–23. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Hori O, Brett J, Slattery T, Cao R, Zhang J, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and coexpression of RAGE and amphoterin in the developing nervous system. J. Biol. Chem. 1995;270:25752–61. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 15.Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, et al. Regulation of monocyte migration by amphoterin (HMGB1). Blood. 2004;104:1174–82. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- 16.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 2005;174:7506–15. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 17.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 18.Silva E, Arcaroli J, He Q, Svetkauskaite D, Coldren C, et al. HMGB1 and LPS induce distinct patterns of gene expression and activation in neutrophils from patients with sepsis-induced acute lung injury. Intensive Care Med. 2007;33:1829–39. doi: 10.1007/s00134-007-0748-2. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, et al. A critical cysteine is required for HMGB1 binding to TLR4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. USA. 2010;107:11943–47. doi: 10.1073/pnas.1003893107. [HMGB1 activates cytokine production by binding and signaling through TLR4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol. Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 21.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–59. doi: 10.1038/nm1622. [HMGB1 enables cytotoxic T cell responses against tumors mediated by TLR4 signaling.] [DOI] [PubMed] [Google Scholar]
- 22.Tsung A, Zheng N, Jeyabalan G, Izuishi K, Klune JR, et al. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J. Leukoc. Biol. 2007;81:119–28. doi: 10.1189/jlb.0706468. [DOI] [PubMed] [Google Scholar]
- 23.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J. Immunol. 2007;178:6573–80. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 24.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, et al. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–77. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 25.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [HMGB1 is released as an early mediator of ischemia-reperfusion injury.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc. Natl. Acad. Sci. USA. 2009;106:3390–95. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 29.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–25. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tracey KJ. Reflex control of immunity. Nat. Rev. Immunol. 2009;9:418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Investig. 2005;115:1267–74. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagato M, Okamoto K, Abe Y, Higure A, Yamaguchi K. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit. Care Med. 2009;37:2181–86. doi: 10.1097/CCM.0b013e3181a55184. [DOI] [PubMed] [Google Scholar]
- 33.Andersson U, Tracey KJ. HMGB1 in sepsis. Scand. J. Infect. Dis. 2003;35:577–84. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- 34.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 2008;14:567–74. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Liao H, Ochani M, Justiniani M, Lin X, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 36.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007;35:1139–44. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Ochani M, Li J, Qiang X, Tanovic M, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [Recombinant HMGB1 A box improves survival in experimental sepsis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, et al. Systemic inflammation and remote organ injury following trauma require HMGB1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1538–44. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 39.Lutterloh EC, Opal SM, Pittman DD, Keith JC, Jr, Tan XY, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit. Care. 2007;11:R122. doi: 10.1186/cc6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagiwara S, Iwasaka H, Hasegawa A, Asai N, Noguchi T. High-dose intravenous immunoglobulin G improves systemic inflammation in a rat model of CLP-induced sepsis. Intensive Care Med. 2008;34:1812–19. doi: 10.1007/s00134-008-1161-1. [DOI] [PubMed] [Google Scholar]
- 41.Urbonaviciute V, Furnrohr BG, Weber C, Haslbeck M, Wilhelm S, et al. Factors masking HMGB1 in human serum and plasma. J. Leukoc. Biol. 2007;81:67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto Y, Mashiko K, Matsumoto H, Hara Y, Kutsukata N, Yamamoto Y. Relationship between effect of polymyxin B-immobilized fiber and high-mobility group box-1 protein in septic shock patients. ASAIO J. 2007;53:324–28. doi: 10.1097/MAT.0b013e3180340301. [DOI] [PubMed] [Google Scholar]
- 43.Pan P, Cardinal J, Dhupar R, Rosengart MR, Lotze MT, et al. Low-dose cisplatin administration in murine cecal ligation and puncture prevents the systemic release of HMGB1 and attenuates lethality. J. Leukoc. Biol. 2009;86:625–32. doi: 10.1189/JLB.1108713. [DOI] [PubMed] [Google Scholar]
- 44.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 2007;35:2762–68. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 45.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl. Acad. Sci. USA. 2002;99:12351–56. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J. Immunol. 2008;180:8369–77. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- 47.Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am. J. Pathol. 2008;172:1297–307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 49.Maeda S, Hikiba Y, Shibata W, Ohmae T, Yanai A, et al. Essential roles of high-mobility group box 1 in the development of murine colitis and colitis-associated cancer. Biochem. Biophys. Res. Commun. 2007;360:394–400. doi: 10.1016/j.bbrc.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 50.Yang R, Miki K, Oksala N, Nakao A, Lindgren L, et al. Bile high-mobility group box 1 contributes to gut barrier dysfunction in experimental endotoxemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R362–69. doi: 10.1152/ajpregu.00184.2009. [DOI] [PubMed] [Google Scholar]
- 51.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol. Med. 2006;12:105–14. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dave SH, Tilstra JS, Matsuoka K, Li F, Demarco RA, et al. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J. Leukoc. Biol. 2009;86:633–43. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu R, Dong W, Qiang X, Wang H, Blau SA, et al. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Crit. Care Med. 2009;37:2421–26. doi: 10.1097/CCM.0b013e3181a557a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasuda T, Ueda T, Shinzeki M, Sawa H, Nakajima T, et al. Increase of high-mobility group box chromosomal protein 1 in blood and injured organs in experimental severe acute pancreatitis. Pancreas. 2007;34:487–88. doi: 10.1097/MPA.0b013e31804154e4. [DOI] [PubMed] [Google Scholar]
- 55.Kocsis AK, Szabolcs A, Hofner P, Takacs T, Farkas G, et al. Plasma concentrations of high-mobility group box protein 1, soluble receptor for advanced glycation end-products and circulating DNA in patients with acute pancreatitis. Pancreatology. 2009;9:383–91. doi: 10.1159/000181172. [DOI] [PubMed] [Google Scholar]
- 56.Yang R, Shaufl AL, Killeen ME, Fink MP. Ethyl pyruvate ameliorates liver injury secondary to severe acute pancreatitis. J. Surg. Res. 2009;153:302–9. doi: 10.1016/j.jss.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Yuan H, Jin X, Sun J, Li F, Feng Q, et al. Protective effect of HMGB1 A box on organ injury of acute pancreatitis in mice. Pancreas. 2009;38:143–48. doi: 10.1097/MPA.0b013e31818166b4. [DOI] [PubMed] [Google Scholar]
- 58.Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, et al. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J. Gastroenterol. 2006;12:7666–70. doi: 10.3748/wjg.v12.i47.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura T, Fujiwara N, Sato E, Kawagoe Y, Ueda Y, et al. Effect of polymyxin B-immobilized fiber hemoperfusion on serum high mobility group box-1 protein levels and oxidative stress in patients with acute respiratory distress syndrome. ASAIO J. 2009;55:395–99. doi: 10.1097/MAT.0b013e3181a5290f. [DOI] [PubMed] [Google Scholar]
- 60.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–54. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 61.Kim JY, Park JS, Strassheim D, Douglas I, Diaz V, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L958–65. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 62.Gong Q, Xu JF, Yin H, Liu SF, Duan LH, Bian ZL. Protective effect of antagonist of high-mobility group box 1 on lipopolysaccharide-induced acute lung injury in mice. Scand. J. Immunol. 2009;69:29–35. doi: 10.1111/j.1365-3083.2008.02194.x. [DOI] [PubMed] [Google Scholar]
- 63.van Zoelen MA, Ishizaka A, Wolthuls EK, Choi G, van der Poll T, Schultz MJ. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29:441–45. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa EN, Ishizaka A, Tasaka S, Koh H, Ueno H, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2006;174:400–7. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- 65.Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, et al. The role of high mobility group box 1 in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2008;39:440–47. doi: 10.1165/rcmb.2007-0330OC. [DOI] [PubMed] [Google Scholar]
- 66.Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, et al. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am. J. Respir. Crit. Care Med. 2008;178:822–31. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamada T, Torikai M, Kuwazuru A, Tanaka M, Horai N, et al. Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum. 2008;58:2675–85. doi: 10.1002/art.23729. [DOI] [PubMed] [Google Scholar]
- 68.Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune MyD88-dependent signaling drives procatabolic effects of the endogenous TLR2/TLR4 ligands LMW-HA and HMGB1. Arthritis Rheum. 2010;62:2004–12. doi: 10.1002/art.27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2008;46:2598–603. doi: 10.1002/art.10540. [DOI] [PubMed] [Google Scholar]
- 70.Goldstein RS, Bruchfeld A, Yang L, Qureshi AR, Gallowitsch-Puerta M, et al. Cholinergic anti-inflammatory pathway activity and high mobility group box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 2007;13:210–15. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.af Klint E, Grundtman C, Engstrom M, Catrina AI, Makrygiannakis D, et al. Intraarticular glucocorticoid treatment reduces inflammation in synovial cell infiltrations more efficiently than in synovial blood vessels. Arthritis Rheum. 2005;52:3880–89. doi: 10.1002/art.21488. [DOI] [PubMed] [Google Scholar]
- 72.Zetterstrom CK, Jiang W, Wahamaa H, Ostberg T, Aveberger AC, et al. Pivotal advance: inhibition of HMGB1 nuclear translocation as a mechanism for the antirheumatic effects of gold sodium thiomalate. J. Leukoc. Biol. 2008;83:31–38. doi: 10.1189/jlb.0507323. [DOI] [PubMed] [Google Scholar]
- 73.Pullerits R, Jonsson IM, Verdrengh M, Bokarewa M, Andersson U, et al. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 2003;48:1693–700. doi: 10.1002/art.11028. [DOI] [PubMed] [Google Scholar]
- 74.Kokkola R, Li J, Sundberg E, Aveberger AC, Palmblad K, et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48:2052–58. doi: 10.1002/art.11161. [Experimental therapeutics that antagonize HMGB1 attenuate collagen-induced arthritis.] [DOI] [PubMed] [Google Scholar]
- 75.Van de WM, Plaisance S, De Vriese A, Waelkens E, Collen D, et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J. Thromb. Haemost. 2006;4:1813–24. doi: 10.1111/j.1538-7836.2006.02033.x. [DOI] [PubMed] [Google Scholar]
- 76.Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–35. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 77.van Maanen MA, Lebre MC, van der Poll T, Larosa GJ, Elbaum D, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–22. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 78.Östberg T, Wahamaa H, Palmblad K, Ito N, Stridh P, et al. Oxaliplatin retains HMGB1 intranu-clearly and ameliorates collagen type II-induced arthritis. Arthritis Res. Ther. 2008;10:R1. doi: 10.1186/ar2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mouri F, Tsukada J, Mizobe T, Higashi T, Yoshida Y, et al. Intracellular HMGB1 transactivates the human IL1B gene promoter through association with an Ets transcription factor PU.1. Eur. J. Haematol. 2008;80:10–19. doi: 10.1111/j.1600-0609.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 80.Yamoah K, Brebene A, Baliram R, Inagaki K, Dolios G,A, et al. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol. Endocrinol. 2008;22:1141–53. doi: 10.1210/me.2007-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Östberg T, Kawane K, Nagata S, Yang H, Chavan S, et al. Protective targeting of HMGB1 in a spontaneous arthritis model. Arthritis Rheum. 2010;62:2963–72. doi: 10.1002/art.27590. [DOI] [PubMed] [Google Scholar]
- 82.Ulfgren AK, Grundtman C, Borg K, Alexanderson H, Andersson U, et al. Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis Rheum. 2004;50:1586–94. doi: 10.1002/art.20220. [DOI] [PubMed] [Google Scholar]
- 83.Jiang W, Pisetsky DS. Expression of high mobility group protein 1 in the sera of patients and mice with systemic lupus erythematosus. Ann. Rheum. Dis. 2008;67:727–28. doi: 10.1136/ard.2007.074484. [DOI] [PubMed] [Google Scholar]
- 84.Barkauskaite V, Ek M, Popovic K, Harris HE, Wahren-Herlenius M, Nyberg F. Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus. 2007;16:794–802. doi: 10.1177/0961203307081895. [DOI] [PubMed] [Google Scholar]
- 85.Grundtman C, Bruton J, Yamada T, Östberg T, Pisetsky DS, et al. Effects of HMGB1 on in vitro responses of isolated muscle fibers and functional aspects in skeletal muscles of idiopathic inflammatory myopathies. FASEB J. 2010;24:570–78. doi: 10.1096/fj.09-144782. [DOI] [PubMed] [Google Scholar]
- 86.Li J, Xie H, Wen T, Liu H, Zhu W, Chen X. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J. Rheumatol. 2010;37:766–75. doi: 10.3899/jrheum.090663. [DOI] [PubMed] [Google Scholar]
- 87.Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med. 2008;205:3007–18. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ombrellino M, Wang H, Ajemian MS, Talhouk A, Scher LA, et al. Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet. 1999;354:1446–47. doi: 10.1016/S0140-6736(99)02658-6. [DOI] [PubMed] [Google Scholar]
- 89.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, et al. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–94. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 90.Barsness KA, Arcaroli J, Harken AH, Abraham E, Banerjee A, et al. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R592–99. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 91.Goldstein RS, Gallowitsch-Puerta M, Yang L, Rosas-Ballina M, Huston JM, et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock. 2006;25:571–74. doi: 10.1097/01.shk.0000209540.99176.72. [DOI] [PubMed] [Google Scholar]
- 92.Kim JB, Lim CM, Yu YM, Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J. Neurosci. Res. 2008;86:1125–31. doi: 10.1002/jnr.21555. [DOI] [PubMed] [Google Scholar]
- 93.Kim JB, Sig CJ, Yu YM, Nam K, Piao CS, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006;26:6413–21. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J. Cereb. Blood Flow Metab. 2008;28:927–38. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 95.Bonanno G, Raiteri L, Milanese M, Zappettini S, Melloni E, et al. The high-mobility group box 1 cytokine induces transporter-mediated release of glutamate from glial subcellular particles (gliosomes) prepared from in situ-matured astrocytes. Int. Rev. Neurobiol. 2007;82:73–93. doi: 10.1016/S0074-7742(07)82004-6. [DOI] [PubMed] [Google Scholar]
- 96.Pedrazzi M, Raiteri L, Bonanno G, Patrone M, Ledda S, et al. Stimulation of excitatory amino acid release from adult mouse brain glia subcellular particles by high mobility group box 1 protein. J. Neurochem. 2006;99:827–38. doi: 10.1111/j.1471-4159.2006.04120.x. [DOI] [PubMed] [Google Scholar]
- 97.Pedrazzi M, Patrone M, Passalacqua M, Ranzato E, Colamassaro D, et al. Selective proinflamma-tory activation of astrocytes by high-mobility group box 1 protein signaling. J. Immunol. 2007;179:8525–32. doi: 10.4049/jimmunol.179.12.8525. [DOI] [PubMed] [Google Scholar]
- 98.Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P. HMGB-1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine. 2002;18:231–36. doi: 10.1006/cyto.2002.0890. [DOI] [PubMed] [Google Scholar]
- 99.Ito T, Kawahara K, Nakamura T, Yamada S, Nakamura T, et al. High-mobility group box 1 protein promotes development of microvascular thrombosis in rats. J. Thromb. Haemost. 2007;5:109–16. doi: 10.1111/j.1538-7836.2006.02255.x. [DOI] [PubMed] [Google Scholar]
- 100.Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904–16. doi: 10.1096/fj.07-8770com. [Anti-HMGB1 monoclonal antibody therapy ameliorates brain damage during ischemia.] [DOI] [PubMed] [Google Scholar]
- 101.Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, et al. The HMGB1 receptor RAGE mediates ischemic brain damage. J. Neurosci. 2008;28:12023–31. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010;16:413–19. doi: 10.1038/nm.2127. [HMGB1-TLR4 signaling is required for seizures following intracortical kainic acid.] [DOI] [PubMed] [Google Scholar]
- 103.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 104.Ramasamy R, Yan SF, Schmidt AM. Stopping the primal RAGE reaction in myocardial infarction: capturing adaptive responses to heal the heart? Circulation. 2008;117:3165–67. doi: 10.1161/CIRCULATIONAHA.108.784397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oozawa S, Mori S, Kanke T, Takahashi H, Liu K, et al. Effects of HMGB1 on ischemia-reperfusion injury in the rat heart. Circ. J. 2008;72:1178–84. doi: 10.1253/circj.72.1178. [DOI] [PubMed] [Google Scholar]
- 106.Kitahara T, Takeishi Y, Harada M, Niizeki T, Suzuki S, et al. High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc. Res. 2008;80:40–46. doi: 10.1093/cvr/cvn163. [DOI] [PubMed] [Google Scholar]
- 107.Germani A, Limana F, Capogrossi MC. Pivotal advances: high-mobility group box 1 protein—a cytokine with a role in cardiac repair. J. Leukoc. Biol. 2007;81:41–45. doi: 10.1189/jlb.0306165. [DOI] [PubMed] [Google Scholar]
- 108.Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20:2565–66. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 109.Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, et al. HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc. Pathol. 2007;16:136–43. doi: 10.1016/j.carpath.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 110.Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, et al. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler. Thromb. Vasc. Biol. 2004;24:2320–25. doi: 10.1161/01.ATV.0000145573.36113.8a. [DOI] [PubMed] [Google Scholar]
- 111.Rouhiainen A, Imai S, Rauvala H, Parkkinen J. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb. Haemost. 2000;84:1087–94. [PubMed] [Google Scholar]
- 112.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–60. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 113.Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, et al. High mobility group 1 B-box mediates activation of human endothelium. J. Intern. Med. 2003;254:375–85. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 114.Chung KY, Park JJ, Kim YS. The role of high-mobility group box-1 in renal ischemia and reper-fusion injury and the effect of ethyl pyruvate. Transplant. Proc. 2008;40:2136–38. doi: 10.1016/j.transproceed.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 115.Wang Q, Ding Q, Zhou Y, Gou X, Hou L, et al. Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology. 2009;110:1279–86. doi: 10.1097/ALN.0b013e3181a160d6. [DOI] [PubMed] [Google Scholar]
- 116.Cardinal J, Pan P, Dhupar R, Ross M, Nakao A, et al. Cisplatin prevents high mobility group box 1 release and is protective in a murine model of hepatic ischemia/reperfusion injury. Hepatology. 2009;50:565–74. doi: 10.1002/hep.23021. [DOI] [PubMed] [Google Scholar]
- 117.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol. Med. 2008;14:476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ilmakunnas M, Tukiainen EM, Rouhiainen A, Rauvala H, Arola J, et al. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl. 2008;14:1517–25. doi: 10.1002/lt.21573. [DOI] [PubMed] [Google Scholar]
- 119.Huang Y, Yin H, Han J, Huang B, Xu J, et al. Extracellular Hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am. J. Transplant. 2007;7:799–808. doi: 10.1111/j.1600-6143.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- 120.Messmer D, Yang H, Telusma G, Knoll F, Li J, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 2004;173:307–13. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 121.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 122.Bassi R, Giussani P, Anelli V, Colleoni T, Pedrazzi M, et al. HMGB1 as an autocrine stimulus in human T98G glioblastoma cells: role in cell growth and migration. J. Neurooncol. 2008;87:23–33. doi: 10.1007/s11060-007-9488-y. [DOI] [PubMed] [Google Scholar]
- 123.Liang X, Romo-Vivar A, Schapiro NE, Loughran P, Thorne SH, et al. Ethyl pyruvate administration inhibits hepatic tumor growth. J. Leukoc. Biol. 2009;86:599–607. doi: 10.1189/jlb.0908578. [DOI] [PubMed] [Google Scholar]
- 124.Hreggvidsdottir HS, Ostberg T, Wahamaa H, Schierbeck H, Aveberger AC, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J. Leukoc. Biol. 2009;86:655–62. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 125.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J. Immunol. 2008;180:2531–37. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 126.Zetterstrom CK, Bergman T, Rynnel-Dagoo B, Erlandsson HH, Soder O, et al. High mobility group box chromosomal protein 1 (HMGB1) is an antibacterial factor produced by the human adenoid. Pediatr. Res. 2002;52:148–54. doi: 10.1203/00006450-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 127.Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 128.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–39. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dai Y, Wong B, Yen YM, Oettinger MA, Kwon J, Johnson RC. Determinants of HMGB proteins required to promote RAG1/2-recombination signal sequence complex assembly and catalysis during V(D)J recombination. Mol. Cell. Biol. 2005;25:4413–25. doi: 10.1128/MCB.25.11.4413-4425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 131.Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, et al. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am. J. Physiol. Cell Physiol. 2006;290:C990–99. doi: 10.1152/ajpcell.00308.2005. [DOI] [PubMed] [Google Scholar]
- 132.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Respir. Crit. Care Med. 2004;170:1310–16. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 133.Schlueter C, Weber H, Meyer B, Rogalla P, Roser K, et al. Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am. J. Pathol. 2005;166:1259–63. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, et al. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ. Res. 2007;100:204–12. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 135.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, et al. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J. Cell Biol. 2001;152:1197–206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-κB activation. J. Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J. Leukoc. Biol. 2009;85:779–87. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 138.Tzeng HP, Fan J, Vallejo JG, Dong JW, Chen X, et al. Negative inotropic effects of high-mobility group box 1 protein in isolated contracting cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1490–96. doi: 10.1152/ajpheart.00910.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hagiwara S, Iwasaka H, Uchino T, Noguchi T. High mobility group box 1 induces a negative inotropic effect on the left ventricle in an isolated rat heart model of septic shock: a pilot study. Circ. J. 2008;72:1012–17. doi: 10.1253/circj.72.1012. [DOI] [PubMed] [Google Scholar]
- 140.Zhou Z, Han JY, Xi CX, Xie JX, Feng X, et al. HMGB1 regulates RANKL-induced osteoclasto-genesis in a manner dependent on RAGE. J. Bone Miner. Res. 2008;23:1084–96. doi: 10.1359/JBMR.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–11. [PubMed] [Google Scholar]
- 142.O'Connor KA, Hansen MK, Rachal PC, Deak MM, Biedenkapp JC, et al. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003;24:254–65. doi: 10.1016/j.cyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 143.Bruchfeld A, Qureshi AR, Lindholm B, Barany P, Yang L, et al. High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD). Mol. Med. 2008;14:109–15. doi: 10.2119/2007-00107.Bruchfeld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Parkkinen J, Raulo E, Merenmies J, Nolo R, Kajander EO, et al. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J. Biol. Chem. 1993;268:19726–38. [PubMed] [Google Scholar]
- 145.Ding BS, Hong N, Christofidou-Solomidou M, Gottstein C, Albelda SM, et al. Anchoring fusion thrombomodulin to the endothelial lumen protects against injury-induced lung thrombosis and inflammation. Am. J. Respir. Crit. Care Med. 2009;180:247–56. doi: 10.1164/rccm.200809-1433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cai B, Chen F, Ji Y, Kiss L, de Jonge WJ, et al. Alpha7 cholinergic-agonist prevents systemic inflammation and improves survival during resuscitation. J. Cell. Mol. Med. 2008;13:3774–85. doi: 10.1111/j.1582-4934.2008.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]