Abstract
Diet and exercise could be an essential part of the treatment of non-insulin dependent diabetes mellitus (NIDDM). The effects of a strict dietary restriction (800–1,200 Kcal/day) with exercise (2-hour walk per day) on glycemic control were evaluatedin NIDDM patients. A short-term study was performed on 147 cases In these cases, the duration of hospitalization was 7–14 days. We achieved glycemic control [fasting blood sugar (FBS) less than 140 mg/dl] in 112 cases (76%). Among them, 78 (53%) were controlled with diet and exercise only and in 34 (23%), control was induced with oral gliquidone treatment for about 5 days A long-term study was done on 76 cases who followed our program for from 1 to 12 months (mean : 3.8 months) after discharge. Glycemic control was maintained in 56 (74%) in spite of the insignificance of the amount of weight reduction. Glycemic control was significantly related to the duration of diabetes, diabetic complications and the peak C-peptide level, but was unrelated to the initial body weight, FBS levels and HbA1c levels. These data indicate that a program of diet and exercise must be an integral part in the treatment of NIDDM. This was true, especially, for patients who have had adequate insulin secretory capacity few diabetic complications, short duration of disease, and no previous history of oral hypoglycemic agents or insulin therapy.
Keywords: NIDDM, Diet, Exercise
INTRODUCTION
The majority of patients with diabetes mellitus (DM) are classified as non-insulin dependent diabetes mellitus (NIDDM). This group retains significant endogenous insulin secretory capacity. Although oral hypoglycemic agents or insulin may be necessary for the glycemic control of NIDDM in some patients, diet and exercise could be an essential part of treatment.1–5) However, there have been few reports from Korea on the effects of diet and exercise as therapy for the control of DM.
In this paper, the effect of strict diet and exercise therapy on glycemic control studied in 147 NIDDM patients is reported. An effort was made to find other possible factors related to glycemic control.
SUBJECTS AND METHODS
1. Subjects
The subjects were 147 NIDDM patients, which included 71 males and 76 females, 98 non-obese NIDDM and 49 obese NIDDM. They had been admitted to Severance Hospital in the Yonsei University Medical Center during the period ranging from February 1984 to April 1985. All subjects were classified according to the National Diabetes Data Group protocol.6) Ages ranged from 26 to 67 years (mean : 54.5 years). Subjects with concomitant infection, diabetic nephropathy (urine protein more than 550mg/24 hours),7) diabetic gangrene, coronary heart disease or cerebrovascular disease were excluded.
2. Methods
All of the subjects were placed on a restricted diet with vitamin supplements and were advised to exercise. According to their body weight, the caloric intake, adjusted to 800–1,200Kcal/day, was 50% carbohydrate (CHO), 30% tat and 20% protein. The exercise schedule consisted of walking for more than 2 hours a day. Fasting blood sugar (FBS) and body weight were monitored every morning. FBS under 140mg% was decided upon as signtying that the disease was being under control. The subjects whose FBS indicated control were defined as a success group, and the others which were brought under control with oral hypoglycemic agents (gliquidone) became members of the group after 5 days of treatment. The subjects who were given hypoglycemic agents were subdivided into an induction group and a failure group, according to their FBS levels. The subjects of the success group and the induction group were together considered to be the short-term success group. They were encouraged to exercise and stay on a strict diet after discharge. Among the subjects followed for more than 1 month (range: 1–12 months, mean 3.8 months), the long-term success group was separated from the long-term failure group by their FBS levels (Fig. 1).
Fig. 1.
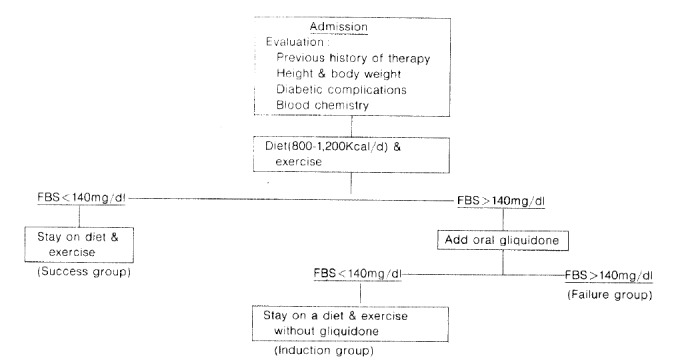
Therapeutic approach of NIDDM patients.
The body weight percentages were calculated as follows :
Statistical analysis were done, using the Student’s t-test and the chi-square. Significance was set at the .05 level. Values are expressed as mean ± S.D. in the tables.
RESULTS
The short-term success group totaled 112 cases (76%). This included 78 cases (53%) who belonged to the success group and 34 cases (23%) who belonged to the induction group (Table 2). The long-term success rate in obese NIDDM patients was slightly greater than that of non-obese NIDDM patients, but not significantly so (Table 2, 3). Our program did not result in significant weight reduction in any groups (Table 4).
Table 2.
Glycemic Control during Hospitalization
| Type | Short-term success group
|
Failure group | Total | |
|---|---|---|---|---|
| Success group | Induction group | |||
| Non-obese | ||||
| NIDDM | 56 | 16 | 26 | 98 |
| Obese | ||||
| NIDDM | 22 | 18 | 9 | 49 |
|
| ||||
| Total(%) | 78(53) | 34(23) | 35(24) | 147 |
Table 3.
Glycemic Control in Long-term Follow-up Study
| Type | Success group | Failure group | Total |
|---|---|---|---|
| Non-obese | |||
| NIDDM | 31(66)* | 16(34) | 47(100) |
| Obese | |||
| NIDDM | 25(86) | 4(14) | 29(100) |
|
| |||
| Total | 56(74) | 29(26) | 76(100) |
Means percent of total patients
Table 4.
Body Weight Changes
| Type | Body weight (% of ideal weight)
|
Weight loss | ||
|---|---|---|---|---|
| Initial | Discharged | Long-term | ||
| Non-obese NIDDM | ||||
| Success group | ||||
| Short-term | 150.4 ± 8.1* | 103.1 ± 7.9 | 2.2 ± 1.8$ | |
| Long-term | 106.0 ± 7.4** | 105.0 ± 7.6 | 103.0 ± 8.5 | 3.0 ± 2.7$$ |
| Failure group | ||||
| Short-term | 103.3 ± 7.6* | 102.0 ± 7.4 | 1.4 ± 1.2$ | |
| Long-term | 104.6 ± 8.7** | 103.2 ± 7.9 | 103.1 ± 9.1 | 1.5 ± 1.3$$ |
| Obese NIDDM | ||||
| Success group | ||||
| Short-term | 128.9 ± 9.4# | 124.0 ± 9.4 | 4.5 ± 2.8& | |
| Long-term | 130.3 ± 10.5## | 124.9 ± 10.2 | 120.3 ± 11.9 | 10.4 ± 4.6&& |
| Failure group | ||||
| Short-term | 131.1 ± 7.4# | 125.0 ± 9.2 | 5.9 ± 2.1& | |
| Long-term | 127.0 ± 82## | 128.3 ± 12.3 | 120.0 ± 6.2 | 6.8 ± 0.6&& |
p> .05
p> .05
p> .05
p> .05
In both types of NIDDM, the duration of DM in the short-term and long-term success groups was significantly shorter than that in the short-term and long-term failure groups (Table 5). Previous history of having taken oral hypoglycemic agents and complications in the short-term and the long-term success groups was significantly more numerous than those in the short-term and the long-term failure groups (Table 6, 7).
Table 5.
Duration of Diabetes
| Type | Duration (yrs)
|
|
|---|---|---|
| Success group | Failure group | |
| Non-obese NIDDM | ||
| Short-term | 4.1 ± 3.2* | 7.6 ± 6.3* |
| Long-term | 2.3 ± 1.7** | 7.9 ± 7.1** |
| Obese NIDDM | ||
| Short-term | 4.4 ± 2.7# | 8.5 ± 7.6# |
| Long-term | 2.6 ± 2 1## | 6.7 ± 1.2## |
p < .05
p < .05
p < .05
p < .05
Table 6.
Previous History of the Treatment for Diabetes
| Type | Oral agents and or insulin(%)
|
|
|---|---|---|
| Success group | Failure group | |
| Non-obese NIDDM | ||
| Short-term | 31* | 89* |
| Long-term | 23** | 75** |
| Obese NIDDM | ||
| Short-term | 35# | 67# |
| Long-term | 32## | 100## |
p < .05
p < .05
p < .05
p < .05
Table 7.
The Prevalance of Chronic Diabetic Complications
| Type | Neuropathy and or retinopathy(%)
|
|
|---|---|---|
| Success group | Failure group | |
| Non-obese NIDDM | ||
| Short-term | 40* | 73* |
| Long-term | 29** | 63** |
| Obese NIDDM | ||
| Short-term | 30# | 78# |
| Long-term | 32## | 75## |
p < .05
p < .05
p < .05
p < .05
There were no significant differences in initial FBS and HbA1c levels among the different groups (Table 8, 9). There was no significant difference between the basal or the peak C-peptide levels of the short-term success and those of the short-term failure groups of both types of NIDDM (Table 10). However, the peak C-peptide levels of the long-term success groups were significantly higher than those of the long-term failure groups (p<.05) (Table 11).
Table 8.
Initial Fasting Blood Glucose Levels
| Type | Fasting blood glucose level (mg/dl)
|
|
|---|---|---|
| Success group | Failure group | |
| Non-obese NIDDM | ||
| Short-term | 198.7 ± 52.7* | 226.5 ± 70.6* |
| Long-term | 208.1 ± 50.4** | 198.7 ± 51.9” |
| Obese NIDDM | ||
| Short-term | 226.5 ± 54.5# | 213.5 ± 70.6# |
| Long-term | 206.9 ± 63.2## | 219.0 ± 73.6## |
p > .05
p > .05
p > .05
p > .05
Table 9.
Initial HbA1c Levels
| Type | HbA1c level (%)
|
|
|---|---|---|
| Success group | Failure group | |
| Non-obese NIDDM | ||
| Short-term | 8.5 ± 1.5* | 9.3 ± 1.5* |
| Long-term | 9.1 ± 1.8** | 8.6 ± 1.4** |
| Obese NIDDM | ||
| Short-term | 8.5 ± 1.2# | 8.7 ± 1.4# |
| Long-term | 8.2 ± 1.2## | 8.5 ± 1.1## |
p > .05
p > .05
p > .05
p > .05
Table 10.
Basal and Peak Serum C-peptide Levels in Short-term Success and Failure Groups
| Type | C-peptide level (ng/ml)
|
|
|---|---|---|
| Basal | Peak | |
| Non-obese NIDDM | ||
| Success group | 2.23 ± 0.86* | 5.25 ± 2.54# |
| Failure group | 2 01 ± 0.71* | 3.44 ± 0.81# |
| Obese NIDDM | ||
| Success group | 2.36 ± 0.97** | 5.38 ± 1.75## |
| Failure group | 2.19 ± 1.24** | 3.89 ± 1.61## |
p > .05
p > .05
p > .05
p > .05
Table 11.
Basal and Peak Serum C-peptide Levels in Long-term Success and Failure Groups
| Type | C-peptide level (ng/ml)
|
|
|---|---|---|
| Basal | Peak | |
| Non-obese NIDDM | ||
| Success group | 2.26 ± 0.94* | 5.93 ± 2.94# |
| Failure group | 1.78 ± 0.45* | 3.19 ± 0.89# |
| Obese NIDDM | ||
| Success group | 2.37 ± 0.75** | 5.95 ± 2.01## |
| Failure group | 1.88 ± 0.40** | 3.65 ± 0.41## |
p > .05
p > .05
p < .05
p < .05
DISCUSSION
Insulin resistance and, to a lesser extent, the reduced secretion of insulin are known to be the central pathogenic mechanisms of NIDDM.8–13) These patients seldom develop ketosis, even in the absence of insulin therapy and are not dependent on exogenous insulin for immediate survival. In view of this, the addition of a program of regular exercise to dietary restriction and weight reduction could be an integral part of the treatment of NIDDM.14)
A strict caloric restriction could result in weight reduction, improved insulin secretion, improved insulin binding to receptor, decreased insulin resistance, and/or improved glucagon secretion. The dietary regimens employed consisted of 800–2,300Kcal/day for from 4 weeks to 6 months.15–18) In addition to the attention given total caloric intake, a great deal of attention was given to the individual components comprising the diabetic diet. Previous attempts to restrict total CHO intake are no longer deemed advisable. Most authorities now advocate liberalization of CHO intake from 50 to 55% of the total calories.19) Also, dietary fiber can reduce glycemic excursions and diminish insulin secretion. This is thought to be due to delayed gastric emptying and overall slowing of the rate of CHO digestion and absorption. In our studies, all subjects were given dietary regimens which included 50% CHO.
Exercise is commonly recommended in the treatment of NIDDM as a means of increasing energy expenditure and insulin sensitivity. Body fat loss, a sense of well-being, increases in aerobic capacity, increases in HDL-cholesterol level and adipocyte lipolysis, and decreases in blood pressure in hypertensive patients, psychologic stress and plasma triglyceride are also benefits which can be reaped from exercise.20) The general guidelines for aerobic conditioning are to exercise 3–5 days per week for 15–60 min per exercise session at a work intensity corresponding to 50–85% maximal oxygen consumption, or 60–90% of maximal heart rate reserve.21,22) The specific kind, amount and duration of exercise should be selected according to physical condition of the individual.23) Patients with advanced complications were excluded from our study because of the possibility that microangiopathic complications might be aggravated.24) The amount of exercise in our regimen was greater than those in others.25,26) Glycemic control was achieved in about 70% of patients. The glycemic control by exercise might be the result of the increase of glycogen repletion in the muscles. The improvement of the peripheral sensitivity to insulin might be due to increased non-oxidative glucose disposal in muscle tissue.14,26–29) The results of glycemic control in non-obese NIDDM on long-term follow-up study can partially be explained by the lesser energy expenditure during exercise than in obese NIDDM.
In this study, the induction group achieved glycemic control with oral gliquidone for about 5 days (induction therapy). Several studies have revealed that a short-term program of insulin therapy improved blood sugar level or glucose homeostasis, insulin secretion, post-receptor defect and basal hepatic glucose production.30–33) These results indicate that the restoration to normal of or improvement of hyperglycemia itself may induce the improvement in the post-receptor defect.
Several factors might be related to the glycemic control. In our study, the glycemic control was significantly related to the previous history of oral hypoglycemic agents, the duration of DM or the extent of diabetic complications, but not to the initial body weight, or FBS or HbA1c levels. Turkington et al.34) reported that patients previously treated with oral hypoglycemic agents or insulin responded poorly to the dietary therapy, as our patients did. Nagulesparan et al.35) and Garvey et al.33) reported that the longer the duration of DM, which is commonly associated with advanced diabetic complications, the poorer the response to therapy. In our study, the patient with a shorter duration of disease (5 years or less) and lesser diabetic complications were better controlled than those with a longer duration of disease and more complications.
It was suggested that patients with a higher capacity of insulin secretion responded better to diet and exercise therapy.34,36,37) In our study, the peak C-peptide levels were significantly higher in the long-term success group than in the long-term failure group.
In this study, weight loss in the success groups was slightly, but not significantly, greater than that in the failure groups. The addition of a program of strict weight reduction by dietary restriction and exercise may be needed to establish the proper guidelines for diabetic control. Such a program will bring an added benefit from the educational standpoint, in the form of better compliance and better glycemic control.
Table 1.
Age and Sex Distribution
| Age(yrs) | Non-obese NIDDM
|
Obese NIDDM
|
Total | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| 20–29 | 0 | 2 | 0 | 0 | 2 |
| 30–39 | 4 | 2 | 1 | 0 | 7 |
| 40–49 | 15 | 10 | 2 | 7 | 34 |
| 50–59 | 25 | 17 | 6 | 15 | 63 |
| 60–69 | 15 | 8 | 3 | 15 | 41 |
|
| |||||
| Total | 59 | 39 | 12 | 37 | 147 |
REFERENCES
- 1.Huh KB. Exercise therapy in diabetes mellitus. J Korean Diabetes Association. 1985;9:5. [Google Scholar]
- 2.Groop L, Haino K. The combination of insulin and sulfonylureas. Acta Endocrinol. 1979;91:33. [Google Scholar]
- 3.Lipson LG, Lipson M. The therapeutic approach to the obese maturity onset diabetic patients. Arch Intern Med. 1984;144:135. [PubMed] [Google Scholar]
- 4.Skyler JS. Non-insulin dependent diabetes mellitus a clinical strategy. Diabetes Care. 1984;7(Suppl 1):118. [PubMed] [Google Scholar]
- 5.Sonksen PH, Lowy C, Perkins JR, Urns HS. Non-insulin dependent diabetes: 10 year outcome in relation to initial response to diet and subsequent sulfonylurea therapy. Diabetes Care. 1984;7(Suppl 1):59. [PubMed] [Google Scholar]
- 6.National Diabetes Data Group. Classification and diagnosis of diabetes and other categories of glucose intolerance. Diabetes. 1979;28:1039. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 7.Viberti G, Keen H. The pattern of proteinuria in diabetes mellitus. Diabetes. 1984;33:686. doi: 10.2337/diab.33.7.686. [DOI] [PubMed] [Google Scholar]
- 8.Kolterman OG, Gray RS, Griffin J, Burstein P, Insel J, Scarlett JA, Olefsky JM. Receptor and postreceptor defects contribute to the insulin resistance in non-insulin dependent diabetes mellitus. J Clin Invest. 1981;68:957. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolterman OG, Insel J, Saekow M, Olefsky JM. Mechanism of insulin resistance in human obesity. J Clin Invest. 1980;65:1272. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeifer MA, Haler JB, Porte D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981;70:579. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- 11.Rizza RA, Mandarino LJ, Gerich JE. Mechanism and significance of insulin resistance in non-insulin dependent diabetes mellitus. Diabetes. 1981;30:990. doi: 10.2337/diab.30.12.990. [DOI] [PubMed] [Google Scholar]
- 12.Ward WK, Beard JC, Halter JB, Pfeifer MA, Porte D., Jr Pathophysiology of insulin secretion in non-insulin dependent diabetes mellitus. Diabetes Care. 1984;7:491. doi: 10.2337/diacare.7.5.491. [DOI] [PubMed] [Google Scholar]
- 13.Reaven GM. Insulin secretion and insulin action in non-insulin dependent diabetes mellitus. Which defect is primary? Diabetes Care. 1984;7:17. [PubMed] [Google Scholar]
- 14.Horton ES. Metabolic aspects of exercise and weight reduction. Med Sci Sports Exerc. 1986;18:10. [PubMed] [Google Scholar]
- 15.Hadden DR, Montgomery DAD, Skelly RJ, Trimble ER, Wewver JA, Wilso EA, Buchanan KD. Maturity onset diabetes mellitus: Response to intensive-dietary management. Br Med J. 1975;3:276. doi: 10.1136/bmj.3.5978.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanik S, Marcus R. Insulin secretion improves following dietary control of plasma glucose in severely hyperglycemic obese patients. Metabolism. 1980;29:4. doi: 10.1016/0026-0495(80)90008-6. [DOI] [PubMed] [Google Scholar]
- 17.Salvage PJ, Bemion LJ, Flock EV, Nagulesparan M, Mott D, Roth J, Unger RH, Bennett PH. Diet induced improvement of abnormalities in insulin and glucagon secretion and in insulin recreptor binding in diabetes mellitus. J Clin Endocrinol Metab. 1979;48:830. doi: 10.1210/jcem-48-6-999. [DOI] [PubMed] [Google Scholar]
- 18.Hughes TA, Gwyne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release, and resistance and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med. 1984;77:7. doi: 10.1016/0002-9343(84)90429-7. [DOI] [PubMed] [Google Scholar]
- 19.Arkz RA. Nutritional management of diabetic diet in diabetes mellitus: Therapy and practice. 3rd ed. New York: Medical Publishing Co; 1983. p. 539. [Google Scholar]
- 20.Sheldahl LM. Special ergometric techniques and weight reduction. Med Sci Sports Exerc. 1986;18:25. [PubMed] [Google Scholar]
- 21.American College of Sports Medicine. Position statement on the recommended quantity of exercise for developing and maintaining fitness in healthy adults. Med Sci Sports Exerc. 1978;10:vii–x. [PubMed] [Google Scholar]
- 22.Pollock ML. How much exercise is enough? Phys Sport Med. 1978;6:50. [Google Scholar]
- 23.Vranic M, Berger M. Exercise and diabetes mellitus. Diabetes. 1979;28(Suppl 1):147. doi: 10.2337/diab.28.2.147. [DOI] [PubMed] [Google Scholar]
- 24.McMillian DE. Exercise and diabetic microangiopathy. Dibetes. 1979;28(Suppl 1):103. doi: 10.2337/diab.28.1.s103. [DOI] [PubMed] [Google Scholar]
- 25.Bogardus H, Ravussin E, Robbins DC, Wolfe RR, Horton ES, Sims EAH. Effects of physical training and diet therapy on carbohydrate metabolism in patients with glucose intolerance and non-insulin dependent diabetes mellitus. Diabetes. 1984;33:311. doi: 10.2337/diab.33.4.311. [DOI] [PubMed] [Google Scholar]
- 26.Reitmann JS, Vasquez B, Klimes I, Nagulesparan M. Improvement of glucose homeostasis after exercise training in non-insulin dependent diabetes. Diabetes Care. 1984;7:434. doi: 10.2337/diacare.7.5.434. [DOI] [PubMed] [Google Scholar]
- 27.Calles J, Cunningham JJ, Nelson L, Brown N, Nadel E, Sherwin RS, Felig P. Glucose turnover during recovery from intensive exercise. Diabetes. 1983;32:734. doi: 10.2337/diab.32.8.734. [DOI] [PubMed] [Google Scholar]
- 28.Piehl K, Acolffson S, Nazor K. Glycogen storage and glucagon synthetase activity in trained and untrained muscle of man. Acta Physiol Scand. 1974;90:779. doi: 10.1111/j.1748-1716.1974.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 29.Zinmann B, Vranic M. Exercise and diabetes mellitus. Diabetes. 1979;28(Suppl 1):107. doi: 10.2337/diab.28.1.s107. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg H, Rayfield EJ. Effect of insulin therapy on insulin resistance in type II diabetic subjects. Diabetes. 1981;30:739. doi: 10.2337/diab.30.9.739. [DOI] [PubMed] [Google Scholar]
- 31.Hidaka H, Nagulesparan M, Klimes K, Clark R, Sasak H, Aronoff SL, Vasquez B, Rubenstein AH, Unger RH. Improvement of insulin secretion but not insulin resistance. J Clin Endocrinol Metab. 1982;54:217. doi: 10.1210/jcem-54-2-217. [DOI] [PubMed] [Google Scholar]
- 32.Andrew WJ, Vasquez B, Nagulesparan M, Klimes I, Foley J, Unger R, Reaven GM. Insulin therapy in obese non-insulin dependent diabetes mellitus induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984;33:634. doi: 10.2337/diab.33.7.634. [DOI] [PubMed] [Google Scholar]
- 33.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- 34.Turkington RW, Estokowski A, Link M. Secretion of insulin or C-peptide A predictor of insulin dependence of obese diabetes. Arch Intern Med. 1982;142:1102. [PubMed] [Google Scholar]
- 35.Nagulesparan M, Salvage PJ, Bennion LJ, Unger RH, Bennett PH. Diminished effect of caloric restriction on control of hyperglycemia with increasing known duration of type II diabetes mellitus. J Clin Endocrinol Metab. 1981;53:560. doi: 10.1210/jcem-53-3-560. [DOI] [PubMed] [Google Scholar]
- 36.Rendell M. C-peptide level as a criterion in treatment of maturity onset diabetes. J Clin Endocrinol Metab. 1983;57:1198. doi: 10.1210/jcem-57-6-1198. [DOI] [PubMed] [Google Scholar]
- 37.Lim SK, Park HS, Kim KR, Lee HC, Hong CS, Huh KB. A study for classification of primary diabetes mellitus. J Korean Diabetes Association. 1985;9:41. [Google Scholar]


