Version Changes
Revised. Amendments from Version 1
We have revised our manuscript to make some small text amendments, in response to Charles Chiu's comments.
Abstract
Metagenomic sequence data can be used to detect the presence of infectious viruses and bacteria, but normal microbial flora make this process challenging. We re-analyzed metagenomic RNA sequence data collected during a recent outbreak of acute flaccid myelitis (AFM), caused in some cases by infection with enterovirus D68. We found that among the patients whose symptoms were previously attributed to enterovirus D68, one patient had clear evidence of infection with Haemophilus influenzae, and a second patient had a severe Staphylococcus aureus infection caused by a methicillin-resistant strain. Neither of these bacteria were identified in the original study. These observations may have relevance in cases that present with flaccid paralysis because bacterial infections, co-infections or post-infection immune responses may trigger pathogenic processes that may present as poliomyelitis-like syndromes and may mimic AFM. A separate finding was that large numbers of human sequences were present in each of the publicly released samples, although the original study reported that human sequences had been removed before deposition.
Keywords: microbiome, metagenomics; neurological infections; computational biology; next-generation sequencing; sequence alignment
Background
Metagenomic shotgun sequencing, in which DNA or RNA is extracted from a tissue sample and then sequenced, has the potential to detect a wide range of infections. Deep whole-genome shotgun (WGS) sequencing can detect bacteria, viruses, and eukaryotic pathogens with equal effectiveness, as long as the infectious agent is similar to a species that has been previously sequenced. Sequencing databases already contain thousands of known species, and as this number grows, the sensitivity of WGS will grow as well.
In 2014, a large outbreak of infection with enterovirus D68 was associated with both severe respiratory illness and acute paralysis, which the U.S. Centers for Disease Control and Prevention (CDC) named acute flaccid myelitis (AFM) 1. Samples collected from 48 patients were sequenced and shown to form a novel strain, Clade B1, based on phylogenetic analysis of 180 complete enterovirus D68 sequences 2. The same study conducted metagenomic sequencing of cerebrospinal fluid (CSF) and/or nasopharyngeal (NP) swabs from 22 of these patients and found enterovirus D68 in some NP samples that were positive based on PCR testing.
The identification of species from a WGS sample is a challenging problem that has spurred the development of multiple new computational methods 3– 5. Because of the large size of next-generation sequencing data sets, these methods need to be very fast, but in the context of clinical diagnosis, they also need to be accurate. We downloaded the 31 next-generation sequencing (NGS) samples from the Greninger et al. 2 study (NCBI accession SRP055445) and re-analyzed them using a computational pipeline based on the recently developed Kraken metagenomic analysis software 4, a very fast and sensitive system that can be customized to use a database containing any species whose sequences are available.
Alternative infectious diagnoses in two subjects
Among the 22 subjects for which NGS data were available, we found at least two that had far greater numbers of sequences (reads) from a bacterial pathogen than from enterovirus D68. Neither subject had been reported in 2 as having a bacterial infection.
In one subject, US/CA/09-871, reported by Greninger et al. 2 as positive for enterovirus D68 through PCR and metagenomic NGS, we found in the NP swab sample an overwhelming presence of bacterial sequences from Haemophilus influenzae, a known cause of meningitis and neurological complications that was a common infection prior to the development of an effective vaccine.
Specifically, we identified 2,389,621 reads from H. influenzae in this subject, with the closest similarity to strain R2846. These reads comprise 93% of all microbial reads identified at the species level in the sample. Greninger et al. 2 reported 2,742 reads (in their Supplementary Table 4) matching enterovirus D68 2 but did not report finding any H. influenzae reads from this sample. Our analysis found 1,330 reads matching enterovirus D68.
To confirm the identity of these reads, we aligned them separately to the complete genome of H. influenzae R2846, and we found that the reads completely covered the genome. Dividing the genome into 100 kilobase windows, depth of coverage varied from 266–828 reads/100Kbp, with far deeper coverage as expected at the 16S ribosomal RNA genes.
The enterovirus D68 isolated from patient US/CA/09-871 differed from the others in that it appeared in 2009, well before the 2014 outbreak, and that it grouped with Clade C, phylogenetically distinct from Clade B1 that was associated with AFM. This patient was reported 2 as having respiratory illness but not AFM. The sequence evidence here suggests that the patient might have had complications from H. influenzae-associated infection, although no clinical or CSF data was available for our re-analysis.
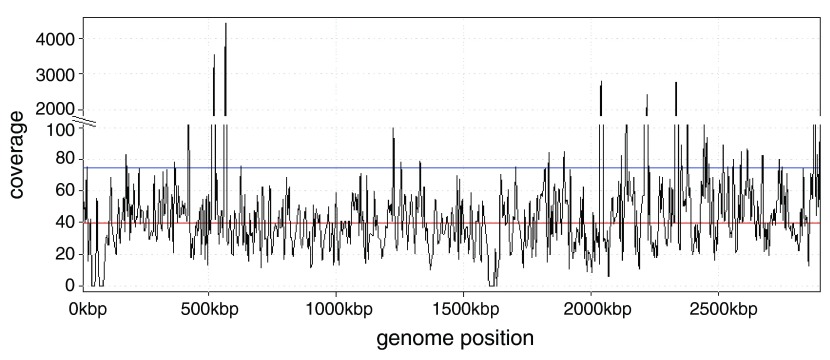
In a second subject, US/CA/12-5837, we found a strikingly large number of reads from Staphylococcus aureus in the NP swabs. The two separate NGS files associated with this subject contained 6,858,453 and 1,343,806 reads, comprising 70% and 84% (respectively) of all non-human reads identified at the species level in each sample. The closest match was S. aureus subsp. aureus MRSA252, a methicillin-resistant strain. The coverage was deep enough, approximately 40X, that it would be possible to assemble this genome separately from the reads here ( Figure 1). Greninger et al. 2 reported 2,790 reads from enterovirus D68 in this subject (our analysis found 1,641) but did not report any from S. aureus.
Figure 1. Depth of read coverage of the S. aureus MRSA252 genome using reads identified in the NGS sample from subject US/CA/12-5837.
High peaks correspond to 16S rRNA genes. Red line: median coverage; blue line: mean coverage.
Patient US/CA/12-5837 was sampled in 2012, two years before the outbreak of AFM, although this patient was described in Greninger et al. 2 as positive for enterovirus D68 based on clinical PCR testing and metagenomic sequencing. This patient is reported to be one of the first patients with enterovirus-D68-positive AFM 2, but the sequence evidence indicates a severe S. aureus infection that might explain at least some of the patient’s symptoms. S. aureus has been implicated in neurological complications such as myelitis 6 and meningitis 7 by mechanisms that involve not only direct invasion into the central nervous system (CNS), but also immunopathogenic responses triggered by superantigens that can target the CNS 8. At a minimum, S. aureus infection was overlooked by the previous analysis. Although the potential role of bacterial infection in the neurological disease that affected these two subjects is difficult to assess because of the lack of clinical and CSF information, its involvement as a pathogenic co-factor should be evaluated.
Human reads included in database submission
The metagenomics data (NCBI accession SRP055445) released by Greninger et al. 2 comprise 43 files which cover 22 of the 48 subjects from their study (in their Supplementary Table 1); the study did not conduct NGS for all subjects. Our metagenomics pipeline identifies human reads at the same time that it searches for pathogens; therefore we scanned the data for human as well as microbial content. Greninger et al. 2 reported that all human sequences had been removed from these files. We found, however, that all samples contained large numbers of human reads, ranging from a low of 18,215 to a high of 6,159,868. These comprised as few as 0.5% to as many as 95.6% of the reads in each sample, as shown in Table 1.
Table 1. Human reads found in metagenomic NGS samples from which human sequences were supposed to have been removed.
Shown are the number of reads in each sample that clearly match the human genome and do not match any microbial species. AFM: acute flaccid myelits; NP: nasopharyngeal swap; CSF: cerebrospinal fluid.
| Isolate | Run ID | Source | Number
of human reads |
%human |
|---|---|---|---|---|
| US/CA/12-5641 | SRR1919640 | NP | 6,159,868 | 85.4 |
| US/CA/12-5641 | SRR1919641 | NP | 1,427,490 | 90.8 |
| US/CA/12-5806 | SRR1919642 | NP | 164,876 | 89.8 |
| US/CA/12-5806 | SRR1919643 | CSF | 202,677 | 95.5 |
| US/CA/12-5807 | SRR1919644 | NP | 160,719 | 94.1 |
| US/CA/12-5807 | SRR1919645 | CSF | 383,094 | 24.2 |
| US/CA/12-5809 | SRR1919646 | NP | 65,635 | 95.4 |
| US/CA/12-5809 | SRR1919647 | NP | 456,228 | 70.4 |
| US/CA/12-5837 | SRR1919648 | NP | 4,662,958 | 20.2 |
| US/CA/12-5837 | SRR1919649 | NP | 1,251,672 | 28.6 |
| US/CA/14-5999 | SRR1919650 | CSF | 3,046,664 | 89.9 |
| US/CA/14-5999 | SRR1919651 | NP | 1,407,842 | 71.0 |
| US/CA/14-5999 | SRR1919933 | NP | 174,140 | 68.5 |
| US/CA/14-6000 | SRR1919652 | CSF | 746,831 | 91.1 |
| US/CA/14-6000 | SRR1919653 | NP | 164,638 | 0.6 |
| US/CA/14-6000 | SRR1919934 | NP | 19,469 | 0.5 |
| US/CA/14-6007 | SRR1919654 | CSF | 352,391 | 85.4 |
| US/CA/14-6010 | SRR1919655 | CSF | 426,172 | 93.2 |
| US/CA/14-6010 | SRR1919656 | NP | 1,194,587 | 38.8 |
| US/CA/14-6010 | SRR1919935 | NP | 144,391 | 36.7 |
| US/CA/14-6013 | SRR1919657 | NP | 544,276 | 87.4 |
| US/CA/14-6013 | SRR1919658 | NP | 1,636,067 | 83.9 |
| US/CA/14-6013 | SRR1919936 | NP | 213,180 | 79.8 |
| US/CA/14-6067 | SRR1919659 | CSF | 567,263 | 3.9 |
| US/CA/14-6067 | SRR1919937 | CSF | 66,076 | 2.3 |
| US/CA/14-6070 | SRR1919660 | CSF | 578,579 | 4.3 |
| US/CA/14-6070 | SRR1919938 | CSF | 88,153 | 3.2 |
| US/CA/14-6102 | SRR1919661 | CSF | 791,143 | 82.4 |
| US/CA/14-6102 | SRR1919939 | CSF | 92,723 | 78.2 |
| US/CO/13-60 | SRR1919662 | CSF | 519,456 | 95.7 |
| US/CO/13-60 | SRR1919940 | CSF | 79,477 | 93.4 |
| US/CO/14-86 | SRR1919663 | CSF | 155,058 | 38.4 |
| US/CO/14-86 | SRR1919941 | CSF | 18,215 | 26.5 |
| US/CO/14-88 | SRR1919664 | NP | 453,411 | 3.8 |
| US/CO/14-88 | SRR1919942 | CSF | 39,899 | 2.7 |
| US/CO/14-93 | SRR1919665 | CSF | 758,650 | 96.6 |
| US/CO/14-93 | SRR1919943 | CSF | 123,250 | 95.3 |
| US/CO/14-94 | SRR1919666 | NP | 835,689 | 96.1 |
| US/CO/14-94 | SRR1919944 | NP | 131,998 | 95.2 |
| US/CO/14-95 | SRR1919667 | CSF | 352,679 | 2.8 |
| US/CA/11-1767 | SRR1919639 | Culture | 1,030,900 | 33.7 |
| US/CA/10-786 | SRR1919638 | NP | 130,044 | 0.5 |
| US/CA/09-871 | SRR1919637 | CSF | 384,285 | 11.0 |
The inclusion of human sequence data in the files deposited at NCBI was likely a result of a computational method (SURPI 5) that was insufficiently sensitive. Although the exact cause cannot be determined here, it is well known that sequence alignment algorithms often trade speed for sensitivity; e.g., by allowing fewer mismatches, an aligner can process reads at a much higher rate, at the cost of missing some alignments. It is less clear why the very large numbers of matches to two bacteria were missed; for both these bacteria, complete genomes from multiple strains are available in GenBank. We used both the Kraken system 4 and the Bowtie2 aligner 9 to ensure both sensitivity and speed in our analysis.
Release of sequence data is highly valuable, if not essential, for reproducibility and validation of sequencing-based studies. Failure to filter human reads from a sample is not uncommon; a recent study 10 found that Human Microbiome Project samples, from which human DNA was supposed to have been removed, contain up to 95% human sequence. This suggests that future efforts to deposit microbiome data need to employ more sensitive computational screens in order to avoid the unintentional release of human sequence data.
Methods
Sequences were extracted from SRP055445 and each file was separately run through the Kraken program version 0.10.6-beta ( https://github.com/DerrickWood/kraken) 4, which identifies species by comparison with a database of all 31-bp sequences in all species. The database included the human genome (version GRCh38.p2), all complete bacterial and viral genomes, selected fungal pathogens, and known laboratory vector sequences from the NCBI UniVec database ( http://www.ncbi.nlm.nih.gov/tools/vecscreen/univec). Percentages of bacterial and viral reads in each sample were re-computed after excluding human and vector sequences. Reads matching more than one species were classified at the genus level or above. Reads from H. influenzae and S. aureus were re-aligned using Bowtie2 version 2.2.5 9, a very fast and sensitive program for alignment of NGS reads to a reference genome, with the --local option. Bowtie2 was also used to re-align all reads from US/CA/12-5837 and US/CA/09-871 to the sequence of multiple enterovirus D68 strains (GenBank accessions JX101846.1, AY426531.1, KM851231.1, KM892500.1, KM892501.1, KM881710.2, KP745751.1, KP745755.1, KP745757.1, KP745760.1, KP745764.1, KP745766.1, and KP745767.1). We report the highest number of reads matching any one of these strains.
Funding Statement
This work was supported in part by the National Institutes of Health under grant R01-HG007196 and by the U. S. Army Research Office under grant number W911NF-14-1-0490.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v2; ref status: indexed
References
- 1. Centers for Disease Control and Prevention. Notes from the field: acute flaccid myelitis among persons aged ≤21 years - United States, August 1-November 13, 2014. MMWR Morb Mortal Wkly Rep. 2015;63(53):1243–1244. [PMC free article] [PubMed] [Google Scholar]
- 2. Greninger AL, Naccache SN, Messacar K, et al. : A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect Dis. 2015;15(6):671–82. 10.1016/S1473-3099(15)70093-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brady A, Salzberg SL: Phymm and PhymmBL: metagenomic phylogenetic classification with interpolated Markov models. Nat Methods. 2009;6(9):673–6. 10.1038/nmeth.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood DE, Salzberg SL: Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15(3):R46. 10.1186/gb-2014-15-3-r46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naccache SN, Federman S, Veeraraghavan N, et al. : A cloud-compatible bioinformatics pipeline for ultrarapid pathogen identification from next-generation sequencing of clinical samples. Genome Res. 2014;24(7):1180–92. 10.1101/gr.171934.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saini M, Prasad K, Ling LM, et al. : Transverse myelitis due to Staphylococcus aureus may occur without contiguous spread. Spinal Cord. 2014;52(Suppl 2):S1–2. 10.1038/sc.2014.69 [DOI] [PubMed] [Google Scholar]
- 7. Aguilar J, Urday-Cornejo V, Donabedian S, et al. : Staphylococcus aureus meningitis: case series and literature review. Medicine (Baltimore). 2010;89(2):117–25. 10.1097/MD.0b013e3181d5453d [DOI] [PubMed] [Google Scholar]
- 8. Stach CS, Herrera A, Schlievert PM: Staphylococcal superantigens interact with multiple host receptors to cause serious diseases. Immunol Res. 2014;59(1–3):177–81. 10.1007/s12026-014-8539-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langmead B, Salzberg SL: Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ames SK, Gardner SN, Marti JM, et al. : Using populations of human and microbial genomes for organism detection in metagenomes. Genome Res. 2015. 10.1101/gr.184879.114 [DOI] [PMC free article] [PubMed] [Google Scholar]