Abstract
Background
B43-pokeweed antiviral protein (B43-PAP) is a high affinity anti-CD19 immunotoxin that is capable of causing apoptotic death in B-lineage leukemic cells with a drug resistant phenotype. B43-PAP exhibited in vivo anti-leukemic activity in preclinical studies as well as on a single agent phase I clinical trial. This pediatric phase I/II study evaluated the toxicity profile and efficacy of B43-PAP immunotoxin in combination with standard induction chemotherapy in children and adolescents with relapsed CD19 positive B-lineage acute lymphoblastic leukemia (B-ALL). Pharmacokinetic profile and immunogenicity of B43-PAP were assessed.
Experimental Design
B43-PAP in combination with standard 3 and 4-drug induction chemotherapy was administered on days 9-13 and 21-25 of a 28-day treatment course with vincristine, prednisone, L-asparaginase, daunomycin and intrathecal methotrexate. Thirty patients with relapsed B-lineage ALL were enrolled on study CCG-0957.
Results
Grade III/IV non-hematologic dose limiting toxicities (DLT) were encountered in 4 patients evaluable for toxicity and included myalgias, motor dysfunction, pulmonary toxicity and elevated liver transaminase. DLT occurred only with the 4-drug regimen. Fourteen patients achieved a complete remission at the end of induction among the 20 patients evaluable for response.
Conclusion
B43-PAP in combination with standard induction chemotherapy can be safely administered and exhibits clinical anti-leukemic activity against relapsed B-lineage ALL.
Keywords: Anti-CD19 Immunotoxin, Immunotherapy, Relapsed B-lineage ALL
Introduction
B-lineage acute lymphoblastic leukemia (B-ALL) is the most common form of cancer in children and adolescents (1). Although the cure rate for B-ALL approaches 80% in most of the published studies and is predicted to reach 90% with contemporary therapy, significant challenges remain for children with poor prognostic indicators (2-6) as well as for children who develop relapsed disease despite intensive multi-agent chemotherapy. In particular for patients who relapse while on therapy or shortly after completing treatment, the overall survival is very poor (7-18). A rapid and complete reduction of the leukemia-cell burden during upfront induction chemotherapy may prevent drug resistance. Incorporating new agents in combination with standard active cytotoxic chemotherapy may improve the survival outcome in ALL.
CD19, a B-lineage specific surface receptor expressed on leukemic cells in 85% of patients with B-ALL, is present at a high density and shows a high affinity for recombinant anti-CD19 monoclonal antibodies. As CD19 is not expressed on non-hematopoietic tissue or hematopoietic progenitor cells, it is an attractive molecular target for biotherapy in B-ALL (19-21).
CD19 is constitutively associated with the multi-drug resistance associated P-glycoprotein (P-gp) (22, 23) and disruption of this association with a CD19 monoclonal antibody has been reported to impair the drug efflux function of P-gp. Therefore, anti-CD19 antibodies may act as chemosensitizing agents.
B43-pokeweed antiviral protein immunotoxin (B43-PAP) is an investigational high affinity anti-CD19 monoclonal antibody linked to the ribosome inhibitory hemitoxin, pokeweed antiviral protein (PAP) (24). Upon binding to the CD19 antigen the PAP toxin moiety is internalized and irreversibly inhibits protein synthesis leading to cellular apoptosis (24, 25). In preclinical studies, B43-PAP exhibited potent anti-leukemic activity both in vitro and in vivo (25-28). Phase I evaluation of B43-PAP as single agent therapy demonstrated responses, including complete remissions, at nontoxic dose levels (29-33).
The primary aims of this pediatric phase I/II study were to evaluate the toxicity profile and determine the efficacy of B43-PAP immunotoxin in combination with standard induction chemotherapy in children and adolescents with relapsed CD19 positive B-ALL.
Materials and Methods
Eligibility Criteria
Informed consent was obtained from the patient, parent or legal guardian and the trial was approved by the institutional review board at participating centers. Patients with relapsed immunophenotypically confirmed B-ALL, defined as >50% CD19 antigen positive bone marrow blasts by flow cytometry or through immunophenotyping, ≤ 21 years of age at the time of initial diagnosis with measurable disease (>25% leukemic blasts in the bone marrow) were eligible for this trial.
Patients were fully recovered from the toxic effects of prior therapy with 2 weeks having elapsed following the administration of intermediate or high dose chemotherapy, 4 weeks if nitrosourea was administered. Patients were required to have adequate organ function defined as a serum creatinine ≤ 1.5 × the upper limit of normal (ULN) or creatinine clearance or GFR ≥ 70 ml/min/1.73 m2, total bilirubin ≤1.5 × the ULN, serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) < 2.5 × the ULN, cardiac shortening fraction ≥ 27% or ejection fraction >50%, pulse oximetry > 94% or corrected carbon monoxide diffusing capacity of >70% and central nervous system (CNS) toxicity ≤ grade 1. Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 and life expectancy of ≥ 2 months were required. Patients positive for human anti-mouse antibody (HAMA) or human anti-PAP antibody (HAPA) were eligible. Patients with active uncontrolled infection, diabetes mellitus, serious medical conditions, HIV seropositivity, pregnant or nursing females or those previously treated with B43-PAP immunotoxin were excluded.
Treatment Regimen
B43-PAP (1 or 1.5 mg/m2 according to escalation design) was administered intravenously (IV) over one hour on days 9-13 and 21-25 of a 28 day treatment course in combination with standard 4-drug ALL induction chemotherapy consisting of vincristine (1.5 mg/m2 IV days 0, 7, 14 and 21), prednisone (60 mg/m2/day orally days 0-27 followed by a 10 day taper), daunomycin (25 mg/m2 IV days 0, 7, 14 and 21) and L-asparaginase (6000 u/m2 intramuscularly beginning day 3, 3 times per week for 9 total doses) with intrathecal methotrexate as CNS prophylaxis (dose based on age; days 0, 14 and 28 if CNS negative; days 0, 7, 14, 21 and 28 if CNS positive). Patients received a single 28-day course of treatment. During study enrollment, data in murine models demonstrated 3-drug induction chemotherapy (vincristine, prednisone, L-asparaginase) with B43-PAP led to a better disease free survival rate as compared to the 4-drug regimen. The 3-drug induction regimen was also thought to potentially decrease myelosuppression and subsequently the risk of serious infection. As a result the protocol was modified. Patients enrolled after July 17, 1998 received B43-PAP, beginning at 1.5 mg/m2 with planned escalation to 2 mg/m2, administered with vincristine, prednisone and L-asparaginase (Table 1). The treatment regimens were administered under BBIND-3864.
Table 1.
Patient Characteristics
| Characteristics | 4-Drug Regimen (n=24) | 3-Drug Regimen (n=6) |
|---|---|---|
| Sex: | ||
| Male | 17 | 2 |
| Female | 7 | 4 |
| Age at Diagnosis (years): | ||
| Range | 2-16 | 2-16 |
| Median | 7.5 | 9.5 |
| Age at Enrollment (years): | ||
| Range | 3-17 | 5-16 |
| Median | 10.5 | 12.0 |
| Race: | ||
| Caucasian | 12 | 5 |
| Hispanic | 8 | 1 |
| African-American | 3 | 0 |
| Asian | 1 | 0 |
| Disease at Diagnosis: | ||
| CNS disease | 1 | 0 |
| Testicular disease | 1 | 0 |
| Other | 0 | 0 |
| Prior Treatment Regimen: | ||
| 1 chemotherapy regimen | 11 | 3 |
| 2 chemotherapy regimen | 9 | 2 |
| ≥ 3 chemotherapy regimen | 4 | 1 |
| Radiation Therapy | 9 | 1 |
| HSCT | 8 | 2 |
| B43-PAP dose level: | ||
| 1.0 mg/m2/day | 11 | - |
| 1.5 mg/m2/day | 13 | 5 |
| 2.0 mg/m2/day | - | 1 |
HSCT - hematopoietic stem cell transplant
Protocol directed early systematic management of vascular leak syndrome (VLS) utilized diuretics, albumin and red blood cell transfusions in cases of hypoalbuminemia or hypotension, low dose dopamine if associated renal insufficiency and high dose steroids with additional necessary supportive care if life threatening systemic VLS occurred.
Toxicity Monitoring and Definition of Dose Limiting Toxicity
The Toxicity and Complications Criteria and the Biological Toxicity Scale (available by request from the COG Operations Office) were utilized to grade adverse events. Patients treated on protocol through day 14 were considered evaluable for toxicity, as were patients who received B43-PAP and experienced does limiting toxicity (DLT) resulting in removal from protocol therapy regardless of time on study. DLT was defined for the 4-drug regimen as grade 3 or 4 non-hematopoietic toxicity that did not resolve to ≤ grade 2 by the next B43-PAP dose for cycle 1 (days 9-13) or by day 35 for cycle 2 (days 21-25).
Response Evaluation
A bone marrow specimen on day 28 of the treatment cycle was obtained and morphologic response determined by the treating institution. A complete response (CR) was defined as ≤ 5% bone marrow blasts (M1) with no extramedullary disease and recovery of peripheral counts and a partial response (PR) defined as disappearance of circulating blasts with <25% bone marrow blast cells (M2) and recovery of peripheral counts which persists for 4 weeks. Progressive disease (PD) was an increase of at least 25% in the absolute number of circulating leukemic blasts from baseline or failure to obtain < 25% marrow blasts after one treatment course. Patients with stable disease (SD) did not meet criteria for CR, PR, or PD.
Dose Escalation
The traditional 3+3 design was used with the following modification (34). If, at the first stage for a dose, three patients were enrolled, a fourth patient could be enrolled if toxicity data were incomplete for the initial 3. If all four were evaluable and 0 or 1 patients experienced DLT, escalation proceeded according to the traditional design where 0 of 3 experienced DLT. Otherwise, dose escalation proceeded according to the escalation rules for 3 evaluable patients. Simulation studies demonstrated the statistical properties of this modification were similar to that of the traditional 3+3 design.
Preparation of B43-PAP Immunotoxin
B43-PAP was prepared from Phytolacca americana plants by ammonium sulfate precipitation and purified by ion exchange chromatography. The anti-CD19 monoclonal antibody was produced in vitro by hollow fiber technology and purified by affinity chromatography. PAP toxin and the anti-CD19 monoclonal antibody were modified via their free amino groups prior to their intermolecular conjugation. Modified PAP was reacted with modified monoclonal antibody resulting in a sulfhydryl-disulfide exchange reaction and yielding disulfide linked B43-PAP immunotoxin. B43-PAP was subjected to preparative gel filtration chromatography and cation exchange chromatography to obtain a highly purified, sterile, and pyrogen-free immunotoxin preparation with less than 5% free antibody contamination and less than 0.5% free PAP contamination. The final product displayed a high affinity for and a very potent anti-leukemic activity against B lineage leukemia cells (35).
Measurement of B43-PAP Immunotoxin Levels in Patient Blood Samples
Peripheral blood samples (1 mL/time point) were collected prior to and one hour after the daily administration of B43-PAP immunotoxin. Plasma from these blood samples was then used to measure the levels of chemically intact B43-PAP immunotoxin by quantitative solid-phase ELISA using previously published procedures (35). The intact B43-PAP concentrations in the plasma samples were determined from standard curves that were generated by linear regression analysis using varying amounts of purified B43-PAP immunotoxin standard.
Results
CCG-0957 was opened for enrollment in April 1996. The study was closed May 1999. The study was closed prematurely due to an unanticipated cessation in the production of study drug and continuing uncertainty regarding the resumption of production. Data current to August 2008 was used for this analysis.
Patient Population
Thirty patients, 19 males and 11 females ranging 2 to 16 years of age at the time of initial diagnosis, with relapsed or refractory B-ALL were eligible and enrolled on this trial between July 1996 and March 1999. One patient was enrolled on study on two occasions. At the time of the initial enrollment the patient did not receive B43-PAP therapy, but did receive when enrolled the second time. All but 2 patients had isolated bone marrow disease at diagnosis; 1 patient also had CNS involvement and 1 testicular involvement. A majority of patients (n=25) had received one or two prior chemotherapy treatment regimens while 5 patients had been treated with 3 or more regimens. Ten patients received prior radiation therapy; 4 received total body irradiation (TBI) containing preparative regimen for allogeneic hematopoietic stem cell transplant (HSCT), 1 TBI and whole brain, 2 whole brain and 3 craniospinal radiation. Ten patients had undergone an allogeneic HSCT prior to enrollment; 2 with matched unrelated donors, 1 with a matched sibling donor and 7 not specified. Patient characteristics are presented in Table 1.
B43-PAP Immunotoxin Levels
Intact B43-PAP levels were measured in the pre- and post-treatment blood samples from patients with relapsed B-lineage ALL. Peak plasma levels of intact B43-PAP immunotoxin greater than the target 100 ng/mL “apoptotic” concentration were achieved in 7 of 9 patients treated at 1 mg/m2 immunotoxin dose level and in all 8 patients treated at 1.5 mg/m2 dose level.
Toxicity
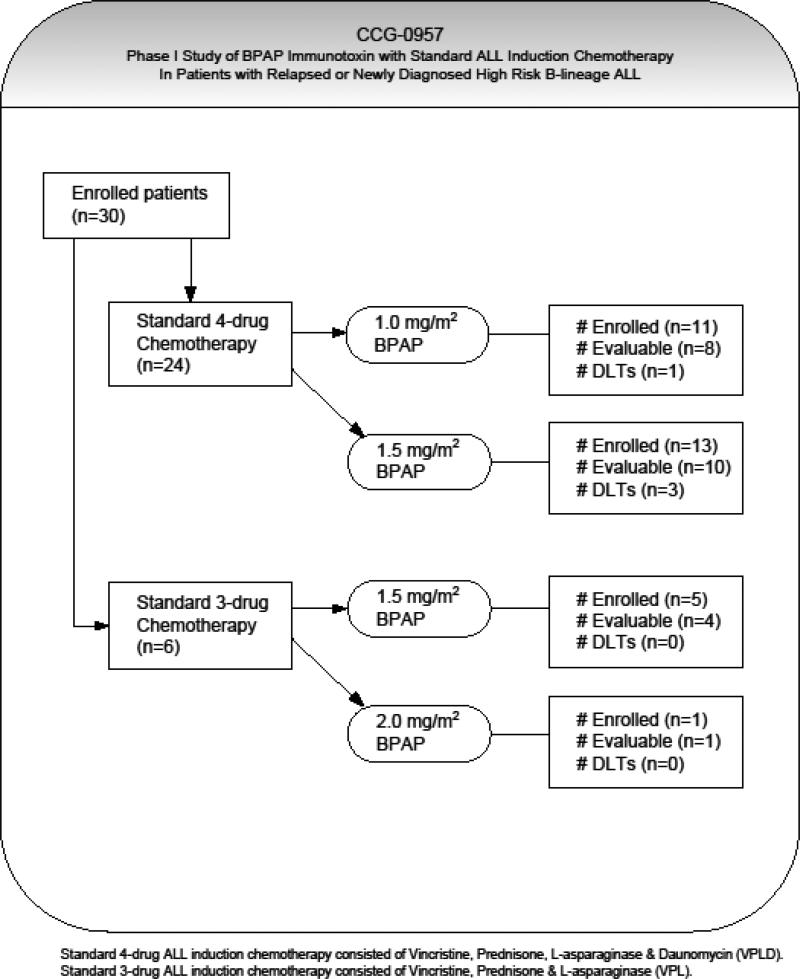
Twenty-four patients were treated with B43-PAP in combination with the 4-drug regimen (VPLD, Figure 1). At the initial B43-PAP dose level of 1.0 mg/m2/day, 8 patients were evaluable for toxicity and determination of dose escalation while 3 did not complete the first 5 day cycle of B43-PAP due to underlying organ dysfunction or toxicity related to another chemotherapeutic agent and were considered inevaluable. Of the first 4 evaluable patients enrolled and treated at this dose level, one patient developed dose limiting myalgias and motor dysfunction with weakness (Table 2). The dose level was expanded to include 4 additional evaluable patients without further DLT observed; therefore the dose was escalated to 1.5 mg/m2/day. In the initial cohort of 3 evaluable patients enrolled at this second dose level, one developed DLT of pulmonary toxicity, progressing to multi-organ failure requiring discontinuation of therapy. This dose level was expanded to eventually include 7 additional evaluable patients. Two patients were not evaluable due to underlying organ dysfunction or toxicity unrelated to B43-PAP. Two additional DLTs were seen in this expansion cohort; grade 4 transaminase (AST and ALT) elevation and irreversible grade 3 ALT elevation. Prior to further dose escalation of B43-PAP in combination with the 4-drug regimen, the protocol was amended and daunomycin was removed from protocol therapy.
Figure 1.
Protocol dose escalation schema, enrollment status and frequency of dose limiting toxicities observed with B43-PAP immunotoxin and standard 3 and 4-drug chemotherapy.
Table 2.
Dose Limiting Toxicities
| Dose Level | Evaluable Patients | Patients with DLT | Description (grade) |
|---|---|---|---|
| 4 Drug Regimen | |||
| 1.0 mg/m2/day | 8 | 1 | Myalgias (4), CNS motor dysfunction (3) |
| 1.5 mg/m2/day | 10 | 3 | Pulmonary (3/4), liver transaminase elevation (3/4) |
| 3 Drug Regimen | |||
| 1.5 mg/m2/day | 4 | 0 | |
| 2.0 mg/m2/day | 1 | 0 | Closed without determining MTD |
Six patients in total received B43-PAP in combination with the 3-drug regimen, 5 were evaluable for toxicity while 1 was inevaluable secondary to a CNS event prior to receiving any protocol therapy. There were no DLTs seen in the 4 patients enrolled at the 1.5 mg/m2/day dose level and the one patient treated at the 2.0 mg/m2/day dose level. The study was closed prior to completion due to B43-PAP supply constraints; therefore, with further enrollment and dose escalations terminated the MTD in combination with 3-drug induction chemotherapy could not be determined.
The most common grade 3 and 4 toxicities were hematologic consisting of thrombocytopenia (13.2% of all grade 3 and 4 adverse events on both treatment regimen), neutropenia (9.6%), leucopenia (6.6%), anemia (5.9%) and lymphopenia (5.9%), but also included elevated ALT (7.4%) and elevated bilirubin (5.1%). Grade 3 and 4 non-hematologic adverse events attributable to B43-PAP are summarized by treatment regimen and dose level (see Supplemental Toxicity Table). There were no drug related deaths.
Disease Response
Twenty of the thirty patients enrolled were considered evaluable for disease response (Table 3). Nine patients did not complete both 5-day B43-PAP cycles (days 9-13 and 21-25 of a 28-day treatment course) and were deemed inevaluable for response analysis due to organ dysfunction preventing the initiation of B43-PAP (n=4), DLT attributable to B43-PAP (n=2), toxicity related to other chemotherapy agents (n=2) and sepsis unrelated to B43-PAP (n=1). Four patients did not begin B43-PAP therapy. Of the 5 patients who received at least one dose of B43-PAP with standard induction chemotherapy one had a PR and 4 were unable to undergo bone marrow evaluation on day 28 so response could not be assessed. One additional patient had a hypoplastic day 28 bone marrow specimen that could not adequately be interpreted for response determination. In total, 14 patients (70%) were considered to have a CR and 2 a PR for an overall response rate of 80%. Ten of the patients with a CR and the 2 patients with a PR were treated with the 4-drug regimen in combination with B43-PAP for an overall response rate of 80% for this regimen. The remaining 4 patients with a CR were treated with the 3-drug regimen in combination with B43-PAP for a response rate of 80% in this limited cohort. Four patients (20%) had disease progression. In continued follow up for those patients who achieved a CR or PR at day 28, 6 went on to eventually die of leukemia with time of death ranging 4.7-23.2 months following study enrollment. Two patients died of infection (4.8-15 months from enrollment), 2 of unknown causes (2.6-7.9 months from enrollment), one from cardiac arrest (4.8 months from enrollment) and one from leukemia and sepsis (6.4 months from enrollment). Three patients, all with a CR at day 28, were alive at the time of last follow up, which ranged 34.8-94 months (2.9-7.7 years) from enrollment. One patient continued treatment with 5 cycles of cytotoxic chemotherapy and a course of immunotherapy, one proceeded directly to a matched sibling HSCT and one received a course of cytotoxic chemotherapy followed by a matched sibling HSCT.
Table 3.
Disease Response
| Response | 4-Drug Regimen (n=24) | 3-Drug Regimen (n=6) | Overall Response |
|---|---|---|---|
| Complete Response (CR) | 10 (67%) | 4 (80%) | 14 (70%) |
| Partial Response (PR) | 2 (13%) | - | 2 (10%) |
| Not CR/PR | 3 (20%) | 1 (20%) | 4 (20%) |
| Not Evaluable | 9 | 1 | 10 |
Discussion
Despite treatment success, relapsed ALL remains a significant cause of cancer related death. Patients with recurrent and refractory disease have a poor prognosis and new therapies are needed to augment standard salvage regimens. Targeted treatment modalities such as immunotoxin therapy represent a strategy to selectively affect leukemia cells avoiding toxicities, which overlap those of cytotoxic agents, particularly myelosuppression. B43-PAP immunotoxin was selected for combination with ALL induction chemotherapy based on in vitro and in vivo anti-leukemic activity; tolerable toxicity profile demonstrated in a single agent phase I trial and the combination represented a reasonable regimen for future upfront protocols.
B43-PAP was well tolerated in the setting of combination therapy particularly with the 3-drug induction regimen. Notably, 8 patients developed grade 3 or 4 VLS with only 1 patient reaching grade 4 severity resulting in pulmonary dose limiting toxicity and multiorgan failure. Severe VLS, a well described adverse event associated with antibody-immunotoxin administration, was not frequently observed on this trial perhaps due to the aggressive and pre-emptive fluid and medication interventions specified in the protocol. Hemolytic uremic syndrome (HUS), an inflammatory reaction following endothelial injury with platelet-thrombin deposits occluding small blood vessels resulting in platelet consumption, microangiopathic hemolytic anemia and acute renal failure, has been reported with the immunotoxin administration. No cases of HUS were reported on this trial and only one patient demonstrated significant renal toxicity (Grade 4 elevation in creatinine), a patient with multi-organ failure.
Disease responses among relapsed patients were seen at both B43-PAP dose levels. A greater number of patients with a first disease relapse had a documented response, 9 of 10 patients with a CR (90%) as compared to those with multiply relapsed disease, response rate 70% (5 CR and 2 PR). Of those patients in first disease relapse, 6 were early (< 36 months from initial diagnosis) and 4 were late (> 36 months) relapses. The patient that did not achieve a CR was an early disease relapse. Of note, 1 patient who had been treated with 6 previous regimens did respond, achieving a CR following B43-PAP in combination with 3-drug therapy, and subsequently underwent an allogeneic matched unrelated donor HSCT. There was no difference in the number of patients that had undergone prior HSCT when comparing patients with disease response to those with complete disease progression, 25% in both cohorts. Patients with disease progression were males and tended to be slightly younger at study enrollment, median age 8.5 years, whereas patients demonstrating disease response were older with a median age of 11.6 years at enrollment and a 1:1 male: female ratio. As with toxicity a comparison of response rates between the 3 and 4-drug treatment regimen is not meaningful due to early closure of the study and small sample sizes.
The response rate of 80% observed overall and individually on both the 3 and 4-drug treatment regimens is comparable to previous studies of various salvage treatment regimen in patients with relapsed ALL achieving re-induction CR rates ranging 68 to 95% (15, 16, 36-40). In multiply relapsed patients, those receiving 2 or more prior treatment regimens, the complete response rate was 50% (overall response rate 70%) on this trial, a slight increase over the re-induction CR rates of 37-42% observed in this patient cohort treated with salvage regimen (15, 18, 41).
As the target apoptotic B43-PAP concentration of 100 ng/mL was exceeded and complete remissions were obtained at both dose levels, no further dose escalation was undertaken and the 1 mg/m2 dose level was chosen for combination with VPLD in newly diagnosed high risk ALL patients with a poor early response to chemotherapy, defined as patients with >25% blasts on day 7 bone marrow evaluation of induction therapy.
Several additional clinical trials evaluating monoclonal antibody therapy have been completed or are ongoing in patients with refractory leukemia and/or lymphoma. The anti-CD20 monoclonal antibody rituximab has demonstrated objective responses in patients with relapsed and refractory B-cell non-Hodgkin lymphoma and mature B-ALL, including heavily pre-treated patients. The agent is incorporated in ongoing pediatric clinical trials (42-43). Epratuzumab, a monoclonal antibody targeting CD22 positive cells, resulted in an 83% response rate when used in combination with cytotoxic chemotherapy on a feasibility Children's Oncology Group (COG) pilot study in relapsed ALL (44). This agent continued in development in a phase 2 COG trial. Blinatumomab, a bi-specific T-cell engager that specifically targets the CD19 antigen, is in current pediatric clinical trials. Monoclonal antibodies targeting CD20, CD25, CD52 and combination CD19/CD22 are also potential anticancer agents (45-47). Other modalities of immunotherapy such as chimeric antigen receptor (CAR) T-cell therapies are currently under evaluation in hematologic malignancies and have been shown to be effective in relapsed disease (48).
This phase I study of B43-PAP was the first evaluation of feasibility of immunotoxin therapy combined with cytotoxic agents in children with relapsed ALL. Despite the unexpected cessation of production of B43-PAP and the uncertainty of its reappearance as an investigational agent, it provided the proof of principle supporting investigations of combination therapy with immune-targeted agents.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Fatih Uckun, Children's Hospital Los Angeles, Los Angeles, California for supplying B43-PAP Immunotoxin (BB-IND-3864) for this trial.
This work was supported by study grants CA98543 (Chair's Grant), CA180886 (NCTN Operation Center Grant), CA98413 (Statistics and Data Center Grant), CA180899 (NCTN Statistics and Data Center Grant.
Footnotes
Conflict of Interest Statement: None declared.
References
- 1.Stanulla M, Schrappe M. Treatment of childhood acute lymphoblastic leukemia. Semin Hematol. 2009;46(1):52–63. doi: 10.1053/j.seminhematol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Trigg ME, Sather H, Reaman GH, Tubergen DG, Steinherz PG, Gaynon PS, Uckun FM, Hammond GD. Ten-year survival of children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Leukemia and Lymphoma. 2008;49(6):1142–54. doi: 10.1080/10428190802074593. [DOI] [PubMed] [Google Scholar]
- 3.Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, Freyer DR, Mattano LA, hastings CA, Rubin CM, Bertolone K, Franklin JL, Heerema NA, Mitchell TL, Pyesmany AF, La MK, Edens C, Gaynon PS. Early postinduction intensification therapy improves survival for children and adolescents with high risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;111(5):2548–55. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reaman GH, Sposto R, Sensel MG, Lange BJ, Feusner JH, Heerema NA, Leonard M, Holmes EJ, Sather HN, Pendergrass TW, Johnstone HS, O'Brien RT, Steinherz PG, Zeltzer PM, Gaynon PS, Trigg ME, Uckun FM. Treatment outcome and prognostic factors for infants with acute lymphoblastic leukemia treated on two consecutive trials of the Children's Cancer Group. J Clin Oncol. 1999;17(2):445–455. doi: 10.1200/JCO.1999.17.2.445. [DOI] [PubMed] [Google Scholar]
- 5.Schultz KR, Pullen J, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, Carroll AJ, Heerema NA, Rubnitz JE, Loh ML, Raetz EA, Winick NJ, Hunger SP, Carroll WL, Gaynon PS, Camitta BM. Risk- and response-based classification of childhood B-precurosr acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uckun FM, Nachman JB, Sather HN, Sensel MG, Kraft P, Steinherz PG, Lange B, Hutchinson R, Reaman GH, Gaynon PS, Heerema NA. Poor treatment outcome of Philadelphia chromosome-positive pediatric acute lymphoblastic leukemia despite intensive chemotherapy. Leuk Lymphoma. 1999;33(1-2):101–106. doi: 10.3109/10428199909093730. [DOI] [PubMed] [Google Scholar]
- 7.Gaynon PS, Harris RE, Altman AJ, Bostrom BC, Brenneman JC, Hawks R, Steele D, Zipf T, Stram DO, Villaluna D, Trigg ME. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group Study CCG-1941. J Clin Oncol. 2006;24:3150–3156. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]
- 9.Bailey LC, Lange BJ, Rheinhold SR, Bunin NJ. Bone marrow relapse in paediatric acute lymphoblastic leukemia. Lancet Oncol. 2008;9(9):873–83. doi: 10.1016/S1470-2045(08)70229-8. [DOI] [PubMed] [Google Scholar]
- 10.Malempati S, Gaynon PS, Sather H, La MK, Stork LC. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children's Oncology Group study CCG-1952. J. Clin Oncol. 2007;25:5800–7. doi: 10.1200/JCO.2007.10.7508. [DOI] [PubMed] [Google Scholar]
- 11.Roy A, Cargill A, Love S, Moorman AV, Stoneham S, Lim A, Darbyshire PJ, Lancaster D, Hann I, Eden T, Saha V. Outcome after first relapse in childhood acute lymphoblastic leukemia – lessons from the United Kingdom R2 trial. Br J. Hematol. 2005;130:67–75. doi: 10.1111/j.1365-2141.2005.05572.x. [DOI] [PubMed] [Google Scholar]
- 12.Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, Mann G, Hahlen K, Gobel U, Klingebiel T, Ludwig WD, Henze G. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005;23:7942–50. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 13.Uckun FM, Morar S, Qazi S. Vinorelbine-Based Salvage Chemotherapy for Therapy-Refractory Aggressive Leukemias. Brit J Haematol. 2006;135(4):500–508. doi: 10.1111/j.1365-2141.2006.06338.x. [DOI] [PubMed] [Google Scholar]
- 14.Chessells JM, Veys P, Kempski H, et al. Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123:396–405. doi: 10.1046/j.1365-2141.2003.04584.x. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–50. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivera GK, Zhou Y, Hancock ML, et al. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 2005;103:368–76. doi: 10.1002/cncr.20743. [DOI] [PubMed] [Google Scholar]
- 17.Sadowitz PD, Smith SD, Shuster J, et al. Treatment of late bone marrow relapse in children with acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1993;81:602–9. [PubMed] [Google Scholar]
- 18.Harned TM, Gaynon P. Relapsed acute lymphoblastic leukemia: current status and future opportunities. Curr Oncol Rep. 2008;10:453–8. doi: 10.1007/s11912-008-0070-3. [DOI] [PubMed] [Google Scholar]
- 19.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18:385–97. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 20.Uckun FM, Jaszcz W, Ambrus JL, et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988;71:13–29. [PubMed] [Google Scholar]
- 21.Uckun F. M. Regulation of human B-cell ontogeny (Review). Blood. 1990;76:1908–1923. [PubMed] [Google Scholar]
- 22.Ghetie MA, Marches R, Kufert S, Vitetta ES. An anti-CD19 antibody inhibits the interaction between P-glycoprotein (P-gp) and CD19, causes P-gp to translocate out of lipid rafts, and chemosensitizes a multi-drug-resistant lymphoma cell line. Blood. 2004;104:178–183. doi: 10.1182/blood-2003-12-4255. [DOI] [PubMed] [Google Scholar]
- 23.Ghetie MA, Ghetie V, Vitetta ES. Anti-CD19 antibodies inhibit the function of the P-gp pump in multidrug-resistant B lymphoma cells. Clinical Cancer Research. 1999;5:3920–3927. [PubMed] [Google Scholar]
- 24.Uckun FM. Immunotoxins for the treatment of leukemia (Review). Brit J Haematol. 1993;85:435–438. doi: 10.1111/j.1365-2141.1993.tb03329.x. [DOI] [PubMed] [Google Scholar]
- 25.Waddick KG, Myers DE, Gunther R, Chelstrom LM, Chandan-Langlie M, Irvin JD, Tumer N, Uckun FM. In vitro and in vivo antileukemic activity of B43-pokeweed antiviral protein against radiation-resistant human B-cell precursor leukemia cells. Blood. 1995;86:4228–4233. [PubMed] [Google Scholar]
- 26.Uckun FM, Chelstrom LM, Irvin JD, Finnegan D, Gunther R, Young J, Kuebelbeck V, Myers DE, Houston LL. In vivo efficacy of B43 (anti-CD19)-pokeweed antiviral protein immunotoxin against BCL-1 murine B-cell leukemia. Blood. 1992;79:2649–2661. [PubMed] [Google Scholar]
- 27.Uckun FM, Gajl-Peczalska KJ, Kersey JH, Houston LL, Vallera DA. Use of a novel colony assay to evaluate the cytotoxicity of an immunotoxin containing pokeweed antiviral protein against blast progenitor cells freshly obtained from patients with common B-lineage acute lymphoblastic leukemia. J. Exp. Med. 1986;163:347. doi: 10.1084/jem.163.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, Richards JO, Vostiar I, Joyce PF, Repp R, Desjarlais JR, Zhukovsky EA. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68(19):8049–57. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- 29.Uckun FM, Messinger Y, Chen CL, O'Neill K, Myers DE, Goldman F, Hurvitz C, Casper JT, Levine A. Treatment of therapy-refractory B-lineage acute lymphoblastic leukemia with an apoptosis-inducing CD19-directed tyrosine kinase inhibitor. Clin Cancer Res. 1999;(12):3906–13. [PubMed] [Google Scholar]
- 30.Masson E, Yanishevski Y, Myers D, Langlie M, Covalciuc C, Evans WE, Uckun FM. Pharmacokinetics of B43-PAP immunotoxin in children with refractory acute lymphoblastic leukemia (ALL). Blood. Suppl. 1995;86:769a. Abstract 3065. [Google Scholar]
- 31.Seibel NL, Sather H, Steinherz PG, De Laat C, Ettinger LJ, Freyer D, et al. Upfront treatment with B43 PAP immunotoxin in newly diagnosed children with higher risk ALL. Children's Cancer Group. Blood. 1961;2000;96:721a. [Google Scholar]
- 32.Seibel NL, Krailo M, O'Neill K, Franklin J, Uckun F, Reaman GH. Phase I study of B43-PAP immunotoxin in combination with standard 4-drug induction for patients with CD19+ acute lymphoblastic leukemia (ALL) in relapse, a Children's Cancer Group Study. Pediatric Research. 1999;45:5. Abstract 195. [Google Scholar]
- 33.Pasqualucci L, Flenghi L, Terenzi A, Bolognesi A, Stirpe F, Bigerna B, Falini B. Immunotoxin therapy of hematological malignancies. Hematologica. 1995;80:546–556. [PubMed] [Google Scholar]
- 34.Lin Y, Shih WJ. Statistical properties of the traditional algorithm based designs for phase I cancer clinical trials. Biostatistics. 2001;2:203–215. doi: 10.1093/biostatistics/2.2.203. [DOI] [PubMed] [Google Scholar]
- 35.Myers DE, Irvin JD, Smith RS, Kuebelbeck VM, Uckun FM. Production of a pokeweed antiviral protein (PAP)-containing immunotoxin, B43-PAP, directed against the CD19 human B lineage lymphoid differentiation antigen in highly purified form for human clinical trials. J Immunol Methods. 1991;136(2):221–37. doi: 10.1016/0022-1759(91)90009-5. [DOI] [PubMed] [Google Scholar]
- 36.Henze G, Fengler R, Hartmann R, et al. Six-year experience with a comprehensive approach to the treatment of recurrent childhood acute lymphoblastic leukemia (ALL-REZ BFM 85). A relapse study of the BFM group. Blood. 1991;78:1166–72. [PubMed] [Google Scholar]
- 37.Lawson SE, Harrison G, Richards S, et al. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol. 2000;108:531–43. doi: 10.1046/j.1365-2141.2000.01891.x. [DOI] [PubMed] [Google Scholar]
- 38.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected]. J Clin Oncol. 2008;26:3971–8. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy A, Cargill A, Love S, et al. Outcome after first relapse in childhood acute lymphoblastic leukaemia - lessons from the United Kingdom R2 trial. Br J Haematol. 2005;130:67–75. doi: 10.1111/j.1365-2141.2005.05572.x. [DOI] [PubMed] [Google Scholar]
- 40.Sadowitz PD, Smith SD, Shuster J, et al. Treatment of late bone marrow relapse in children with acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1993;81:602–9. [PubMed] [Google Scholar]
- 41.Ko RH, Ji L, Barnette P. Outcome of Patients Treated for Relapsed or Refractory Acute Lymphoblastic Leukemia: A Therapeutic Advances in Childhood Leukemia Consortium Study. J Clin Oncol. 2010;28(4):648–54. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2009;52(2):177–81. doi: 10.1002/pbc.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attias D, Weitzman S. The efficacy of rituximab in high-grade pediatric B-cell lymphoma/leukemia: a review of available evidence. Curr Opin Pediatr. 2008;20(1):17–22. doi: 10.1097/MOP.0b013e3282f424b0. [DOI] [PubMed] [Google Scholar]
- 44.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study. J Clin Oncol. 2008;26:3756–62. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoelzer D. Targeted therapy with monoclonal antibodies in acute lymphoblastic leukemia. Curr Opin Oncol. 2013;25(6):701–6. doi: 10.1097/CCO.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 46.Portell CA, Advani AS. Novel targeted therapies in acute lymphoblastic leukemia. Leuk Lymphoma. 2014;55(4):737–48. doi: 10.3109/10428194.2013.823493. [DOI] [PubMed] [Google Scholar]
- 47.Le Jeune C, Thomas X. Antibody-based therapies in B-cell lineage acute lymphoblastic leukaemia. Eur J Haematol. 2014 Jun 30; doi: 10.1111/ejh.12408. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Maude Sl, Frey N, Shaw PA, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.