Abstract
Calcium-activated potassium ion channel-3.1 (KCa3.1) plays a pivotal role in the potassium-calcium exchange involved in atopy. This study aimed to explore the impact of lentiviral-mediated shRNA silencing KCa3.1 on allergic response in a murine allergic rhinitis (AR) model. The BALB/c mice were divided into four groups: untreated AR group, negative control AR group, lentiviral KCa3.1-shRNA treated AR group and normal control group. Concentrations of ovalbumin (OVA)-specific IgE, histamine and leukotrienes C4 (LTC4) in serum, and IL-4, IL-9 and IL-17 in nasal lavage fluid (NLF) were analyzed. Goblet cells and mast cells were counted. KCa3.1 positive cells were counted after immunolabelling by immunofluorescence method. KCa3.1, Mucin 5AC (MUC5AC), and tryptase mRNA levels were determined using real-time polymerase chain reaction. Furthermore, P815 cell line was used to explore the role and mechanism of lentiviral KCa3.1-shRNA on mast cells. The results showed that LV-KCa3.1-shRNA intervention effectively attenuated allergic responses in LV-KCa3.1-shRNA treated mice. LV-KCa3.1-shRNA intervention effectively suppressed KCa3.1 levels and phosphorylation of AKT in P815 cells, leading to the downregulation of tryptase, IL-6 and IL-8 levels. LV-KCa3.1-shRNA intervention effectively attenuated the allergic responses in AR and suppressed mast cell activity by inhibiting PI3K/AKT signaling pathway.
Allergic rhinitis (AR) is a chronic inflammatory upper airway disease characterized by sneezing, rhinorrhea and stuffy nose, and AR could negatively impact patient’s quality of life1,2. There is ample evidence that goblet cell hyperplasia, mast cell infiltration and lymphocytes disorders are involved in the pathogenesis of AR3,4,5. However, the precise pathogenesis of AR remains uncertain. Accumulating evidences indicate that antihistamine agents and corticosteroids are effective in the treatment of AR6,7, however, the transient efficacy and drug tolerance of them make AR not easy to cure8. It is acknowledged that allergen immunotherapy (also named as desensitization) can alleviate allergic symptoms by inducing specific immunologic tolerance in the AR sufferer with one specific allergen, however, many patients are allergic to more than one allergen, and this treatment is time-consuming and expensive, and it has the risk of triggering serious allergic reactions. Hence, a novel safe, effective and inexpensive treatment for AR is urgently needed.
RNA interference (RNAi) is a gene-specific RNA degradation process mediated by short interfering RNA (siRNA) and is a promising therapeutic tool directed against a potential therapeutic target in the allergic disorders, infectious diseases and cancers9,10,11. A short hairpin RNA (shRNA) is a precursor of siRNA and is composed of siRNA sequence and hairpin loop sequence12,13. It has been established that shRNA was more stable than siRNA and was more readily integrated into the plasmid or viral vectors14. Lentivirus-mediated shRNA using lentiviral vector yielded an efficient and stable response of the target gene silencing both in vitro and in vivo15.
It is well established that calcium ion (Ca2+) is essential for regulating most cell activities involving cell growth, transformation, and migration. There is ample evidence that upregulation of intracellular Ca2+ participates in the proliferation of airway epithelial cells, smooth muscle cells and lymphocytes involved in asthma16,17,18. It has been illustrated that sustained Ca2+ influx through Ca2+ release-activated Ca2+ channel (CRAC) requires sufficient electrical driving force which is conserved by efflux of potassium ion (K+) and intermediate Ca2+-activated K+ channel (KCa3.1, also named as KCNN4) has been proven to mainly modulate the efflux of K+ and plays a pivotal role in the potassium-calcium exchange involved in the allergic and inflammatory responses19. Nevertheless, whether KCa3.1 is involved in the pathophysiology of AR and the inhibition of KCa3.1 could suppress the allergic response in AR are unclear. Hence, the present study was aimed to explore the expression and role of KCa3.1 in the pathogenesis of AR and to further assess the therapeutic effect of lentivirus-mediated shRNA silencing of KCa3.1 in a murine AR model. Furthermore, P815 cell line was used to explore the role and mechanism of lentiviral KCa3.1-shRNA on mast cells.
Results
Effects of LV-KCa3.1-shRNA on nasal symptoms in a murine AR model
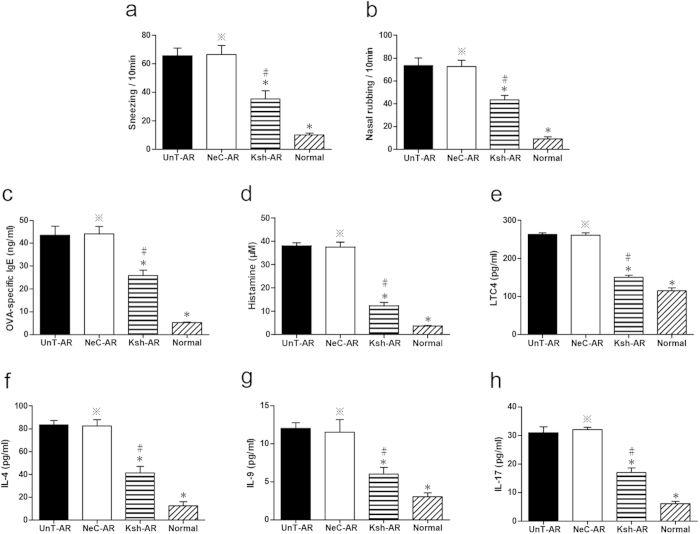
As indicated in Fig. 1a,b, significant elevation of frequencies of sneezes and nasal rubbing were found in untreated AR mice compared with normal control mice, respectively (P < 0.01), and LV-KCa3.1-shRNA group showed lower frequencies of sneezes and nasal rubbing compared with untreated AR or negative control group, respectively (P < 0.01).
Figure 1. Sneezes and nasal rubbing in UnT-AR group, NeC-AR group, Ksh-AR and normal control group.
(a–b). Concentrations of OVA-specific IgE, histamine and LTC4 in serum, and IL-4, IL-9 and IL-17 in NLF assayed by ELISA (c–h). One-way ANOVA test was employed for intergroup comparison. *P < 0.01 vs UnT-AR group. #P < 0.01 vs NeC-AR group. *P > 0.05 vs UnT-AR group.
Effects of LV-KCa3.1-shRNA on OVA-specific IgE, histamine and LTC4 in serum and cytokine levels in NLF
To further illustrate the roles of LV-KCa3.1-shRNA on mast cell, Th2, Th9 and Th17 cells in AR, serum OVA-specific IgE, histamine and LTC4, IL-4 (Th2-derived cytokine), IL-9 (Th9-derived cytokine) and IL-17 (Th17-derived cytokine) levels in NLF were assayed by ELISA. As illustrated in Fig. 1c–h, significantly elevation of serum OVA-specific IgE, histamine and LTC4 levels, and IL-4, IL-9 and IL-17 levels in NLF from untreated AR group were found compared with normal control mice. LV-KCa3.1-shRNA treatment resulted in the reduction of OVA-specific IgE, histamine, LTC4, IL-4, IL-9 and IL-17 levels in LV-KCa3.1-shRNA treated group compared with untreated AR or negative control group, respectively (P < 0.01). These findings were consistent with the changes in the nasal symptoms following LV-KCa3.1-shRNA treatment.
Goblet cell hyperplasia, mast cell infiltration and KCa3.1 immunoreactivity in nasal tissues
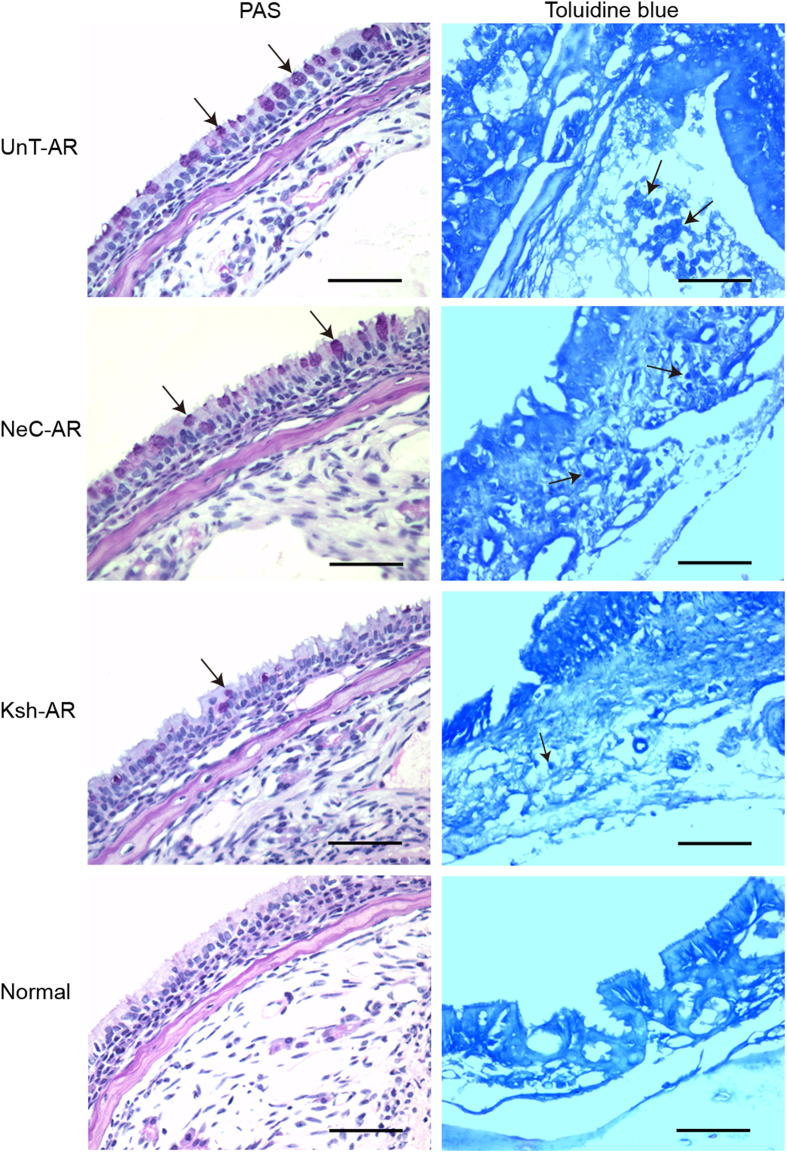
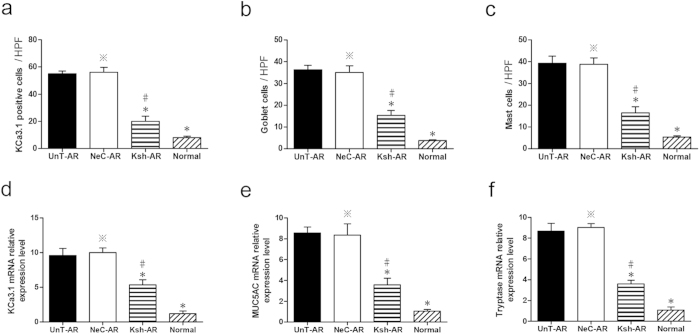
As indicated in Fig. 2 and Fig. 3b,c, significant elevation of goblet cell and mast cell numbers were found in untreated AR mice compared with normal control mice (P < 0.01), and LV-KCa3.1-shRNA group showed less goblet cell and mast cell numbers compared with untreated AR or negative control group, respectively (P < 0.01), indicating that LV-KCa3.1-shRNA treatment reduced goblet cell hyperplasia and mast cell infiltration in AR mice.
Figure 2. Goblet cell and mast cell numbers in UnT-AR group, NeC-AR group, Ksh-AR and normal control group.
Arrows indicate goblet cell or mast cell. Scale bar = 50 μm.
Figure 3. Analysis of KCa3.1 protein immunolabelling, goblet cell and mast cell numbers.
(a–c) KCa3.1, MUC5AC and tryptase mRNA levels assayed by real-time PCR (d–f). One-way ANOVA test was employed for intergroup comparison. *P < 0.01 vs UnT-AR group. #P < 0.01 vs NeC-AR group. *P > 0.05 vs UnT-AR group.
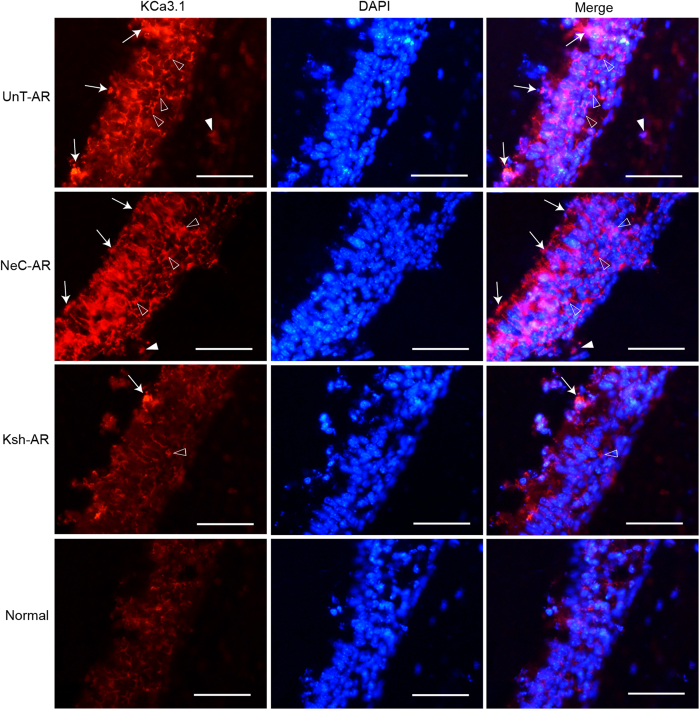
As depicted by immunofluorescence staining (Fig. 4), KCa3.1 positive cells were mainly epithelial cells among mucosal surface and surrounding basal lamina, and sub-mucosal inflammatory cells in the nasal mucosa. KCa3.1 was significantly overexpressed in AR group compared with control group; notably, LV-KCa3.1-shRNA treatment resulted in the reduction of KCa3.1 protein levels in LV-KCa3.1-shRNA treated group compared with untreated AR or negative control group, respectively (P < 0.01). These results were consistent with the changes in the nasal symptoms and cytokine levels following LV-KCa3.1-shRNA treatment.
Figure 4. KCa3.1 immunolabelling assayed by immunofluorescence in UnT-AR group, NeC-AR group, Ksh-AR and normal control group.
Arrows indicate KCa3.1 positive mucosal epithelial cells. Empty triangles indicate KCa3.1 positive epithelial cells surrounding basal lamina. Filled triangles indicate KCa3.1 positive sub-mucosal inflammatory cells. Scale bar = 50 μm.
Moreover, KCa3.1 positive cell numbers were positively correlated with goblet cell or mast cell numbers respectively (Spearman’s test, r = 0.821, p = 0.023 and r = 0.865, p = 0.012).
Effects of LV-KCa3.1-shRNA on KCa3.1, MUC5AC and tryptase mRNA levels in AR mice
To further illuminate the roles of LV-KCa3.1-shRNA on goblet cell and mast cell in AR mice, mRNA levels of KCa3.1, MUC5AC, which is mainly secreted by goblet cell20, and tryptase, known as a key mast cell marker which mainly exists in the secretory granules of mast cell21, were assayed by real-time PCR.
As showed in Fig. 3d–f, significant upregulation of KCa3.1, MUC5AC and tryptase mRNA levels were found in AR mice compared with normal control mice (P < 0.01); Of note, LV-KCa3.1-shRNA treatment suppressed KCa3.1, MUC5AC and tryptase mRNA levels in LV-KCa3.1-shRNA treated group compared with untreated AR or negative control group, respectively (P < 0.01). Moreover, KCa3.1 mRNA level was positively correlated with MUC5AC or tryptase mRNA level, respectively (Spearman’s test, r = 0.738, p = 0.037 and r = 0.833, p = 0.01). These results were consistent with the findings of histopathologic examination following LV-KCa3.1-shRNA treatment.
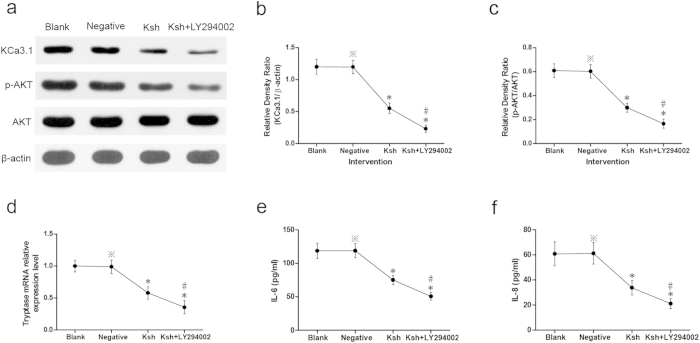
Effects of LV-KCa3.1-shRNA on KCa3.1 levels and phosphorylation of AKT in P815 cells
To further illustrate the roles and mechanism of LV-KCa3.1-shRNA on mast cell, P815 cell line was intervened with LV-KCa3.1-shRNA and LY294002 serving as a specific inhibitor of PI3K/AKT signaling pathway. As indicated in Fig 5a–c, strong bands of KCa3.1 and p-AKT protein were found in the blank control or negative control group, respectively; whereas weak bands were detected in LV-KCa3.1-shRNA group or LV-KCa3.1-shRNA +LY294002 group; notably, weaker bands were found in LV-KCa3.1-shRNA +LY294002 group compared with LV-KCa3.1-shRNA group. KCa3.1 levels (KCa3.1/β-actin) and phosphorylation of AKT (p-AKT/AKT) were suppressed by LV-KCa3.1-shRNA, and LY294002 enhanced these effects.
Figure 5. Results of P815 cell culture and intervention experiments.
Western blotting analysis of KCa3.1 levels (KCa3.1/β-actin) and phosphorylation of AKT (p-AKT/AKT) (a–c) tryptase mRNA levels assayed by real-time PCR (d). Concentrations of IL-6 and IL-18 in the culture supernatants (e–f). One-way ANOVA test was employed for intergroup comparison. *P < 0.01 vs blank control group. #P < 0.01 vs Ksh group. *P > 0.05 vs blank control group. The cropped blots images are shown in the figure and the full-length blots images are presented in Supplementary Information.
Effect of LV-KCa3.1-shRNA on tryptase mRNA level in P815 cells
As showed in Fig. 5d, significant downregulation of tryptase mRNA level was found in LV-KCa3.1-shRNA group or LV-KCa3.1-shRNA+LY294002 group (P < 0.01), indicating that P815 cells were inactivated by LV-KCa3.1-shRNA and LY294002 enhanced the inhibition.
Effects of LV-KCa3.1-shRNA on cytokines released from P815 cells
As showed in Fig. 5e,f, significant downregulation of IL-6 and IL-8 levels were found in LV-KCa3.1-shRNA group or LV-KCa3.1-shRNA+LY294002 group (P < 0.01), indicating that IL-6 and IL-8 levels released from P815 cells were suppressed by LV-KCa3.1-shRNA and LY294002 enhanced the inhibition.
Discussion
It has been proved that goblet cell hyperplasia, mast cell infiltration, Th-2, Th-9 and Th-17 polarization are recognized as important features of AR3,5,22,23. In the present study, significantly increased goblet cell hyperplasia, mast cell infiltration, IL-4, IL-9 and IL-17 levels were found in AR mice compared with normal control mice, which is consistent with previous reports3,5,22,23. In addition, MUC5AC and tryptase mRNA levels were significantly upregulated in AR mice compared with normal control mice, further confirming that goblet cell hyperplasia and mast cell infiltration are involved in the pathogenesis of AR.
Our previous reports demonstrated that Orai1, an essential component of the CRAC was upregulated and led to elevation of intracellular Ca2+ concentration in a murine AR model and target inhibition of Orai1 could effectively alleviate murine AR through reducing Ca2+ influx24,25. Recently, there is emerging evidence that KCa3.1 has been implicated as an amplifier of CRAC current and could sustain sufficient membrane potential and electrochemical driving force for Ca2+ influx by modulating K+ efflux26,27. Previous studies have demonstrated that targeted inhibition of KCa3.1 gene could effectively suppress the allergic responses in asthma and inhibit cell proliferation in cancers28,29. In our present study, as indicated in Figs 1, 2, 3 and Fig. 3a–f, LV-KCa3.1-shRNA could effectively alleviate allergic symptoms of AR mice by reducing goblet cell hyperplasia, mast cell infiltration, OVA-specific IgE, histamine, LTC4, IL-4, IL-9, IL-17, KCa3.1, MUC5AC and tryptase levels. These results show that LV-KCa3.1-shRNA intervention may suppress goblet cell hyperplasia and mast cell infiltration, and inactivate plasma cells, Th2 ,Th9 and Th17 cells.
Considerable data indicate that mast cells play central roles in the pathologic change of nasal mucosal tissue and pathophysiologic process in AR30,31,32. Accumulating evidence has shown that upregulation of intracellular Ca2+ concentration is essential for mast cell activation33,34 and treatment of mast cells with carbon dioxide suppresses degranulation via repression of intracellular calcium levels35. KCa3.1 channels are required for mast cell recruitment and activation36. To date, there are many lines of evidence indicating that PI3K/AKT signaling pathway plays an essential role in the maturation and activation of mast cells37,38,39 and PI3K/AKT signaling pathway inhibitor LY294002 suppresses mast cells degranulation and activation40,41. In the present study, to further illustrate the roles and mechanism of LV-KCa3.1-shRNA on mast cell, P815 cell line was treated with LV-KCa3.1-shRNA and PI3K/AKT signaling pathway inhibitor LY294002. As depicted in Fig. 5a–f, LV-KCa3.1-shRNA group showed lower levels of KCa3.1, AKT phosphorylation, tryptase, IL-6 and IL-8 compared with blank control or negative control group, respectively and LY294002 enhanced these effects. These results indicate that the roles of LV-KCa3.1-shRNA on mast cells may be mediated via inactivation of the PI3K/AKT signaling pathway in mast cells. Our findings are in agreement with previous findings demonstrating that PI3K/AKT signaling pathway inhibitor LY-294002 exerted inhibitory effects on the mast cell activation41,42,43.
Some weaknesses and limitations of our study should be interpreted. First, relatively small sample size was included in this study and studies with large sample sizes are warranted to further clarify these findings. Second, it is necessary to perform whole genome microarray to assess the off target effects of LV-KCa3.1-shRNA in future studies.
In conclusion, LV-KCa3.1-shRNA could effectively alleviate murine allergic rhinitis by suppressing KCa3.1, leading to the reduction of goblet cell hyperplasia and mast cell infiltration, as well as attenuation of allergic responses and inflammatory factors. The role of LV-KCa3.1-shRNA on mast cells may be mediated via inactivation of the PI3K/AKT signaling pathway in mast cells. The targeted inhibition of KCa3.1 gene mediated by lentiviral vector may be a novel promising approach in AR gene therapy.
Methods
Construction of lentiviral KCa3.1-shRNA vector
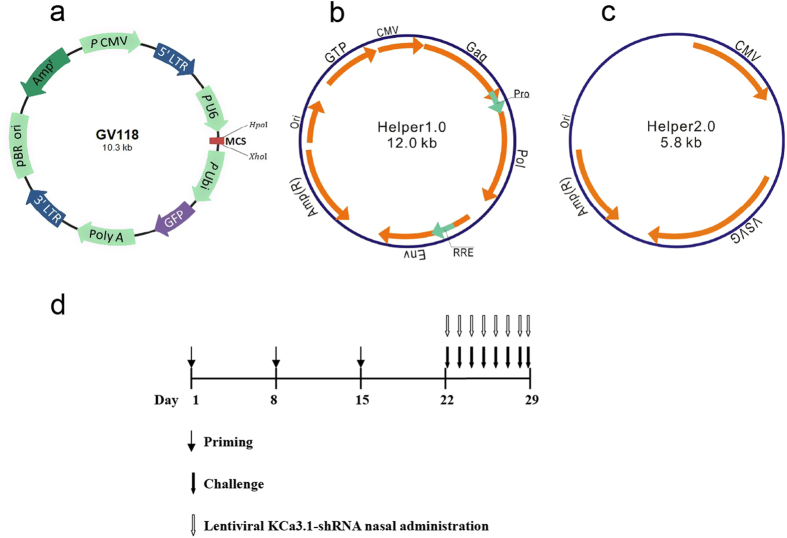
The shRNA targeting sequence for KCa3.1 (KCa3.1-shRNA) was 5′-TCATGATGGACATCCATTA-3′ and the scrambled RNA sequence (negative-shRNA) used as negative control was 5′-TTCTCCGAACGTGTCACGT-3′. The GV118 vector encoding KCa3.1-specific shRNA, pHelper 1.0 vector, and pHelper 2.0 vector (Fig. 6a–c, GeneChem, Shanghai, China) were co-transfected into the 293T cell line using lipofectamine 2000 (Invitrogen, Carlsbad, USA) to generate re-constructed lentiviruses according to the manufacturer’s instructions. After 48 hours, the supernatants were collected and re-constructed lentiviruses were harvested by centrifugation and then stored at −80 °C for further experiments.
Figure 6. Lentiviral vector system.
(a–c) GV-118 vector encoding sustained RNA expression (a) pHelper 1.0 vector, encoding a viral structural protein (b) and pHelper 2.0 vector, encoding a viral capsid protein (c). Schematic diagram of a murine AR model and LV-KCa3.1-shRNA treatment (d).
Murine AR model and LV-KCa3.1-shRNA treatment
A total of 48 female BALB/c mice (6–8 weeks of age, Shanghai Experimental Animal Center, Shanghai, China) were included in this study and they were kept in a specific-pathogen free facility. Animal experimental protocols were approved by the Animal Care and Use Committee of Eye and ENT Hospital of Fudan University and the experiments were carried out in accordance with the approved guidelines. The mice were divided four groups (n = 12 per group): untreated AR (UnT-AR) group, negative control AR group (NeC-AR), lentiviral KCa3.1-shRNA treated AR group (LV-KCa3.1-shRNA treated AR group, Ksh-AR) and normal control group. The murine AR model was established as described previously24. Briefly, mice were sensitized with 0.2 mL suspension comprising 0.5 mg/mL ovalbumin (OVA, Sigma-Aldrich, USA) and 20 mg/mL aluminum hydroxide (Sinopharm Chemical Reagent Co. Ltd., China) by intraperitoneal injection on Day 1, 8, and 15, respectively. Then, the mice were challenged daily with 20 μl OVA solution (40 mg/ml in normal saline) by intranasal instillation on Days 22 to 29 (Fig. 6d). To improve gene transfection efficiency, the mice were instilled intranasally with 4μl lysophosphatidylcholine (1% w/v in PBS, Sigma-Aldrich, USA) prior to delivery of the lentiviral vector system, as described previously44. Mice in lentiviral KCa3.1-shRNA treated group were instilled intranasally with lentivirus mediated KCa3.1-shRNA (20 μl, 1 × 108 TU/ml) and the mice in negative control group were given with the same dose of lentivirus mediated negative-shRNA.Mice in normal control group were administrated with normal saline.
Nasal symptoms evaluation and sample preparation
On Day 29, immediately after the final OVA provocation, nasal symptoms involving frequency of sneezes and nasal rubbing were assessed for 10 minutes. The mice were observed and reviewed independently by three blinded reviewers whose inter-interviewer variability was not statistically significant. After 24 hours, the mice were killed and the blood samples were harvested by cardiac puncture and were centrifuged to obtain serum for enzyme-linked immunosorbent assay (ELISA). Tracheas of the mice were partially resected and a 22-gauge catheter was inserted via the tracheal opening to the nasopharynx in order to perfuse the nasal passages from choana to nostril with 1 ml PBS. Nasal lavage fluid (NLF) was collected from the nares and centrifuged, and the supernatants were collected and stored at −80 °C for ELISA. Nasal mucosas were assayed by Periodic Acid-Schiff (PAS) stainning, toluidine blue staining, immunofluorescence staining, real-time PCR or western blotting methods.
ELISA assay
Serum OVA-specific immunoglobulin E (IgE), histamine and leukotrienes C4 (LTC4) levels were assayed using specific ELISA kit (BlueGene Biotech, Shanghai, China) and concentrations of IL-4, IL-9 and IL-17 in NLF or IL-6 and IL-8 in the cell culture supernatants were assayed with specific ELISA kits (eBioscience Inc., USA). Each sample was analyzed in triplicate.
PAS, toluidine blue and immunofluorescence staining
The mice were decapitated and the heads were fixed with 4% paraformaldehyde for 2 days and decalcified for 21 days in 10% ethylene diamine tetra acetic acid (EDTA).Then the samples were paraffin-embedded and serially sectioned. The sections were stained with PAS for counting goblet cell numbers, stained with toluidine blue for assessing mast cell infiltration, and stained with immunofluorescence for assessing KCa3.1 immunolabelling. Following deparaffinization and rehydration, the sections were rinsed using PBS for three times and then microwaved for antigen retrieval in EDTA buffer (PH8.0). The sections were incubated with primary antibody (rabbit anti-mouse KCa3.1, 1:100 dilution, Abcam) at 37 °C for 60 min, rinsed for three times and incubated with secondary antibody (cy3-labeled goat anti-rabbit IgG, 1:300 dilution, Abcam) for 30 min. Then, 4,6-diamidino-2-phenylindole (DAPI) was added for nuclear staining. Goblet cell, mast cell and KCa3.1 positive cell numbers were microscopically counted under high-powered field (HPF, ×400), and five fields of each section were randomly selected and mean value per HPF was calculated. All pathology slides images were reviewed independently by three blinded reviewers whose inter-interviewer variability was not statistically significant.
RNA extraction and reverse transcription
After homogenization of the tissues or cells, TRIzol reagent was added to extract total RNA. The purity and integrity were assessed by measuring absorbance ratios at 260/280 nm (1.8 ~ 2.0 was considered eligible) and agarose gel electrophoresis, respectively. For reverse transcription, PrimeScript™ 1st Strand cDNA Synthesis Kit (TaKaRa) was applied to reversely transcribe two micrograms total RNA into cDNA as a template for real-time PCR.
Real-time PCR
SYBR Green PCR master mix (TaKaRa) was employed to assay KCa3.1, mucin 5AC (MUC5AC) and tryptase mRNA levels. Primers were designed and synthesized (Sangon Biotech, Shanghai, China). Each sample was analysed in triplicate. KCa3.1 (120 bp) primers were: forward primer, 5–AAGCACACTCGA AGGAAGGA–3 and reverse primer, 5–ATTCACTTGTTCCCGGAGCT –3. MUC5AC (193 bp) primers were: forward primer, 5–AGGGCTCTGTGACAAC TACC–3 and reverse primer, 5–TGGGGTGTGGGTAGAAGAAC–3. Tryptase (142 bp) primers were: forward primer, 5–CCGCCACCATTTCCTTTGAA–3 and reverse primer, 5–GTCCTTCATTCCCAGCACAC–3.The internal control GAPDH (186 bp) primers were: forward primer, 5–CAACTCCCACTCTTCCACCT–3 and reverse primer, 5–CTTGCTCAGTGTCCTTGCTG–3. Relative mRNA level was expressed as the relative fold change and calculated using the formula: 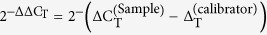 where each
where each 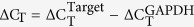 . One sample from a normal control mouse was designated as a calibrator.
. One sample from a normal control mouse was designated as a calibrator.
Mast cell culture and intervention
Culture of the mouse mastocytoma cell line P815 cells (ATCC, Manassas, VA, USA) were performed as described previously45. Briefly, P815 cells were cultured with Dulbecco’s modified Eagle’s medium (DMEM, ATCC, Manassas, VA, USA) containing 10% fetal bovine serum (FBS), 1.5 mg/ml sodium bicarbonate, 4.5 mg/ml glucose, 4 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C in 5% CO2, humidified atmosphere. Before intervention, P815 cells were cultured in the serum-free basal medium for at least 24 h. The cells were divided into four groups and then incubated with 10 μl PBS (blank control), 10 μl negative-shRNA (1 × 108 TU/ml, negative control), 10 μl 1 × 108 TU/ml lentiviral KCa3.1-shRNA (Ksh), or 10 μl 1 × 108 TU/ml lentiviral KCa 3.1-shRNA supplemented with 20 μM phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway inhibitor LY294002 (Cell Signaling Technology) for 12 h, respectively. Then, the cell pellets were harvested for assaying KCa3.1, p-AKT and AKT protein levels using western blotting and tryptase mRNA levels using real-time PCR and the culture supernatants were centrifuged and collected for detecting IL-6 and IL-8 protein levels by ELISA.
Western blotting
The cells were homogenized and lysed in RIPA buffer (Pierce) and then centrifugated at 14000 rpm for 15 min at 4 °C. The supernatant involving the total protein were collected. Following quantification of protein concentration, the samples were loaded on SDS–PAGE gels (Invitrogen) for protein separation and subsequently electroblotted onto PVDF membranes (Millipore) under the same experimental conditions. After blocking of excess protein-binding sites with 5% skimmed milk for 1 h, the PVDF membranes were incubated with the primary antibody (rabbit anti-mouse KCa3.1, 1:1000 dilution, Abcam; rabbit anti-mouse p-AKT and AKT, 1:1000 dilution, Cell Signaling Technology) at 4 °C overnight. After TBST washing, the secondary goat anti-rabbit IgG-HRP antibody (Abcam) was added for 1 h incubation. Lastly, immunoreactions were visualised by the ECL kit (Pierce). β-actin was used as an internal control. Densitometric analyses were performed using Gel-Pro Analyzer 4.0 software (Media Cybernetics).
Statistical analysis
Statistical analysis softwares (GraphPad Prism 5.0 and SPSS version 20.0 software) were applied to conduct the analyses. One-way ANOVA test followed by Dunnett’s tests were employed for intergroup comparison of all the continuous variables for them had normal distribution. Correlations were assessed by Spearman’s test. Values were shown as mean ± standard error (SE). Two-sided P value < 0.05 was deemed statistically significant.
Additional Information
How to cite this article: Lin, H. et al. Lentiviral shRNA against KCa3.1 inhibits allergic response in allergic rhinitis and suppresses mast cell activity via PI3K/AKT signaling pathway. Sci. Rep. 5, 13127; doi: 10.1038/srep13127 (2015).
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81470672, 81271060 and 30973281).
Footnotes
Author Contributions H.L. carried out experiments, performed data analysis and prepared the manuscript; J.L., C.Y. and L.H. carried out experiments and participated in the data analysis; C.Z. designed the study and prepared the manuscript.
References
- Bellanti J. A. & Settipane R. A. The burden of allergic rhinitis on patients’ quality of life. Allergy Asthma Proc. 33 Suppl 1, S112 (2012). [DOI] [PubMed] [Google Scholar]
- Hellings P. W. et al. Uncontrolled allergic rhinitis and chronic rhinosinusitis: where do we stand today? Allergy. 68, 1–7 (2013). [DOI] [PubMed] [Google Scholar]
- Ouyang Y. et al. TGF-beta signaling may play a role in the development of goblet cell hyperplasia in a mouse model of allergic rhinitis. Allergol Int. 59, 313–319 (2010). [DOI] [PubMed] [Google Scholar]
- Poggi A., Canevali P., Contatore M. & Ciprandi G. Higher frequencies of CD161+ circulating T lymphocytes in allergic rhinitis patients compared to healthy donors. Int Arch Allergy Immunol. 158, 151–156 (2012). [DOI] [PubMed] [Google Scholar]
- Won Jung H., Jung J. K., Weon Cho C., Kang J. S. & Park Y. K. Antiallergic effect of KOB03, a polyherbal medicine, on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse and human mast cells. J Ethnopharmacol. 142, 684–693 (2012). [DOI] [PubMed] [Google Scholar]
- Lohia S., Schlosser R. J. & Soler Z. M. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta-analysis. Allergy. 68, 569–579 (2013). [DOI] [PubMed] [Google Scholar]
- Stokes J. R. et al. The effects of an H3 receptor antagonist (PF-03654746) with fexofenadine on reducing allergic rhinitis symptoms. J Allergy Clin Immunol. 129, 409–412, 412 e401-402 (2012). [DOI] [PubMed] [Google Scholar]
- Raphael G. D. et al. Efficacy, safety, and optimal dose selection of beclomethasone dipropionate nasal aerosol for seasonal allergic rhinitis in adolescents and adults. Curr Med Res Opin. 29, 1329–1340 (2013). [DOI] [PubMed] [Google Scholar]
- Hosoya K. et al. Gene silencing of STAT6 with siRNA ameliorates contact hypersensitivity and allergic rhinitis. Allergy. 66, 124–131 (2011). [DOI] [PubMed] [Google Scholar]
- Salzano G. et al. Polymeric micelles containing reversibly phospholipid-modified anti-survivin siRNA: a promising strategy to overcome drug resistance in cancer. Cancer Lett. 343, 224–231 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T. P., Mayer G. & Wolf P. In vivo siRNA targeting of CD28 reduces UV-induced DNA damage and inflammation. J Invest Dermatol. 134, 861–864 (2014). [DOI] [PubMed] [Google Scholar]
- Van De Weijer M. L. et al. A high-coverage shRNA screen identifies TMEM129 as an E3 ligase involved in ER-associated protein degradation. Nat Commun. 5, 3832 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z. et al. Adenovirus vectors lacking virus-associated RNA expression enhance shRNA activity to suppress hepatitis C virus replication. Sci Rep. 3, 3575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar N., Munoz D., Hoy J. & Lokeshwar B. L. Use of shRNA for stable suppression of chemokine receptor expression and function in human cancer cell lines. Methods Mol Biol. 1172, 209–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J. H., Jeong E. S. & Choi Y. K. Therapeutic effects of lentivirus-mediated shRNA targeting of cyclin D1 in human gastric cancer. BMC Cancer. 14, 175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier H. et al. Activation of store-operated calcium entry in airway smooth muscle cells: insight from a mathematical model. PLoS One. 8, e69598 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. T., Gilmore R. C., Watson M. J., Archer C. B. & Tarran R. 17beta-Estradiol inhibits phosphorylation of stromal interaction molecule 1 (STIM1) protein: implication for store-operated calcium entry and chronic lung diseases. J Biol Chem. 288, 33509–33518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. et al. Impaired Ca(2)(+) regulation of CD4(+)CD25(+) regulatory T cells from pediatric asthma. Int Arch Allergy Immunol. 156, 148–158 (2011). [DOI] [PubMed] [Google Scholar]
- Girodet P. O. et al. Ca(2+)-activated K(+) channel-3.1 blocker TRAM-34 attenuates airway remodeling and eosinophilia in a murine asthma model. Am J Respir Cell Mol Biol. 48, 212–219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W. et al. Interleukin-17A promotes MUC5AC expression and goblet cell hyperplasia in nasal polyps via the Act1-mediated pathway. PLoS One. 9, e98915 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaiher J. et al. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv Immunol. 122, 211–252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri-Aria K. T., Pilette C., Jacobson M. R., Watanabe H. & Durham S. R. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. J Allergy Clin Immunol. 116, 73–79 (2005). [DOI] [PubMed] [Google Scholar]
- Tsvetkova-Vicheva V. M., Gecheva S. P., Komsa-Penkova R., Velkova A. S. & Lukanov T. H. IL-17 producing T cells correlate with polysensitization but not with bronchial hyperresponsiveness in patients with allergic rhinitis. Clin Transl Allergy. 4, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Zhao X., Yan W. & Qi W. Influence of Orai1 intervention on mouse airway epithelium reactions in vivo and in vitro. Ann Allergy Asthma Immunol. 108, 103–112 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Y., Lin L. & Zheng C. Downregulation of Orai1 expression in the airway alleviates murine allergic rhinitis. Exp Mol Med. 44, 177–190 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X. et al. Positive feedback regulation of agonist-stimulated endothelial Ca2+ dynamics by KCa3.1 channels in mouse mesenteric arteries. Arterioscler Thromb Vasc Biol. 34, 127–135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Pollock C. A. & Chen X. M. Role of the potassium channel KCa3.1 in diabetic nephropathy. Clin Sci (Lond). 127, 423–433 (2014). [DOI] [PubMed] [Google Scholar]
- Yu Z. H. et al. Targeted inhibition of KCa3.1 channel attenuates airway inflammation and remodeling in allergic asthma. Am J Respir Cell Mol Biol. 48, 685–693 (2013). [DOI] [PubMed] [Google Scholar]
- Panyi G., Beeton C. & Felipe A. Ion channels and anti-cancer immunity. Philos Trans R Soc Lond B Biol Sci. 369, 20130106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong K. T. et al. Anti-allergic effect of a Korean traditional medicine, Biyeom-Tang on mast cells and allergic rhinitis. BMC Complement Altern Med. 14, 54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y. et al. Sequential engagement of FcepsilonRI on Mast Cells and Basophil Histamine H(4) Receptor and FcepsilonRI in Allergic Rhinitis. J Immunol. 190, 539–548 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y., Nakao A., Han D. & Zhang L. Transforming growth factor-beta1 promotes nasal mucosal mast cell chemotaxis in murine experimental allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 74, 117–123 (2012). [DOI] [PubMed] [Google Scholar]
- Yang B. et al. Polydatin attenuated food allergy via store-operated calcium channels in mast cell. World J Gastroenterol. 19, 3980–3989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. H. et al. Sparassis crispa suppresses mast cell-mediated allergic inflammation: Role of calcium, mitogen-activated protein kinase and nuclear factor-kappaB. Int J Mol Med. 30, 344–350 (2012). [DOI] [PubMed] [Google Scholar]
- Strider J. W., Masterson C. G. & Durham P. L. Treatment of mast cells with carbon dioxide suppresses degranulation via a novel mechanism involving repression of increased intracellular calcium levels. Allergy. 66, 341–350 (2011). [DOI] [PubMed] [Google Scholar]
- Cruse G., Duffy S. M., Brightling C. E. & Bradding P. Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax. 61, 880–885 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. W. et al. PI3K/AKT signaling is essential for communication between tissue-infiltrating mast cells, macrophages, and epithelial cells in colitis-induced cancer. Clin Cancer Res. 19, 2342–2354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Yang H. & He S. TNF increases expression of IL-4 and PARs in mast cells. Cell Physiol Biochem. 26, 327–336 (2010). [DOI] [PubMed] [Google Scholar]
- Ching T. T., Hsu A. L., Johnson A. J. & Chen C. S. Phosphoinositide 3-kinase facilitates antigen-stimulated Ca(2+) influx in RBL-2H3 mast cells via a phosphatidylinositol 3,4,5-trisphosphate-sensitive Ca(2+) entry mechanism. J Biol Chem. 276, 14814–14820 (2001). [DOI] [PubMed] [Google Scholar]
- Zhang H., Yang H., Ma W., Zhang Z. & He S. Modulation of PAR expression and tryptic enzyme induced IL-4 production in mast cells by IL-29. Cytokine. 61, 469–477 (2013). [DOI] [PubMed] [Google Scholar]
- Lam R. S. et al. Phosphatidylinositol-3-kinase regulates mast cell ion channel activity. Cell Physiol Biochem. 22, 169–176 (2008). [DOI] [PubMed] [Google Scholar]
- Yang H. et al. Cockroach allergen Per a 7 down-regulates expression of Toll-like receptor 9 and IL-12 release from P815 cells through PI3K and MAPK signaling pathways. Cell Physiol Biochem. 29, 561–570 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Induction of IL-13 production and upregulation of gene expression of protease activated receptors in P815 cells by IL-6. Cytokine. 50, 138–145 (2010). [DOI] [PubMed] [Google Scholar]
- Kremer K. L., Dunning K. R., Parsons D. W. & Anson D. S. Gene delivery to airway epithelial cells in vivo: a direct comparison of apical and basolateral transduction strategies using pseudotyped lentivirus vectors. J Gene Med. 9, 362–368 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang H., Yang H., Ma W. & He S. Induction of IL-13 production and upregulated expression of protease activated receptor-1 by RANTES in a mast cell line. Cytokine. 53, 231–238 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.