Vaccines have led to some of the greatest public health achievements in history, including the worldwide eradication of naturally occurring smallpox and the near eradication of polio. In addition, vaccines have contributed to significant reduction in the disease burden imposed by measles, mumps, hepatitis, influenza, diphtheria, and many other infections. The science of vaccinology is dynamic; it unfolds as technology enables scientists to continue to create safer and more effective vaccines. Safety evaluation is integrated into every step of the vaccine research and development process.
The National Institute of Allergy and Infectious Diseases (NIAID) is the lead institute at the National Institutes of Health (NIH) for research and development of vaccines against emerging and reemerging infectious diseases (Text Box 1). Together with partners throughout the federal government, in academia, and in the public and private sectors, NIAID-supported scientists have helped develop many important life-saving vaccines against diseases such as invasive Haemophilus influenzae type b (Hib), pneumococcal pneumonia, meningitis, pertussis, influenza, chickenpox, and hepatitis A and B. Use of these and other vaccines worldwide has made significant contributions to public health by reducing the morbidity and mortality associated with many dreaded infectious diseases (Text Box 2).
TEXT BOX 1.
Vaccine Research at NIH
| The NIH is a pioneer in the discovery and development of vaccines. Its numerous institutes and centers support and conduct basic and applied research, often in partnership with industry, and provide the foundation for advanced development. The NIH supports research conducted by scientists employed by the federal government and scientists outside of the federal government. |
| Basic research provides knowledge that is essential for developing safe and effective vaccine candidates. These candidates are then evaluated for safety and efficacy in animal models and in clinical trials. |
| Goals of the NIH vaccine research and development program include: |
| identification of new vaccine candidates to prevent or ameliorate diseases for which no vaccines currently exist; |
| improvement in the safety and efficacy of existing vaccines; |
| design of novel vaccine approaches and strategies, such as DNA vaccines and strategies based on protein conjugation; |
| development of innovative technologies such as new delivery methods, stabilization techniques, and adjuvants; and |
| research to investigate specific hypotheses on issues related to vaccine safety. |
TEXT BOX 2.
History of Vaccine Research and Development at the NIAID
| In 1962, the NIAID revolutionized the cumbersome, piecemeal approach to vaccine studies by establishing a network of Vaccine and Treatment Evaluation Units (VTEUs) that are still very active today. These clinical evaluation sites are based primarily at university medical research centers, public health departments, and community clinics across the country. The VTEUs can rapidly recruit volunteers for clinical studies and vaccinate them safely and effectively. They conduct studies that include people of all ages and risk categories. The VTEUs played a major role in studies that led to the licensure of vaccines that have signified major public health advances, such as an acellular pertussis vaccine and the first vaccine against H5N1 influenza. The VTEUs have also evaluated vaccine candidates for pneumonia, cholera, malaria, smallpox, and tuberculosis. More recently, they have been called on to conduct critical studies of pandemic influenza vaccines, including those designed to prevent infection with the novel 2009 H1N1 virus. |
| In 1988, the NIAID established the AIDS Vaccine Evaluation Group, a network of testing centers devoted exclusively to HIV vaccines. In 1999, the NIAID built on this network by creating the HIV Vaccine Trials Network (HVTN), a collaboration of investigators all over the world that clinically evaluates candidate HIV vaccines for safety and efficacy. The HVTN includes sites in Africa, Asia, South America, and the Caribbean. The international sites enable studies that examine the effect of differences in genetic makeup, nutrition, access to health care, and HIV subtypes on vaccine response, which is crucial for creating a vaccine that is effective worldwide. |
| In 2000, the NIAID established the Dale and Betty Bumpers Vaccine Research Center (VRC) on the NIH campus in Bethesda, Maryland. The VRC has the capacity to develop vaccines from initial concept to final product. Scientists at the VRC conduct basic research on microbes and the immune system's response to them, design candidate vaccines, and, with their collaborators, evaluate the most promising candidates in preclinical studies and clinical trials. The VRC recently launched a clinical trial to evaluate a prime-boost vaccination regimen for HIV, as well as trials to evaluate seasonal influenza DNA vaccines. A phase I clinical trial of a novel vaccine against the Ebola virus showed that the vaccine was safe and immunogenic. |
STAGES OF VACCINE DISCOVERY, DEVELOPMENT, AND EVALUATION
Discovery, development, and evaluation of vaccines are performed in multiple stages as promising ideas are developed into potential vaccine candidates. Developing a vaccine usually involves collaboration between federal agencies, academia, and industry. The NIAID's role in vaccine development and testing extends from basic research through clinical evaluation (Fig 1).
FIGURE 1.
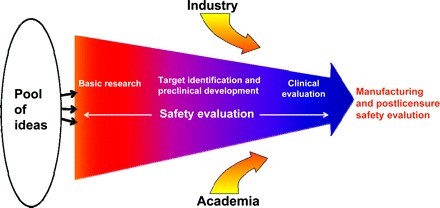
Stages of vaccine research, development, and evaluation. Safety evaluation is integral to every stage of the product-development pathway. This pathway begins with basic research, which involves understanding the pathogen's mechanism of action, the interaction between pathogen and host, and the host response. Target identification entails studying the biological plausibility of particular strategies for creating a vaccine against the pathogen. In preclinical development, the candidate vaccine is tested in vitro and evaluated for safety and biological response in relevant animal models. If the vaccine candidate successfully passes all the preclinical stages, it is then evaluated in humans by using a phased clinical approach. The candidate vaccine can then be passed on to entities outside of the NIH, such as other federal agencies and industry, for advanced development, licensure, manufacturing, and postlicensure safety evaluation.
Basic Research: Understanding Pathogenesis of the Microbe and the Host Response Is Instrumental in Creating Safe and Effective Vaccines
Basic research includes studies that characterize how microbes survive and multiply, elucidate complex host-microbe interactions, and increase understanding of how the host responds. Elucidating immunologic mechanisms and developing novel technologies applicable to vaccine design and development are important areas of basic research supported by the NIAID. Research on innate and adaptive immunity aims to increase our ability to manipulate immune responses through better understanding of the underlying molecular, cellular, and systemic aspects of natural host defenses and antigen-specific immunity.
Another important area of emphasis is research on activation of the innate immune system by vaccine adjuvants and establishment of long-term protective antibody and T-cell memory responses. Basic research in these areas contributes to vaccine safety in 2 important ways. First, it provides the knowledge and analytical tools for understanding the immunologic basis of protective immune responses as well as a framework for approaching studies of adverse events. Second, basic studies of the immunology of pathogen-host interactions contribute to the overall goal of improved vaccine efficacy with fewer adverse effects. For example, identifying the pathogen-specific and nonspecific components of immune responses of diverse subjects to different vaccine formulations has the potential to shed light on the genetics of vaccine responses and adverse effects. Such studies can also provide information on the immunologic basis of inflammation and on adjuvant activity.
Knowledge gained can provide a basis for engineering simpler, better-defined immunogens and live attenuated viral vaccines. Research in animal models enables rapid discovery of immunologic mechanisms and detailed functional analyses. Ultimately, studies must transition to humans for development of new vaccines and adjuvants. The NIAID has been increasingly emphasizing human immunology research to address uniquely human immune mechanisms and the vast genetic diversity of human populations. Future studies are planned to define the capacity and quality of the immune response throughout infancy and childhood and to identify the molecular basis for different immune/physiologic responses to vaccination at different stages of life.
Target Identification: In Vitro and in Vivo Assessments of Safety and Efficacy
Basic research sets the stage for the target-identification phase in which researchers identify portions of the microbe that will stimulate a potentially protective host immune response. Preclinical development includes testing in vitro and in relevant animal models to evaluate safety, immune response, and efficacy before clinical research can begin. The NIAID provides extensive resources for researchers, including a diverse array of preclinical services (eg, access to in vitro and in vivo testing, animal models, samples and reagents, and special patient populations). (For more information on available research resources, visit www.niaid.nih.gov/LabsAndResources/resources.)
Clinical Evaluation for Safety and Efficacy
Once the preclinical data package has been approved by the US Food and Drug Administration, sequential phases of clinical evaluation, each of which includes careful and extensive monitoring for safety, can commence. Phase I, which involves a small number of participants (typically 20–80), is used to evaluate safety and determine the most appropriate dose and dosage. Phase II further evaluates safety and efficacy and continues to determine the appropriate vaccine dose in larger numbers of subjects (usually 100–300 participants). Phase III clinical evaluation, which involves larger numbers of subjects, is used to confirm efficacy, collect additional safety information, and, if applicable, compare with existing vaccines. Phase III studies typically involve 1000 to 3000 participants, although a recent NIAID phase III study of a vaccine against herpes simplex virus had a sample size of 7000. For the vast majority of vaccine candidates that reach phase III clinical trials, substantial safety information has already been obtained, because previous preclinical studies and clinical evaluation have typically eliminated those candidates for which acute safety concerns are immediately evident.
UNDERSTANDING THE HOST IMMUNE RESPONSE
A new dimension in vaccine-safety research is rapidly taking shape as a result of advances in the analysis of human immune responses. Advances include high-throughput gene-expression profiling, methods to study individual T and B cells that are antigen-specific, and integration of vast amounts of data by using bioinformatics and systems biology. For example, individual, antigen-specific human T cells can be isolated by using special reagents. These T cells can then be extensively phenotyped by using gene arrays and next-generation nucleic acid sequencing. Large-scale cloning of human antibodies from individual B cells is providing new understanding of their origin, specificity, and binding characteristics. Innate immune responses of human dendritic cells, macrophages, and other antigen-presenting cells are being phenotyped in detail.
These techniques enable the detailed phenotyping of human responses to various vaccines; the goal is to develop an “encyclopedia” of human immunity. By understanding the molecular and cellular pathways that lead to healthy, protective responses and how they may differ when responses are inadequate, short-lived, or deleterious, vaccine researchers will have the information and tools that enable the clinical testing of new vaccines and provide methods to analyze adverse events.
The NIAID supports several immunology networks that aim to better understand how the human immune system responds to infection and vaccination, as well as databases, reagent resources, and bioinformatics tools for immunologic research. (For more information on NIAID immunology programs, see www.niaid.nih.gov/about/organization/dait/programsNetworks.htm.)
Immune Response to Microbes and Vaccines
Genetic variations in humans may play a significant role in susceptibility to infection and quality of response to vaccinations. Several NIAID programs provide support to further the understanding of variability in human immune responses to infectious pathogens and vaccination. The NIAID supports a network of sites (Population Genetics Analysis Program: Immunity to Vaccines/Infections) that are studying these associations by analyzing protein-expression levels and protein function. For example, 1 site is examining the possible significance of genetic polymorphisms in immune response induced by vaccinia immunization.
The Atopic Dermatitis and Vaccinia Network supports research to reduce the incidence and severity of eczema vaccinatum, a disseminated viral infection that can occur in subjects with atopic dermatitis after smallpox immunization or inadvertent exposure to a vaccinated person. One of the findings from that program is that the cytokines in the skin of patients with atopic dermatitis contribute to a deficiency in antimicrobial peptides, one of the body's innate immune defenses that is essential for protection of skin from infections.
The goal of the Cooperative Centers for Translational Research on Human Immunology and Biodefense is to further knowledge of human immune responses against infectious pathogens and to increase understanding of the molecular mechanisms responsible for both short-term immunity and long-term immune memory. For example, 1 center is examining smallpox immunization in people with cancer or eczema, and another is focused on research on vaccine-induced immunity in the young and aged.
Studies supported by the Immune Function in Children, Elderly, and Immunocompromised Populations program are focused on defining the molecular basis for differential immune capabilities in combating infection or responding to vaccination at different stages of life, or in people with different underlying health problems.
Human Immune Phenotyping and Vaccines
Vaccine-safety research requires the ability to understand factors that may contribute to variable immunization outcomes in recipients across genders, a wide range of ages, ethnic backgrounds, and immune competence. Currently, there is little information on what constitutes a “normal” human immune system, although that is beginning to change. The NIAID recently awarded grants for a new program designed to characterize diverse states of the human immune system (1) after infection, (2) before and after vaccination against an infectious disease, or (3) before and after treatment with an immune adjuvant that targets a known innate immune receptor(s). This program takes advantage of recent advances in systems biology, bioinformatics, and high-throughput multiplex assays that have resulted in the ability to more readily measure immune responses. (For more information, visit www.niaid.nih.gov/news/newsreleases/2010/Pages/HIPROcenters.aspx.)
The potential benefit of human immune phenotyping for vaccine development was illustrated by a recent study in which responses to the yellow fever vaccine were analyzed.1 Using a systems biology approach to understand protection afforded by this well-established vaccine, NIAID-supported scientists identified gene-activity profiles that predicted immunity. Analysis of large data sets identified key genetic patterns; mathematical analysis enabled positive identification of 2 distinct sets of early immune genes that predicted the T-cell response with 90% accuracy and the B-cell response with 100% accuracy. Understanding the response of the human immune system to vaccination may facilitate design and development of better vaccines and identification of correlates of protection and help scientists identify novel approaches to protecting against diseases such as malaria and HIV for which no vaccines are currently available.
Predicting the immunogenicity of a new vaccine is only one of several benefits that may arise from the use of these new technologies. Recent advances in the field of vaccinology have revealed that vaccine responses are often far more robust than previously appreciated. The immunogenicity of a vaccine is not a complete predictor of its efficacy, and it is important to distinguish between response profiles that correlate with vaccine immunogenicity and protection from those that may signal an overly vigorous response. Thus, developing an understanding of human immune profiles that predict vaccine safety and effectiveness in different populations is a critical need.
Adjuvants and the Innate Immune System
The discovery and development of novel adjuvants, which include compounds that stimulate the innate immune system, are important components of the NIAID vaccine research and development program. Receptors of the innate immune system are “hard-wired” to detect elements unique to the microbe, such as components of the cell wall or forms of nucleic acid not found in vertebrates. By using high-throughput library screens to identify potential adjuvant candidates that stimulate an innate immune response, several lead candidates have been identified and subsequently optimized and evaluated in animal models in which they have proven efficacious. The best candidates are now being developed for phase I clinical trials. Knowledge gained from this research is likely to benefit future vaccine development and formulation.
Other NIAID-supported research is focused on increasing understanding of how adjuvants work. Recent research has revealed how the adjuvant alum interacts with, and stimulates, the immune system to help provide protection against infectious diseases. Alum seems to activate a cluster of proteins found in certain immune cells that regulate the release of substances that promote inflammation.2 This discovery, which identifies some of the cellular machinery that helps provoke an effective immune response, may help scientists develop safer and more effective vaccines against a wide variety of pathogens.
Clinical studies conducted by industry have analyzed the safety profile of adjuvants in people with chronic diseases, including those on immunosuppressive therapy. In these studies, autoimmune symptoms did not seem to increase in people given adjuvants, which provides reassurance about their safety.
SAFELY PERFORMING VACCINE-SAFETY RESEARCH
At the NIH, vaccine safety is an integral part of every aspect of the development and evaluation of vaccines for specific diseases. Throughout the clinical research process, vaccine testing focuses on safety. When a study protocol is developed and before it is presented to the research community, safety concerns that would halt the study are clearly delineated. Likewise, measures to protect the safety of individual volunteers and reasons to discontinue a volunteer's participation in the study because of safety concerns are also clearly delineated in the protocol. NIH vaccine research is performed with an investigational new drug application in place with the Food and Drug Administration (FDA). The FDA performs a review of the preclinical data, clinical data, and the protocol design for human volunteer concerns before a study can start.
Before a vaccine study starts, each NIH protocol is also reviewed and approved by an institutional review board. The institutional review board review and approval encompasses the ethics of performing a study and a determination of the balance between the risks and the benefits to each subject. During a vaccine study, each subject is actively questioned about both local and systemic reactions to vaccines. Information about reactions and adverse events is collected for at least 6 weeks and as long as 1 year after vaccination. The clinical investigator and the study staff are the first line of this data collection. Medical monitors, who are clinicians who work at the NIH, review safety data for each clinical study across all study sites. Also, safety-monitoring committees (SMCs) and data safety-monitoring boards (DSMBs) provide a second and independent review of the safety data. On the basis of the SMC or DSMB review of the safety data, the SMC or DSMB can recommend that clinical trials be stopped or modified or continue unchanged. Most NIAID vaccine studies are blinded and randomized, and many are placebo-controlled to maximize the chance of detecting a safety or toxicity signal. After completion of the study, the safety and efficacy data are reviewed to determine if further clinical trials with a vaccine candidate are necessary or warranted.
The NIAID has extensive internal resources to manage clinical trial execution. The focus is on human volunteer safety and the accuracy of the data collected. These resources help design and review the science, collect data, organize safety committees, and keep track of the progress of the studies. The NIAID participates in ongoing activities that include other organizations. Data from trials are submitted to the Vaccine Adverse Events Reporting System or to MedWatch as appropriate. During the recent H1N1 pandemic, the NIAID participated in a government-wide independent review of the safety of the H1N1 vaccine that was coordinated by the National Vaccine Program Office called the National Vaccine Advisory Committee working group: H1N1 Vaccine Safety and Risk Assessment Working Group. The safety data collected during the NIAID-sponsored clinical trials were presented to this group as part of the US government's effort to make the safety profile of the H1N1 vaccine fully transparent.
In addition, the NIH supports research to address specific vaccine-safety research hypotheses. For example, when concerns about the effects of methyl mercury sparked questions about the safety of thimerosal, which contains a different mercury compound (ethyl mercury), the NIH supported studies to compare the 2 substances. Studies included comparison of ethyl and methyl mercury distribution in nonhuman primates and comparative pharmacokinetic and pharmacodynamic studies. Studies of how infants metabolized and excreted thimerosal after routine immunizations were also conducted. The results revealed important differences in the way that infants metabolized the 2 compounds. Ethyl mercury levels in blood and urine were uniformly low in all infants studied and, in many cases, too small to measure. There was no evidence of ethyl mercury accumulation in children between vaccinations. These results show that ethyl mercury is cleared from the body much more quickly than methyl mercury and confirm that methyl mercury is not an appropriate model for assessing risk from thimerosal.3,4
Recently, several NIH institutes, including the NIAID, have collaborated on an ongoing initiative to encourage research to address important scientific questions and issues related to vaccine safety. It is hoped that this type of research will contribute to the overall understanding of issues surrounding vaccines and their safety. Examples of relevant research areas that are encouraged by this initiative include:
detailed evaluation of various host immune/physiologic responses to currently licensed vaccine antigens and/or adjuvant combinations;
evaluation of existing childhood immunization schedules to optimize safe and long-term protective immune memory;
studies that define the capacity and quality of the immune response throughout infancy and childhood (can vaccines be further optimized to minimize the need for secondary immunizations?);
identification of the molecular basis for differential immune/physiologic responses to vaccination at different stages of life or when underlying health problems exist;
studies to determine if there are associations between genetic variations among people and susceptibility to serious adverse events in response to vaccination;
identification of risk factors and biological markers that would allow for assessment of whether there is a relationship between certain diseases or disorders and licensed vaccines;
comparison of the immunologic and physiologic effects of different combinations of vaccines and different schedules; and
creation/evaluation of statistical methodologies to provide rigorous analysis of vaccine-safety data from existing sources including passive reporting systems (eg, Vaccine Safety Datalink, Vaccine Adverse Events Reporting System) and to establish novel approaches to designing studies of vaccine safety.
KEYS TO FUTURE SUCCESS
Despite the many accomplishments in vaccine research, much remains to be done. Millions around the globe suffer illness and death from the relatively new disease HIV/AIDS and the ancient scourges of malaria and tuberculosis. For this reason, the NIH continues to make developing new or improved vaccines for those illnesses a focus of research and development activity. Other areas of focus include devising vaccines against disease-causing agents that either arise naturally, such as West Nile virus, rotavirus, and cytomegalovirus, or that might be deliberately released in an act of bioterrorism. Finding ways to quickly, effectively, and safely produce vaccines against strains of influenza that experts fear may spark a pandemic is also an area of interest area for the NIH.
Global demand for rapid development of a vaccine against the 2009 H1N1 influenza pandemic highlighted the urgency of developing new, faster, more efficient methods of vaccine production. Currently, influenza vaccines produced in the United States rely on egg-based manufacturing methods. Influenza vaccines have been prepared in eggs for >50 years, but the process is lengthy and requires hundreds of millions of fertilized eggs. Cell culture–based vaccines are currently licensed only in Europe, and it may be some time before vaccines produced by using cell cultures are licensed in the United States. The NIH actively supports research to develop new and improved vaccines and vaccine-production technologies for influenza. Innovative vaccine technologies being developed by the NIH and its industry partners include using recombinant DNA to create subunit vaccines in which various influenza virus proteins are selectively produced in cultured cells and are then purified and used in a vaccine. This and other “next-generation” vaccines will require research effort and time to reach commercial levels of manufacturing.
In addition to developing vaccines against classic infectious diseases and emerging microbes, the NIH is working to develop new and improved vaccines against chronic diseases with infectious origins as well as therapeutic vaccines against autoimmune diseases and other immune-mediated conditions. Therapeutic vaccines that could be effective against cancer and other diseases in which immune system activation may be beneficial are also being developed and tested.
Successful vaccine research and development requires collaborative efforts in which each partner plays a unique role. For example, vaccines against NIAID category A through C priority pathogens (microbes and toxins considered to be the most significant threats to the nation's well-being) are of the highest public health priority. However, the private sector has only limited incentives to invest in development of vaccines against these pathogens, given the absence of a substantial commercial market, regulatory hurdles, and extensive clinical trial requirements. To remedy this situation, the NIH offers services to facilitate the movement of promising vaccine candidates through the research and development pathway. These services include preclinical planning, process development and manufacturing, formulation and stability, assay development, sample-testing, in vivo immunogenicity, and vaccine safety and toxicity testing.
New understanding of human immune responses to vaccination is poised to emerge on the basis of better modeling of normal and adverse reactions, novel technologies that can rapidly and accurately determine immunologic characteristics of clinical samples by using only small amounts of material, and highly integrated and comprehensive databases and specimen repositories. The expanding knowledge and capabilities will greatly accelerate the design and development of new efficacious and safe vaccines.
Ultimately, the development of a vaccine from idea through licensure cannot be accomplished by 1 group or organization. True partnerships between government, academia, industry, foundations, and other organizations hold the key to creating safe and effective vaccines that meet the ultimate goal of preventing illness or death in the community.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- NIAID
- National Institute of Allergy and Infectious Diseases
- NIH
- National Institutes of Health
REFERENCES
- 1.Querec TD, Akondy RS, Lee EK, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminum adjuvants. Nature. 2008;453(7198):1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichichero ME, Gentile A, Giglio N, et al. Mercury levels in newborns and infants after receipt of thimerosal-containing vaccines. Pediatrics. 2008;121(2). Available at: www.pediatrics.org/cgi/content/full/121/2/e208 [DOI] [PubMed] [Google Scholar]
- 4.Pichichero ME, Gentile A, Giglio N, et al. Mercury levels in premature and low birth weight newborn infants after receipt of thimerosal-containing vaccines. J Pediatr. 2009;155(4):495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]


