Abstract
IN BRIEF Current therapeutic approaches are only moderately efficacious at preventing the progression of diabetic kidney disease (DKD). As the number of people with DKD continues to rise worldwide, there is an urgent need for novel therapies. A better understanding of the root causes and molecular mechanisms of DKD pathogenesis has enabled the identification of numerous new therapeutic targets, including advanced glycation end products, reactive oxygen species, protein kinase C, and serum amyloid A. Although experimental studies have illustrated the potential of such approaches, challenges in clinical translation remain a barrier in therapeutic development. Advances in preclinical safety and efficacy evaluations and improved delivery systems may aid in clinical translation of novel DKD therapies.
Diabetic kidney disease (DKD) is the leading cause of chronic kidney disease (CKD) resulting in end-stage renal disease (ESRD) and premature death in the developed and developing world (1). In the United States alone, 44% of all cases of ESRD are attributed to DKD. Clinically, CKD is defined as albuminuria (ratio of albumin to creatinine >30 mg/g) and/or impaired kidney function (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) for ≥3 months. In most cases, CKD associated with diabetes is the result of DKD, but kidney disease from other causes also occurs in people with diabetes. The earliest evidence for DKD typically is increased levels of albuminuria, followed by reduction in eGFR. However, DKD is increasingly being recognized by low eGFR without albuminuria. (See related article by Narva and Bilous on p. 162 of this issue.)
DKD develops in ∼30% of people with type 1 diabetes and ∼40% of people with type 2 diabetes (1). In parallel with the rising rates of obesity and diabetes in United States, the prevalence of DKD increased 50% between 1998 and 2008 (2). Worldwide, ∼8% of the adult population has been diagnosed with diabetes. This translates to >366 million people with diabetes, with a projection of 552 million worldwide by 2030. As a result, DKD is also expected to reach pandemic levels (3).
Risks of cardiovascular disease (CVD) and all-cause mortality are strongly related to CKD in general and to DKD in particular. If eGFR is mildly or moderately decreased or albuminuria is increased, patients are 20 times more likely to experience a major CVD event or to die than they are to need kidney replacement therapy in the form of dialysis or transplantation (4). It is a sobering fact that <10% of the population with DKD progresses to ESRD because most die during the long course of this debilitating illness. The financial costs and human suffering associated with DKD have continued to increase despite widespread implementation of therapies to control hyperglycemia and hypertension by renin-angiotensin system (RAS) inhibition. Successful development and deployment of novel therapies for DKD is essential to reverse this trend. DKD presents a serious and rising global health burden, prompting an exigent need for more effective therapeutic approaches.
The State of Current Therapies: A Case for Novel Approaches
Renin-Angiotensin System
Currently, the available therapies for DKD include treatment of hypertension with RAS inhibition, glycemic control, and dietary interventions. Inhibition of the RAS has been the primary therapeutic intervention for DKD for two decades. Several clinical trials demonstrated that administration of single RAS inhibitors, angiotensin II receptor blockers (ARBs), or ACE inhibitors was mod-estly renal-protective in patients withDKD and “overt proteinuria/macro-albuminuria” (generally, urine protein-to-creatinine ratio >500 mg/g or albumin-to-creatinine ratio >300 mg/g) (5–7).
This result prompted testing of the hypothesis that further suppression of the RAS by “dual blockade” (combination therapy with two agents (e.g., ACE inhibitor, ARB, and/or a direct renin inhibitor) may be synergistic and result in greater renal protection (8–10). These clinical trials were stopped early because of safety concerns and high rates of adverse events, particularly hyperkalemia and acute kidney injury with dual therapy (8–10). There were no apparent benefits on outcomes from combination therapy. However, because the trials were stopped prematurely, the efficacy results from these trials are inconclusive. (See the related article by Patney et al. on p. 175 of this issue.) Despite evidence that single RAS inhibition with either an ARB or an ACE inhibitor can reduce the risk of DKD progression, there is increased risk of adverse events with dual RAS blockade and no obvious additional benefit of this form of combination therapy in recent clinical trials. Development of RAS-independent therapies is an important direction for future work.
Glycemic Control
Glycemic control is essential to the optimal management of diabetes and prevention of complications, including DKD in both type 1 and type 2 diabetes. Long-term follow-up of type 1 diabetes patients in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort study, showed durable benefits of intensive glycemic control to an A1C of ∼7% versus standard treatment to an A1C of ∼9% on preventing albuminuria and reduced kidney function (11). After approximately three decades of follow-up, there was also reduction of ESRD risk, although the absolute number of cases was small.
A strong positive association between A1C concentration and incident DKD was found after 11 years of follow-up in type 2 diabetes in the Atherosclerosis Risk in Communities study (12). The association was present in the absence of albuminuria and retinopathy at baseline. Similar to the DCCT/EDIC in type 1 diabetes, several studies in relatively early-onset type 2 diabetes found that controlling hyperglycemia (to an A1C of ∼7% vs. an A1C of ∼9%) prevented new-onset and progressive albuminuria (13–17). More recent studies enrolling older adults (generally >60 years of age) with longstanding type 2 diabetes, including ACCORD (Action to Control Cardiovascular Risk in Diabetes), ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation), and VADT (Veterans Affairs Diabetes Trial), which targeted even lower A1C goals (<6–6.5%), failed to demonstrate benefits of more intensified glycemic control on the primary CVD outcomes, although the risk of albuminuria development or progression was reduced (18,19). The ADVANCE trial eventually showed reduced risk for ESRD after the period of intensive glycemic control in the follow-up ADVANCE-Post Trial observational study, but like the DCCT/EDIC in type 1 diabetes, the absolute number of cases was low. Importantly, the risk of severe hypoglycemia (neurological compromise) was increased ∼2.5-fold in these trials, which greatly restricts use of intensive regimens in this population (1). Limitations of regimens for glycemic control highlight the need for alternate approaches to improve clinical outcomes in DKD.
Novel Therapies: Targeting Pathogenic Mechanisms in the Kidney
Numerous mechanisms driving the development and progression of DKD have been investigated as possible targets for novel therapies (Table 1). In humans and animal models, DKD is characterized by glomerular and tubulointerstitial disease with inflammation and fibrosis figuring prominently. Glomerulosclerosis and tub-ulointerstitial fibrosis culminate in loss of kidney function (20–23). Underlying mechanisms of DKD include hemodynamic and metabolic disturbances leading to activation of myriad mediators with autocrine and paracrine actions in the kidney. Primary among the aberrant metabolic products that drive the DKD process are advanced glycation end products (AGEs) and reactive oxygen species (ROS). These products are key activators for upregulation of proinflammatory and pro-fibrotic mediator production. Multiple cell types produce these mediators, ultimately resulting in the pathogenesis of DKD.
TABLE 1.
Summary of Novel Therapies
| AGEs Therapies | ||||
| Therapeutic | Mechanism of Action | Model | Results | Reference |
| Alagebrium | Crosslink breaker | STZ-induced diabetic mouse | Reduced renal AGE accumulation, glomerular expansion, expression of MCP-1 and ICAM-1 | Watson et al., 2012 (27) |
| Alagebrium | Crosslink breaker | db/db mouse | Reduced oxidative stress in kidneys and activity of PKCα/β | Park et al., 2011 (63) |
| Pyridoxamine | AGE inhibitor | STZ-induced diabetic rat | Attenuated increase in albuminuria and reduced levels of AGE and CML | Degenhardt et al., 2002 (30) |
| Pyridoxamine | AGE inhibitor | Type 2 diabetic KK-Ay/Ta mouse | Improved levels of ACR, reduced glomerular accumulation of CML and reduced renal expression of TGF-β1 | Tanimoto et al., 2007 (29) |
| Pyridoxamine | AGE inhibitor | Human with T1DM or T2DM | Reduced change in serum creatinine and urinary TGF-β1 and AGEs | Williams et al., 2007 (31) |
| Nrf2 Agonists | ||||
| Therapeutic | Mechanism of Action | Model | Results | Reference |
| Sulforaphane | Disruption of the Keap1-Nrf2 complex | STZ-induced diabetic mouse | Attenuated increase in ACR, reduced GBM thickening, mesangial cell proliferation, and renal tubular epithelial damage. Decreased expression of TGF-β1 and CTGF | Cui et al., 2012 (34) |
| Sulforaphane | Disruption of the Keap1-Nrf2 complex | STZ-induced diabetic mouse | Attenuated ACR, glomerulosclerosis, GBM thickening. Reduced renal oxidative stress, TGF-β1, and extracellular matrix deposition | Zheng et al., 2011 (64) |
| MG132 | Induction of Nrf2 via protease inhibition | T1DM OVE26 mouse | Attenuated renal hypertrophy, BUN, and ACR. Reduced glomerular enlargement, expansion of mesangial matrix, and epithelial damage. Decreased renal expression of TGF-β1 and CTGF | Cui et al., 2013 (65) |
| tBHQ | Disruption of the Keap1-Nrf2 complex | STZ-induced diabetic mouse | Reduced renal hypertrophy, fibronectin accumulation, and glomerular malondialdehyde | Li et al., 2011 (41) |
| dh404 | Disruption of the Keap1-Nrf2 complex | STZ-induced diabetic ApoE−/−mice | Attenuated ACR, mesangial expansion, glomerular injury, and improved renal tubular injury in diabetic mice. Reduced oxidative stress and proinflammatory mediators TNF-α, ICAM-1, VCAM-1, and MCP-1 | Tan et al., 2014 (66) |
| PKC Inhibitors | ||||
| Therapeutic | Mechanism of Action | Model | Results | Reference |
| LY333531 (Ruboxistaurin) | PKCβI/II inhibitor | Diabetic rat | Improved eGFR, albumin excretion rate, and retinal circulation in diabetic rats | Ishii et al., 1996 (45) |
| Ruboxistaurin | PKCβI/II inhibitor | (mRen-2)27 rat | Reduced albuminuria, glomerulosclerosis, tubulointerstitial pathology, and expression of TGF-β | Kelly et al., 2003 (47) |
| Ruboxistaurin | PKCβI/II inhibitor | Human with T2DM | Reduced increase in urinary TGF-β:creatinine ratio | Gilbert et al., 2007 (51) |
| Ruboxistaurin | PKCβI/II inhibitor | Human with T2DM | Decreased ACR and attenuated loss of eGFR | Tuttle et al., 2005 (50) |
| Ruboxistaurin | PKCβI/II inhibitor | Human with T2DM | Similar outcomes in patients who received placebo and patients who received ruboxistaurin | Tuttle et al., 2007 (67) |
ACR, albumin-to-creatinine ratio; AGE, advanced glycation end product; ApoE−/−; apolipoprotein E deficient; BUN, blood urea nitrogen; CML, carboxylmethyllysine; GBM, glomerular basement membrane; ICAM-1, intercellular adhesion molecule 1; Keap1, Kelch-like ECH-associated protein; MCP-1, monocyte chemoattractant protein 1; (mRen-2)27, hypertensive Ren-2 transgenic; Nrf2, nuclear factor (erythroid-derived 2)-like 2; PKCα/β, protein kinase C alpha/beta; STZ, streptozotocin; T1DM, type 1 diabetes; T2DM, type 2 diabetes; TGF-β, transforming growth factor β (if a 1 appears, the specific isoform TGF-β1 was studied); TNF-α, tumor necrosis factor alpha; VCAM-1, vascular cell adhesion protein 1.
AGEs
AGEs are modified proteins, peptides, and amino acids that are nonenzymatically glycated and oxidized after interaction of amino groups with aldose sugars. AGEs increase in hyperglycemic conditions and after consumption of foods high in protein, especially animal meats cooked at high temperatures. AGEs are increased in the kidneys of patients with DKD, and serum levels of AGEs correlate with DKD severity (24,25). AGEs are nephrotoxic by mechanisms including inflammation, fibrosis, and apoptosis of kidney cells (26). A major pathway for cellular demise in response to AGEs is via the receptor for AGEs (RAGE), which initiates signals that activate transcription for mediators of these processes.
Several therapies that inhibit the formation of AGEs, or AGE crosslink breakers, have been under investigation for DKD. The crosslink breaker alagebrium reduces inflammation, fibrosis, and overall severity of kidney damage in diabetic mice (27). Although enrollment began for a phase II randomized, placebo-controlled trial of alagebrium in patients with type 1 diabetes and DKD, it was unfortunately terminated early because of business and regulatory considerations (28).
Pyridoxamine (PDX), a derivative of vitamin B6, inhibits the formation of AGEs. In two different rodent models of DKD, administration of PDX improved albuminuria and attenuated increases in serum creatinine (29,30). In an analysis of pooled data from two phase II clinical trials of PDX for 24 weeks’ duration in people with DKD and overt proteinuria, increases in serum creatinine levels were attenuated (31). The pro-fibrotic biomarker, urinary transforming growth factor-beta (TGF-β), was also reduced. The beneficial effect of PDX was most readily observed in patients with type 2 diabetes and a serum creatinine level >1.3 mg/dL. Another phase II, randomized trial of PDX in patients with type 2 diabetic nephropathy did not show benefit on changes in serum creatinine except in patients who had reduced kidney function at the onset of the trial (32). Currently, a phase III clinical trial is being conducted to evaluate the efficacy and safety of PDX for reducing the risk of clinical events (ESRD and eGFR loss of 50%) in patients with DKD and severely increased albuminuria receiving the standard-of-care, background RAS inhibition. Reducing AGEs remains a promising area for development of DKD therapies.
ROS
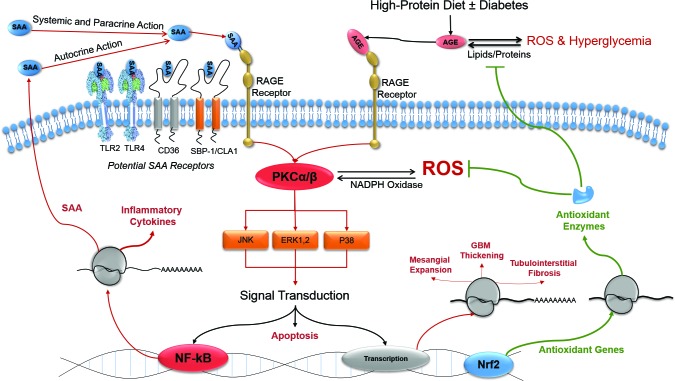
ROS are increased and contribute to induction of cell-signaling pathways that produce inflammatory and fibrotic molecules in the diabetic kidney (33). ROS promote formation of AGEs, which in turn further increase cellular ROS, eliciting an autocrine loop of AGEs and ROS production in the diabetic milieu (Figure 1). ROS also induce signaling cellular cascades, including protein kinase C (PKC) and nuclear factor κB (NF-κB), culminating in the expression of numerous proinflammatory and pro-fibrotic genes (23).
FIGURE 1.
Key mechanistic pathways in DKD. CD36, cluster of differentiation 36; CLA-1, LIMPII analogous-1; ERK, extracellular signal-regulated kinase; GBM, glomerular basement membrane thickening; JNK, c-Jun N-terminal kinase; NADPH, nicotinamide adenine dinucleotide phosphate; Nrf2, nuclear factor (erythroid-derived 2)-like 2; SAA, serum amyloid A; SBP-1, selenium-binding protein 1; TLR2, toll-like receptor 2; TLR4, toll-like receptor 4.
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a transcription factor that functions as a regulator of an endogenous antioxidant system. Compounds that activate Nrf2 have attenuated diabetes-induced kidney hypertrophy, fibrosis, and albuminuria (34).
Bardoxolone methyl (BM), an activator of Nrf2, was studied in the phase III clinical trial Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: the Occurrence of Renal Events (BEACON) (35). This trial was terminated prematurely because of numerous severe adverse events, including increased rates of CVD events, particularly heart failure, as well as higher levels of albuminuria and blood pressure. The consequences of this clinical trial have raised serious concern over the use of this strategy in DKD (36). For example, caution should be exercised when a drug increases, rather than decreases, albuminuria and blood pressure (37). The abrupt increase in eGFR observed in the phase II clinical trial of BM was likely related to glomerular hyperfiltration and raised intraglomerular pressure. Preclinical experiments with two analogs of BM (dh404 and RTA 405) may provide insight into the fate of the BEACON trial (38–40). For example, in a study of RTA 405 in diabetic rats, measures of kidney damage (proteinuria, tubular damage, and glomerulosclerosis) actually worsened. To the contrary, RTA 405 attenuated increases in blood urea nitrogen and creatinine in diabetic rats in another study (40). Overall, there is limited and conflicting evidence from experimental models supporting the use of BM for DKD.
Activation of Nrf2 in the kidneys with sulforaphane (SFN) and tert-butylhydroquinone (tBHQ) has attenuated kidney damage in mouse models of diabetes (34,41). For example, 3 months of SFN reduced albuminuria along with decreases in fibrosis, inflammation, and oxidative stress in the kidneys of diabetic mice (33). Administration of tBHQ significantly reduced kidney weight and proteinuria, as well as decreased kidney levels of fibronectin while concomitantly increasing expression of Nrf2 expression and antioxidant genes (41). Antioxidant therapies, perhaps including Nrf2 activation, may be worthy of further exploration for DKD.
PKC
PKC is activated by a number of diabetes-related stimuli, such as AGEs, hyperglycemia, angiotensin II, and ROS (Figure 1). PKC conveys signals to several downstream targets, including NF-κB, the SMAD/TGF-β axis, and apoptosis systems (42–44). Thus, PKC can be conceived as a nodal point in major signaling pathways of DKD, which makes it an attractive therapeutic target.
PKC has at least 11 isoforms. There has been substantial interest in determining which isoforms lead to DKD. Early studies implicated PKCβ as one of the primary isoforms (45). Administration of the selective PKCβ inhibitor ruboxistaurin (RBX) reduced glomerular hyperfiltration and albuminuria in diabetic rats and reduced kidney fibrosis, mesangial expansion, and glomerulosclerosis in diabetic mice (45–47). PKCα is another isoform implicated in DKD. Inhibition of PKCα abrogated albuminuria but not expression of fibrotic genes or total kidney and glomerular hypertrophy in diabetic mice (48). Recently, studies exploring reduction of both PKCα and PKCβα abolished diabetes-induced renal hypertrophy, podocyte loss, and reduced fibrosis and albuminuria in mice. In all, these data suggest that dual inhibition of PKCαα and PKCβα may be a candidate therapeutic approach (49).
A randomized, controlled phase II clinical trial examined the effects of RBX (32 mg/day) in people with type 2 diabetes and persistent albuminuria (urinary albumin-to-creatinine ratio [UACR] 200–2,000 mg/g), despite therapy with RAS inhibitors (50). UACR decreased significantly and substantially (mean reduction 24%) in those treated with RBX compared to placebo. The UACR-lowering effect of RBX appeared by 1 month. eGFR did not significantly decline in the RBX group, whereas the placebo group lost eGFR at a rate of ∼5 mL/min/1.73 m2 over a 1-year period (50). Urinary TGF-β increased by 43% over 1 year in the placebo group, but not in study participants who received RBX (51).
In a post hoc safety analysis from 11 controlled clinical trials of RBX in diabetic retinopathy, the overall rate of serious adverse events was not increased in the RBX group. Indeed, the placebo group experienced more frequent serious adverse events than the RBX-treated group (23 vs. 20%, respectively) (52). Recently, another post hoc analysis of kidney-related outcomes in phase III clinical trials of RBX for diabetic peripheral neuropathy was conducted. After 3 years, patients with RBX had lower UACRs and higher eGFRs compared to placebo, suggesting that RBX might prevent or delay DKD development (53).
It is important to recognize that RBX has not moved from phase II to phase III clinical trials because of business and regulatory decisions rather than concerns regarding safety or efficacy. Studies of PKC inhibition may yet yield a novel therapy for DKD.
Serum Amyloid A
Serum amyloid A (SAA) is an acute-phase proinflammatory protein expressed in podocytes, mesangial cells, and tubular epithelium that may contribute to inflammatory and apop-totic mechanisms in DKD (54,55). SAA initiates an inflammatory signal-ing cascade that results in upregulation of SAA itself, along with multiple inflammatory cytokines and chemoattractant molecules in podocytes (54,56). This suggests that the podocyte may promote local SAA-mediated inflammatory responses such as monocyte and macrophage recruitment in the glomeruli. SAA expression at mRNA and protein levels are increased in glomerular and tubulointerstitial compartments of diabetic mouse models and patients with DKD (56). Furthermore, SAA may also be a DKD biomarker in that blood levels associate with prevalent albuminuria in people with type 2 diabetes and predict incident albuminuria in type 1 diabetes (57–60). In studies to date, associations of SAA with DKD are independent of traditional risk factors, suggesting that it could add to DKD risk prediction in diabetic patients (56). SAA is an encouraging new candidate for therapeutic intervention and biomarker development in DKD.
Therapeutic Strategies
Combination drug therapies that work synergistically to ameliorate serial pathogenic mechanisms may ultimately prove to be more successful than single therapies. Because adverse safety signals have limited recent attempts at intensified or combinatorial drug regimens, safety and efficacy together must be carefully considered in the preclinical experimental phase and in the design and execution of clinical trials. Additionally, methods for targeting therapies to specific sites and targets of disease, such as nano-particle delivery systems and antisense oligonucleotides, may be useful tools to enhance treatment efficacy and safety (61,62).
Conclusion
DKD is a multifactorial diabetic complication with numerous mech-anistic pathways contributing to disease pathogenesis. Comprehensive mechanistic-based studies and improved strategies for clinical translation are required for the development of safe and effective new treatments. Among many possible targets, AGEs, ROS, PKC, and SAA are promising mechanisms for therapeutic and biomarker development.
Duality of Interest
Dr. Tuttle is a consultant in the area of novel therapies for DKD for Amgen, Eli Lilly and Company, and Noxxon Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
References
- 1.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]
- 2.de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health , National Institute of Diabetes and Digestive and Kidney Diseases. Atlas of End-Stage Renal Disease in the United States. Bethesda, Md, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006 [Google Scholar]
- 4.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998;32(Suppl. 3):S112–S119 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 6.Amann B, Tinzmann R, Angelkort B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care 2003;26:2421–2425 [DOI] [PubMed] [Google Scholar]
- 7.Sjolie AK, Chaturvedi N, Fuller J. [Effect of lisinopril on progression of retinopathy and microalbuminuria in normotensive subjects with insulin-dependent diabetes mellitus]. Ugeskrift for laeger 1999;161:949–952 [PubMed] [Google Scholar]
- 8.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–1903 [DOI] [PubMed] [Google Scholar]
- 9.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372:547–553 [DOI] [PubMed] [Google Scholar]
- 10.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204–2213 [DOI] [PubMed] [Google Scholar]
- 11.Writing Team for the DCCT/EDIC Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BAsh LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absense of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med 2008;168:2440–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shichiri M, Kishikawa H, Ohkubo Y, et al. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000;23(Suppl. 2):B21–B29 [PubMed] [Google Scholar]
- 14.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117 [DOI] [PubMed] [Google Scholar]
- 15.U.K. Prospective Diabetes Study Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 16.U.K. Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 17.Levin SR, Coburn JW, Abraira C, et al. ; Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type 2 Diabetes Feasibility Trial Investigators. Effect of intensive glycemic control on microalbuminuria in type 2 diabetes. Diabetes Care 2000;23:1478–1485 [DOI] [PubMed] [Google Scholar]
- 18.Patel A, Group AC, MacMahon S, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829–840 [DOI] [PubMed] [Google Scholar]
- 19.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 20.Alpers CE, Hudkins KL. Mouse models of diabetic nephropathy. Curr Opin Nephrol Hypertens 2011;20:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mezzano S, Droguett A, Burgos ME, et al. Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int 2003;64(Suppl. 86):S64–S70 [DOI] [PubMed] [Google Scholar]
- 22.Mezzano S, Aros C, Droguett A, et al. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant 2004;19:2505–2512 [DOI] [PubMed] [Google Scholar]
- 23.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106:2067–2072 [DOI] [PubMed] [Google Scholar]
- 24.Ono Y, Aoki S, Ohnishi K, et al. Increased serum levels of advanced glycation end-products and diabetic complications. Diabetes Res Clin Pract 1998;41:131–137 [DOI] [PubMed] [Google Scholar]
- 25.Tanji N, Markowitz GS, Fu C, et al. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol 2000;11:1656–1666 [DOI] [PubMed] [Google Scholar]
- 26.Li JH, Wang W, Huang XR, et al. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol 2004;164:1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson AM, Gray SP, Jiaze L, et al. Alagebrium reduces glomerular fibrogenesis and inflammation beyond preventing RAGE activation in diabetic apolipoprotein E knockout mice. Diabetes 2012;61:2105–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alicic RZ, Tuttle KR. Novel therapies for diabetic kidney disease. Adv Chronic Kidney Dis 2014;21:121–133 [DOI] [PubMed] [Google Scholar]
- 29.Tanimoto M, Gohda T, Kaneko S, et al. Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-A(y)/Ta mice. Metabolism 2007;56:160–167 [DOI] [PubMed] [Google Scholar]
- 30.Degenhardt TP, Alderson NL, Arrington DD, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int 2002;61:939–950 [DOI] [PubMed] [Google Scholar]
- 31.Williams ME, Bolton WK, Khalifah RG, et al. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am J Nephrol 2007;27:605–614 [DOI] [PubMed] [Google Scholar]
- 32.Lewis EJ, Greene T, Spitalewiz S, et al. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol 2012;23:131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006;55:225–233 [PubMed] [Google Scholar]
- 34.Cui W, Bai Y, Miao X, et al. Prevention of diabetic nephropathy by sulforaphane: possible role of Nrf2 upregulation and activation. Oxid Med Cell Longev 2012;2012:821936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013;369:2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011;365:327–336 [DOI] [PubMed] [Google Scholar]
- 37.Himmelfarb J, Tuttle KR. Bardoxolone methyl in type 2 diabetes and advanced chronic kidney disease. N Engl J Med 2014;370:1768–1769 [DOI] [PubMed] [Google Scholar]
- 38.Zoja C, Corna D, Nava V, et al. Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am J Physiol Renal Physiol 2013;304:F808–F819 [DOI] [PubMed] [Google Scholar]
- 39.Chin MP, Reisman SA, Bakris GL, et al. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am J Nephrol 2014;39:499–508 [DOI] [PubMed] [Google Scholar]
- 40.Chin M, Lee CY, Chuang JC, et al. Bardoxolone methyl analogs RTA 405 and dh404 are well tolerated and exhibit efficacy in rodent models of type 2 diabetes and obesity. Am J Physiol Renal Physiol 2013;304:F1438–F1446 [DOI] [PubMed] [Google Scholar]
- 41.Li H, Zhang L, Wang F, et al. Attenuation of glomerular injury in diabetic mice with tert-butylhydroquinone through nuclear factor erythroid 2-related factor 2-dependent antioxidant gene activation. Am J Nephrol 2011;33:289–297 [DOI] [PubMed] [Google Scholar]
- 42.Trushin SA, Pennington KN, Carmona EM, et al. Protein kinase Calpha (PKCalpha) acts upstream of PKCtheta to activate IkappaB kinase and NF-kappaB in T lymphocytes. Mol Cell Biol 2003;23:7068–7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D, Peng F, Zhang B, et al. PKC-beta1 mediates glucose-induced Akt activation and TGF-beta1 upregulation in mesangial cells. J Am Soc Nephrol 2009;20:554–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tossidou I, Starker G, Kruger J, et al. PKC-alpha modulates TGF-beta signaling and impairs podocyte survival. Cell Physiol Biochem 2009;24:627–634 [DOI] [PubMed] [Google Scholar]
- 45.Ishii H, Jirousek MR, Koya D, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science 1996;272:728–731 [DOI] [PubMed] [Google Scholar]
- 46.Koya D, Jirousek MR, Lin YW, et al. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest 1997;100:115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly DJ, Zhang Y, Hepper C, et al. Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 2003;52:512–518 [DOI] [PubMed] [Google Scholar]
- 48.Menne J, Park JK, Boehne M, et al. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-alpha-deficient diabetic mice. Diabetes 2004;53:2101–2109 [DOI] [PubMed] [Google Scholar]
- 49.Menne J, Shushakova N, Bartels J, et al. Dual inhibition of classical protein kinase C-alpha and protein kinase C-beta isoforms protects against experimental murine diabetic nephropathy. Diabetes 2013;62:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuttle KR, Bakris GL, Toto RD, et al. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 2005;28:2686–2690 [DOI] [PubMed] [Google Scholar]
- 51.Gilbert RE, Kim SA, Tuttle KR, et al. Effect of ruboxistaurin on urinary transforming growth factor-beta in patients with diabetic nephropathy and type 2 diabetes. Diabetes Care 2007;30:995–996 [DOI] [PubMed] [Google Scholar]
- 52.McGill JB, King GL, Berg PH, et al. Clinical safety of the selective PKC-beta inhibitor, ruboxistaurin. Expert Opin Drug Saf 2006;5:835–845 [DOI] [PubMed] [Google Scholar]
- 53.Tuttle KR, McGill JB, Bastyr EJ 3rd, et al. Effect of ruboxistaurin on albuminuria and estimated GFR in people with diabetic peripheral neuropathy: results from a randomized trial. Am J Kidney Dis 2015;65:534–636 [DOI] [PubMed] [Google Scholar]
- 54.Meek RL, LeBoeuf RC, Saha SA, et al. Glomerular cell death and inflammation with high-protein diet and diabetes. Nephrol Dial Transplant 2013;28:1711–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meek RL, Benditt EP. Amyloid A gene family expression in different mouse tissues. J Exp Med 1986;164:2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderberg RJ, Meek RL, Hiudkins KL, et al. Serum amyloid A-induced inflammatory responses in podocytes and diabetic kidney disease. Lab Invest 2015;95:250–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller S, Martin S, Koenig W, et al. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia 2002;45:805–812 [DOI] [PubMed] [Google Scholar]
- 58.Scheja L, Heese B, Zitzer H, et al. Acute-phase serum amyloid A as a marker of insulin resistance in mice. Exp Diabetes Res 2008;2008:230837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poitou C, Viguerie N, Cancello R, et al. Serum amyloid A: production by human white adipocyte and regulation by obesity and nutrition. Diabetologia 2005;48:519–528 [DOI] [PubMed] [Google Scholar]
- 60.Overgaard AJ, McGuire JN, Hovind P, et al. Serum amyloid A and C-reactive protein levels may predict microalbuminuria and macroalbuminuria in newly diagnosed type 1 diabetic patients. J Diabetes Complications 2013;27:59–63 [DOI] [PubMed] [Google Scholar]
- 61.Dolman ME, Harmsen S, Pieters EH, et al. Targeting of a platinum-bound sunitinib analog to renal proximal tubular cells. Int J Nanomedicine 2012;7:417–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hauser PV, Pippin JW, Kaiser C, et al. Novel siRNA delivery system to target podocytes in vivo. PLoS One 2010;5:e9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J, Kwon MK, Huh JY, et al. Renoprotective antioxidant effect of alagebrium in experimental diabetes. Nephrol Dial Transplant 2011;26:3474–3484 [DOI] [PubMed] [Google Scholar]
- 64.Zheng H, Whitman SA, Wu W, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 2011;60:3055–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui W, Li B, Bai Y, et al. Potential role for Nrf2 activation in the therapeutic effect of MG132 on diabetic nephropathy in OVE26 diabetic mice. Am J Physiol Endocrinol Metab 2013;304:E87–E99 [DOI] [PubMed] [Google Scholar]
- 66.Tan SM, Sharma A, Stefanovic N, et al. Derivative of bardoxolone methyl, dh404, in an inverse dose-dependent manner lessens diabetes-associated atherosclerosis and improves diabetic kidney disease. Diabetes 2014;63:3091–3103 [DOI] [PubMed] [Google Scholar]
- 67.Tuttle KR, McGill JB, Haney DJ, et al. Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol 2007;2:631–636 [DOI] [PubMed] [Google Scholar]