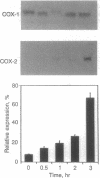
Abstract
Nonsteroidal antiinflammatory drugs (NSAIDs) are widely used for the treatment of inflammatory diseases, but significant side effects such as gastrointestinal erosion and renal damage limit their use. NSAIDs inhibit the enzyme cyclooxygenase (COX), which catalyzes the conversion of arachidonic acid to prostaglandins (PGs) and thromboxane. Two forms of COX have been identified--COX-1, which is constitutively expressed in most tissues and organs, and the inducible enzyme, COX-2, which has been localized primarily to inflammatory cells and tissues. In an animal model of acute inflammation (injection of carrageenan into the footpad), edema was produced that was associated with marked accumulation of COX-2 mRNA and thromboxane. A selective inhibitor of COX-2 (SC-58125) inhibited edema at the inflammatory site and was analgesic but had no effect on PG production in the stomach and did not cause gastric toxicity. These data suggest that selective inhibition of COX-2 may produce superior antiinflammatory drugs with substantial safety advantages over existing NSAIDs.
Full text
PDF

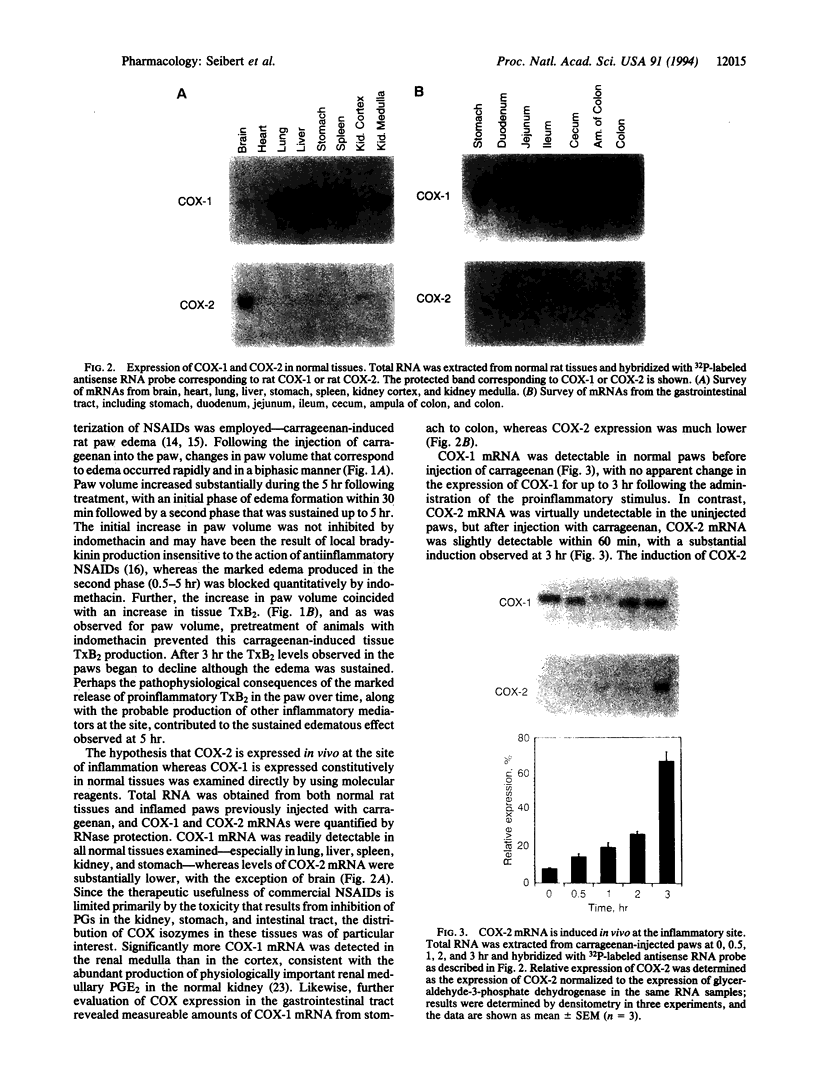
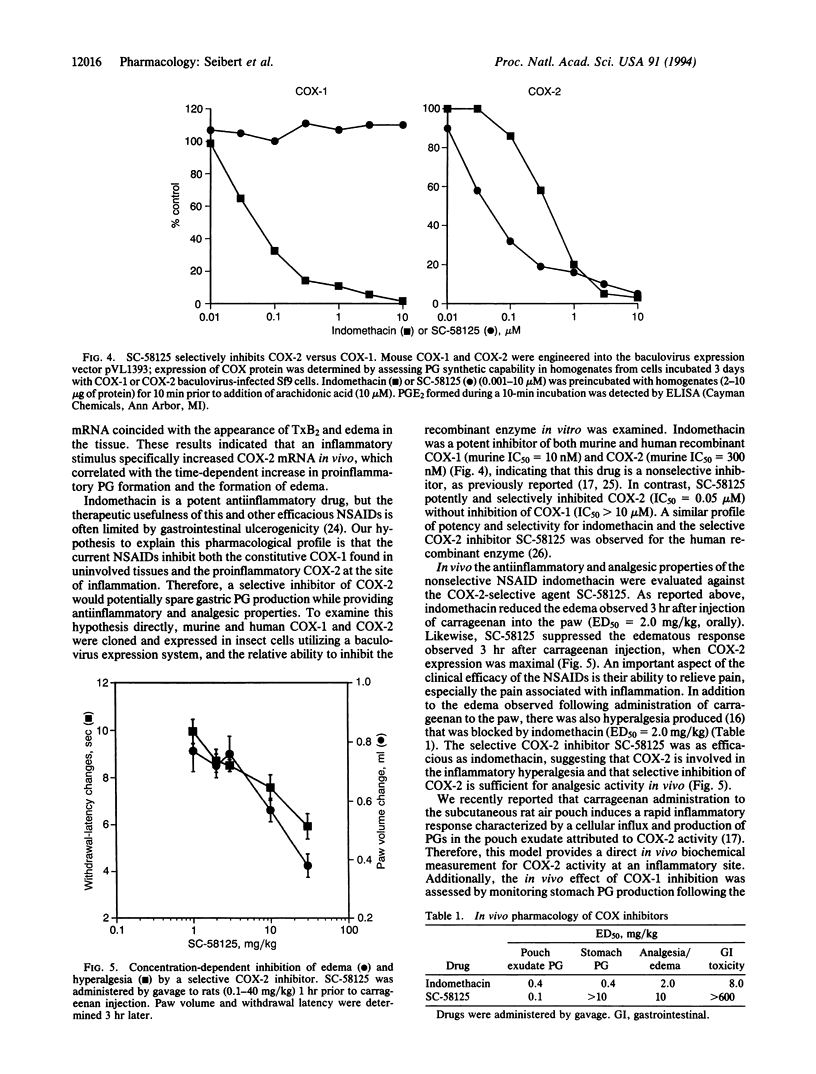

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Howatson A. G., Torrance C. J., Lee F. D., Russell R. I. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 1992 Sep 10;327(11):749–754. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- Brodie D. A., Cook P. G., Bauer B. J., Dagle G. E. Indomethacin-induced intestinal lesions in the rat. Toxicol Appl Pharmacol. 1970 Nov;17(3):615–624. doi: 10.1016/0041-008x(70)90036-0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Hargreaves K., Dubner R., Brown F., Flores C., Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988 Jan;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Kennedy B. P., Chan C. C., Culp S. A., Cromlish W. A. Cloning and expression of rat prostaglandin endoperoxide synthase (cyclooxygenase)-2 cDNA. Biochem Biophys Res Commun. 1993 Dec 15;197(2):494–500. doi: 10.1006/bbrc.1993.2506. [DOI] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Kujubu D. A., Herschman H. R. Dexamethasone inhibits mitogen induction of the TIS10 prostaglandin synthase/cyclooxygenase gene. J Biol Chem. 1992 Apr 25;267(12):7991–7994. [PubMed] [Google Scholar]
- Masferrer J. L., Seibert K., Zweifel B., Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Manning P. T., Hauser S. D., Leahy K. M., Smith W. G., Isakson P. C., Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Seibert K., Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. doi: 10.1172/JCI114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGiff J. C., Itskovitz H. D. Prostaglandins and the kidney. Circ Res. 1973 Nov;33(5):479–488. doi: 10.1161/01.res.33.5.479. [DOI] [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Needleman P., Wyche A., Bronson S. D., Holmberg S., Morrison A. R. Specific regulation of peptide-induced renal prostaglandin synthesis. J Biol Chem. 1979 Oct 10;254(19):9772–9779. [PubMed] [Google Scholar]
- Raz A., Wyche A., Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. doi: 10.1073/pnas.86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Sano H., Hla T., Maier J. A., Crofford L. J., Case J. P., Maciag T., Wilder R. L. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Invest. 1992 Jan;89(1):97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois J., Richards J. S. Purification and characterization of a novel, distinct isoform of prostaglandin endoperoxide synthase induced by human chorionic gonadotropin in granulosa cells of rat preovulatory follicles. J Biol Chem. 1992 Mar 25;267(9):6382–6388. [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Valle V. G., Fagian M. M., Parentoni L. S., Meinicke A. R., Vercesi A. E. The participation of reactive oxygen species and protein thiols in the mechanism of mitochondrial inner membrane permeabilization by calcium plus prooxidants. Arch Biochem Biophys. 1993 Nov 15;307(1):1–7. doi: 10.1006/abbi.1993.1551. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]