Abstract
Background
Acanthus is a unique genus consisting of both true mangrove and terrestrial species; thus, it represents an ideal system for studying the origin and adaptive evolution of mangrove plants to intertidal environments. However, little is known regarding the two respects of mangrove species in Acanthus. In this study, we sequenced the transcriptomes of the pooled roots and leaves tissues for a mangrove species, Acanthus ilicifolius, and its terrestrial congener, A. leucostachyus, to illustrate the origin of the mangrove species in this genus and their adaptive evolution to harsh habitats.
Results
We obtained 73,039 and 69,580 contigs with N50 values of 741 and 1557 bp for A. ilicifolius and A. leucostachyus, respectively. Phylogenetic analyses based on four nuclear segments and three chloroplast fragments revealed that mangroves and terrestrial species in Acanthus fell into different clades, indicating a single origin of the mangrove species in Acanthus. Based on 6634 orthologs, A. ilicifolius and A. leucostachyus were found to be highly divergent, with a peak of synonymous substitution rate (Ks) distribution of 0.145 and an estimated divergence time of approximately 16.8 million years ago (MYA). The transgression in the Early to Middle Miocene may be the major reason for the entry of the mangrove lineage of Acanthus into intertidal environments. Gene ontology (GO) classifications of the full transcriptomes did not show any apparent differences between A. ilicifolius and A. leucostachyus, suggesting the absence of gene components specific to the mangrove transcriptomes. A total of 99 genes in A. ilicifolius were identified with signals of positive selection. Twenty-three of the 99 positively selected genes (PSGs) were found to be involved in salt, heat and ultraviolet stress tolerance, seed germination and embryo development under periodic inundation. These stress-tolerance related PSGs may be crucial for the adaptation of the mangrove species in this genus to stressful marine environments and may contribute to speciation in Acanthus.
Conclusions
We characterized the transcriptomes of one mangrove species of Acanthus, A. ilicifolius, and its terrestrial relative, A. leucostachyus, and provided insights into the origin of the mangrove Acanthus species and their adaptive evolution to abiotic stresses in intertidal environments.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-015-1813-9) contains supplementary material, which is available to authorized users.
Keywords: Abiotic stress, Adaptation, Mangroves, Comparative transcriptome
Background
Tropical intertidal zones are extreme environments characterized by high salinity, drought, hypoxia and high ultraviolet (UV) radiation, which severely limit plant growth, development and reproduction [1]. As the dominant forest community and ecosystem in the coasts, mangrove plants struggle and survive in these environments with remarkable morphological and physiological characteristic, for example, exposed breathing roots, support roots and buttresses, salt-excreting leaves, and viviparous water-dispersed propagules [1, 2]. Understanding the genetic basis underlying those adaptive traits at the genomic level could provide important clues to the molecular mechanisms of stress resistance in marine halophytes. Mangroves are constituent plants of approximately 70 species from 28 genera belonging to 20 families [3]. Studies based on fossils and phylogenetic analysis have suggested that these biogeographically and taxonomically diverse genera are of independent origins in different geologic epochs [4, 5]. However, the divergent time and the species radiation within some genera are still unclear and are of great interest to many botanists.
Comparative analysis among mangrove species and model terrestrial plants using RNA-sequencing has revealed that specific sequence divergence and transcriptional regulation play major roles in response to salt stress in many mangrove species, such as Aegiceras corniculatum [6], Bruguiera gymnorhiza [7, 8] and Sonneratia alba [9]. However, the model plants used in these studies, such as Arabidopsis thaliana and Populus tomentosa, are distantly related to mangroves, which makes it difficult to distinguish adaptive processes from those caused by phylogenetic effects, thus reducing the resolution of selection signals [10].
Among all true mangrove genera, Acanthus is the only genus that includes both mangrove and terrestrial species; therefore, it is an ideal system to investigate the adaptive evolution of mangrove plants to stressful intertidal environments while minimizing phylogenetic influences. This genus consists of three representative mangrove species and approximately 27 terrestrial species [1]. The three mangrove species, which are also called Holly mangroves, are found in the intertidal zones from India to the West Pacific and tropical Australia. Unlike other terrestrial species of Acanthus, which are distributed in the Mediterranean Basin, A. leucostachyus is restrict to South to Southeast Asia and grows under rain forest canopies at the edges of streams 600–1200 m above the sea level [11]. Similarities in geographic distribution and ecological requirements of A. leucostachyus and the congeneric mangrove species suggested A. leucostachyus is a suitable outgroup for illustrating the evolutionary history of the mangrove species in Acanthus.
Nguyen et al. [12, 13] identified 170 genes that are involved in response to salt stress from 628 expressed sequence tags (ESTs) of A. ebracteatus. However, these studies only listed the annotations of these stress-response genes. The role of natural selection in adaptive evolution of mangrove species has not been characterized. In the current study, we performed RNA-sequencing to assess the mangrove species A. ilicifolius and its terrestrial congener A. leucostachyus, to provide insights into the evolutionary process underlying the adaptation of mangroves to intertidal zones. We asked the following specific questions: 1) what is the origination pattern of the mangrove Acanthus taxa and when mangrove species diverged from their terrestrial relatives within this genus? 2) Are there any marked differences in Gene Ontology (GO) classification among the mangrove transcriptome profiles? Finally, 3) which genes have experienced adaptive evolution and contributed to the adaptation of A. ilicifolius to stressful intertidal habitats?
Results
Transcriptome sequencing and de novo assembly
A total of 44.04 million 90-bp and 46.89 million 100-bp paired-end reads were sequenced for A. ilicifolius and A. leucostachyus, respectively (Table 1). The raw data were deposited in the NCBI Sequence Read Archive (SRA) with the accession number SRP053334. To minimize sequencing errors, we individually trimmed each read to its longest contiguous segment, using quality scores of higher than 20 for all remaining segments. After the trimming processes, reads with length of less than 50 bp were removed from each dataset. A total of 34.13 and 38.53 million high-quality reads for A. ilicifolius and A. leucostachyus, respectively, were used for a further de novo assembly. This assembly was performed with Trinity software [14], and generated 97,347 and 90,998 contigs for the two species, respectively. Of them, 24,487 and 19,212 similar contigs were clustered into 4869 and 2633 clusters by TGICL [15] using a default threshold of 0.94 and CD-HIT [16] using a global identity threshold of 0.90. A total of 77,729 and 74,419 contigs were remained in the two datasets, respectively.
Table 1.
Sequencing and assembly statistics for the transcriptome data of Acanthus ilicifolius and A. leucostachyus. No. is short for number
| Assembly results | After removing redundancy and contigs with low coverage or depth | |||
|---|---|---|---|---|
| A. ilicifolius | A. leucostachyus | A. ilicifolius | A. leucostachyus | |
| No. of contigs | 97347 | 90998 | 73039 | 69580 |
| Maximum length of contigs (bp) | 5243 | 11541 | 5243 | 11541 |
| Average length of contigs (bp) | 601 | 980 | 580 | 913 |
| Contig N50 (bp) | 816 | 1699 | 763 | 1580 |
| No. of contigs with length greater than 1 kb | 15755 | 31572 | 10655 | 21776 |
To enhance the reliabilities of the assembly results, we calculated the coverage and the average depth for each contig and removed 4690 and 4839 contigs with less than 75 % sites with at least 2× depth from the two data sets, respectively. At last, 73,039 and 69,580 unigenes with average depths of 32× and 31× were retained for A. ilicifolius and A. leucostachyus, respectively. The average lengths of the remained contigs were 580 and 913 bp and N50 values were 763 and 1580 bp, respectively, suggesting that the transcriptomes for both species were of good quality. The discrepancy in N50 values between the two species may be due to the difference of the genomic complexity. The length distribution of these contigs is shown in Additional file 1.
Phylogenetic analysis and the divergent time estimation
In this study, we estimated the synonymous substitution rate (Ks) of the orthologs between each pair of the two Acanthus species and Avicennia marina (Av. marina). The results showed that the peak of Ks distribution was 0.145 between A. ilicifolius and A. leucostachyus, while it was 0.605 and 0.585 between Av. marina and A. ilicifolius and A. leucostachyus, respectively (Fig. 1a). This unusual high Ks value between A. ilicifolius and its terrestrial congener suggests long-term divergence, albeit within the same genus. Our estimation indicated that A. ilicifolius diverged with A. leucostachyus at approximately 16.8 million years ago (MYA) with a 95 % credibility interval (CI) of 11.6 to 22.1 MY, while the split time of Acanthus and Avicennia was dated at 52.1 MYA (95 % CI: 43.0–60.0). The estimated divergence time between Acanthaceae s.l. and Pedaliaceae was approximately 65.9 MYA (95 % CI: 55.6–73.4).
Fig. 1.
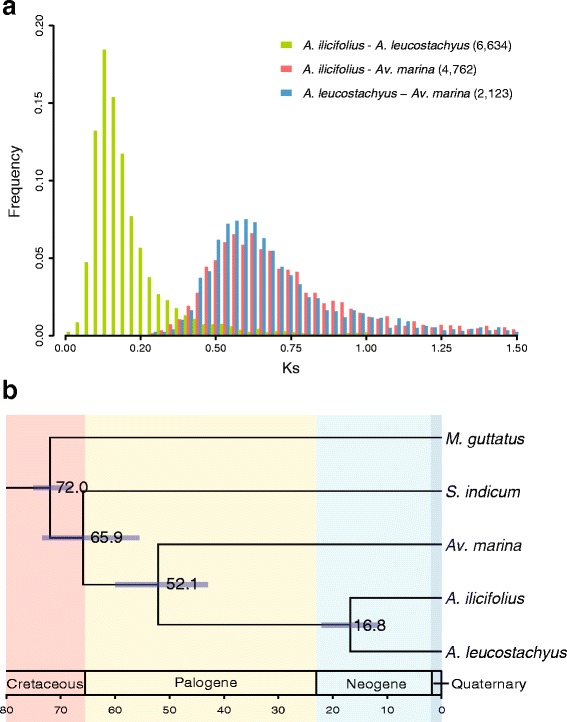
Genetic divergence and the divergent time among Acanthus ilicifolius, A. leucostachyus and Avicennia marina. a Synonymous substitution rates (Ks) distribution of the orthologs between each pair of Acanthus ilicifolius, A. leucostachyus and Av. marina. The numbers in parentheses after the species name indicate the number of orthologs used for Ks distribution plotting. b Divergent time of A. ilicifolius, A. leucostachyus and Av. marina, as well as the two outgroups Sesamum indicum and Mimulus guttatus. The scale bar is 10 million years. The value and purple bar at each node indicate the estimated divergent time (million years) with a 95 % credibility interval
To infer the origin of the mangrove species in Acanthus, we reconstructed the phylogenetic tree for the three mangrove species, A. ilicifolius, A. ebracteatus and A. volubilis, and their terrestrial relatives, A. leucostachyus and two European species A. mollis and A. montanus, based on the combined sequences of four nuclear segments, including three nuclear genes (CL302, CL4763 and c51040) and the nrITS region, and the three chloroplast fragments (trnV-trnM, rpl-rps and trnL-trnF). Maximum likelihood analysis revealed that Acanthus fell into two clades with high bootstrap support (BS); three mangrove species formed one clade, and the three terrestrial species formed the other (BS = 100 and 81 %, respectively; Fig. 2). Within the mangrove clade, A. ebracteatus formed a single clades with a BS value of 80 %. Compared to A. volubilis, A. ilicifolius and A. ebracteatus was close to each other with strong support (BS = 100 %). Within the terrestrial clade, A. mollis is sister to A. montanus with 100 % BS.
Fig. 2.

Phylogenetic tree of six species of Acanthus. The branches of mangroves species were marked in red. Numbers at each node indicate maximum likelihood bootstrap values
Furthermore, we calculated the Ks for the combined sequences of the three nuclear genes and the Kimura two-parameter distances for the nrITS and chloroplast fragments (Additional files 2, 3 and 4). For the three nuclear genes, the average Ks between A. ilicifolius and A. leucostachyus was 0.035. Based on the divergence time of the two species of 16.8 MYA, the synonymous substitution rate within Acanthus was estimated to be 1.04e-9 per synonymous site per year. According to this synonymous substitution rate, the splitting time between the mangrove clade and the two European terrestrial species was estimated at 35.1 MYA. Within the mangrove clade, the divergence time between A. ilicifolius and A. ebracteatus (Ks = 0.0045) was estimated to be 2.2 MYA, while it was estimated to be 2.3 MYA between A. volubilis and the clade of A. ilicifolius and A. ebracteatus (Ks = 0.00475). Comparatively, the divergence of A. leucostachyus and the clade of A. montanus and A. mollis was 0.049, corresponding to 23.6 MYA. Similarly, for nrITS and the combined chloroplast fragments, the mean of the Kimura two-parameter distances between the mangrove and terrestrial clades were 0.096 and 0.029, which are one order of magnitude higher than those among the three mangrove taxa (0.012 and 0.001), respectively.
Functional annotation
To access the GO classification for each gene, the full transcriptome sequences of A. ilicifolius and A. leucostachyus were annotated with the Swiss-Prot database in AgBase [17] with a cutoff e-value of 1e-6. A total of 29,244 (40.04 %) and 25,864 (37.2 %) genes, respectively, were successfully annotated with known GO terms. The GO distributions for the two species are shown in Fig. 3. In general, the GO classifications did not show significant differences between the transcriptome profiles of A. ilicifolius and A. leucostachyus. In the cellular component category, cell and organelle component-related functions were predominant. A total of 26,335 (90.1 %) and 23,206 (89.7 %) genes were assigned to cell and 26,335 (90.1 %) and 23,206 (89.7 %) to cell part for the two species, respectively. There were 21,979 and 11,797 genes in A. ilicifolius and 19,394 and 10,450 genes in A. leucostachyus annotated with organelle and organelle part. In molecular function category, binding and catalytic activity were the most enriched, comprising 16,896 (57.8 %) and 13,629 (46.6 %) genes in A. ilicifolius and 15,054 (58.2 %) and 11,904 (46.0 %) genes in A. leucostachyus, respectively. In the last category, biological process, 26,064 (89.1 %), 23,574 (80.6 %) and 17,676 (60.4 %) genes were annotated to three major GO terms in A. ilicifolius, including cellular and metabolic process and biological regulation, while 22,999 (88.9 %), 20,745 (80.2 %) and 15,475 (59.8 %) genes were assigned to these three terms in A. leucostachyus. It should be noted that 17,694 (60.5 %) and 15,461 (59.8 %) genes were assigned to the GO term response to stimulus, which also comprised a large proportion of the biological process category for both species. These results indicate that functions with enriched annotation may be quite fundamental and essential for plants.
Fig. 3.

Gene Ontology (GO) distributions for Acanthus ilicifolius (red) and A. leucostachyus (blue). Annotation results were mapped to categories in the second level of GO terms, respectively. GO terms that contain less than 1 % of total genes were excluded from this graph. *, p-value <0.05. **, p-value < 0.01
Several GO terms significantly differed between A. ilicifolius and A. leucostachyus. Compared with A. leucostachyus, a lower percentage of genes in the cellular component category were annotated with extracellular region and extracellular region part (p < 0.01), and a higher percentage to symplast (p < 0.05) for A. ilicifolius. In the molecular function category, genes related to molecular transducer activity were more abundant in A. ilicifolius compared with A. leucostachyus (p < 0.05), but those involved in structural molecular activity were less prominent (p < 0.01). In the biological process category, more genes were assigned to multicellular organismal and development processes (p < 0.05) as well as cellular component organization (p < 0.01) in A. ilicifolius than in A. leucostachyus.
Identification of genes under positive selection in A. ilicifolius
We calculated and plotted the non-synonymous to synonymous substitution ratio (Ka/Ks) for the 6634 pairs of orthologs between the two Acanthus species, A. ilicifolius and A. leucostachyus, as shown in Fig. 4. Only 13 pairs were identified with a Ka/Ks ratio that was significantly larger than 1, indicating signals of positive selection in these two species (red dots in Fig. 4). The functional descriptions of the 13 pairs are listed in Additional file 5. Of them, four were found to be involved in the response to biotic and/or abiotic stimuli (bold in Additional file 5).
Fig. 4.

Ka/Ks distribution of 6634 pairs of orthologs between Acanthus ilicifolius and Ac. leucostachyus. The solid line marks Ka/Ks = 1, whereas the red dots mark the genes with Ka/Ks ratio significantly larger than 1
To identify candidate genes under positive selection along the branch of A. ilicifolius, we further tested the 2245 pairs of orthologs among four species, A. ilicifolius, A. leucostachyus, Av. marina and S. indicum, using the improved branch-site likelihood method in the codeml module of PAML [18–20]. A total of 99 genes were identified as positively selected genes (PSGs) in A. ilicifolius. GO annotations revealed that 48 (20.8 %) and 42 (18.2 %) of the 99 genes were assigned to metabolic and cellular process, respectively (Additional file 6a). Of the 99 genes, 18 sequences were assigned to 13 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (15 unique EC numbers) [21]. Detailed annotations were performed on these genes and the results are listed in Additional file 7. Interestingly, 23 genes were annotated with functions involving stimulus responses and reproduction, and 16 were found to be directly involved in response to abiotic stresses (Fig. 5; bold in Additional file 7). These genes fell into three functional groups. The first group consisted of four genes involved in salt-stress resistance. One gene of interest, Ail_c57143_g1_i2, catalyzes the transfer of an amino group from ornithine to the precursor of proline, L-glutamate 5-semialdehyde, which plays a key role in the proline synthesis pathway. Twelve genes with annotations of response to heat and UV stress were assigned to the second groups. Two genes, Ail_c48553_g1_i1 and Ail_c56385_g1_i1, were found to be involved in the last step of the synthesis of glutathione, which is an important antioxidant that protects important cellular components from damage caused by reactive oxygen species (ROS) in plants. The third groups included seven genes involved in seed germination and embryo development.
Fig. 5.

Positively selected and expanded key genes in stress resistance pathways of Acanthus ilicifolius. Genes, products and pathways for responding to salt-stress and for heat and ultraviolet-stress were marked in blue and orange shading, respectively
We further calculated the fragments per kilobase of exon per million fragments mapped (FPKM) [22] for the 23 genes in A. ilicifolius and A. leucostachyus (Additional file 6b). We found that 18 of the 23 genes were expressed at low levels and that their expression levels did not differ between the two species. In contrast, three genes, Ail_c56385_g1_i1, Ail_c48553_g1_i1 and Ail_c52163_g1_i1, were expressed at significantly higher levels in A. ilicifolius than in A. leucostachyus (red double asterisks in Additional file 6b), and two gene, Ail_c53207_g1_i1 and Ail_c51326_g2_i1, showed decreased expression in A. ilicifolius than in A. leucostachyus (blue double asterisks in Additional file 6b).
Discussion
High divergence between the mangrove and terrestrial species within Acanthus
Most genera comprising mangrove species contain exclusively mangroves [3], and each genus is considered to be a monophyletic group. Acanthus is the only genus consisting of both true mangroves and terrestrial plants; thus, it is a good system for dissecting the adaptive evolution of mangroves. In this study, we reconstructed the phylogenetic tree for the genus Acanthus, including three mangrove species and three terrestrial relatives, and Av. marina was used as the outgroup. The topological structure revealed that the mangrove and terrestrial species of Acanthus fell into different clades, with the peak of Ks distribution of 0.145 (Fig. 1a). The pairwise Kimura two-parameter distances of the six Acanthus species also revealed that the divergence between the mangrove and terrestrial species of Acanthus is one order of magnitude higher than that among the three mangrove taxa (Additional files 3 and 4). The splitting time of A. ilicifolius and its terrestrial relative A. leucostachyus was estimated to be 16.8 MYA (Fig. 1b), while 35.1 MYA between the mangrove clade and the two European species. These estimations suggest that the mangrove Acanthus species entered the intertidal environment in Early to Middle Miocene (approximately 23.0 - 11.6 MYA). The transgression that occurred from 18.0 to 16.0 MYA caused dramatic environmental changes and the loss of tropical forests, which may be the major reason for new lineages entering the intertidal environment [23].
In contrast with the high divergence of the mangrove clade and their terrestrial relatives, the three mangrove Acanthus species had quite low interspecific divergence, especially between A. ilicifolius and A. ebracteatus. The average Ks between A. volubilis, and the clade of A. ilicifolius and A. ebracteatus was 0.00475, suggesting that the divergence time between these two clades was 2.3 MYA according to the assumed synonymous substitution rate of 1.04e-9 per synonymous site per year. The two sibling species, A. ilicifolius and A. ebracteatus, show very close but distinct relationship in the phylogenetic trees. This result is in agreement with the taxonomy based on morphological characteristics, including the open inflorescences, white or purple flowers, absent bracteoles and stem without axillary spines of A. ebracteatus [24], although these two species are considered to be the same species by some authors [25]. In this study, the divergence time between A. ilicifolius and A. ebracteatus was estimated as 2.2 MYA, based on the three nuclear genes. Such recent speciation has also been observed in several mangrove genera, such as Sonneratia [26]. However, unlike other mangrove species, A. ilicifolius and A. ebracteatus have nearly identical distributions, suggesting that the primary force driving the divergence of these two species cannot be simply attributed to geographic isolation but that there is a high probability of ecological or adaptive differentiation. However, this hypothesis requires a further testing in the future.
Absence of genetic components specific to the mangrove transcriptomes
Resistance to extreme abiotic stress in the intertidal zone is a complex process involving numerous genes that underlie relevant the morphological and metabolic characteristics across the transcriptome. In the current study, we compared the GO classifications between the transcriptomes of A. ilicifolius and its terrestrial relative, A. leucostachyus. We did not observe any noteworthy differences in GO classifications between these two species, except for a small number of GO terms. Most of the genes were assigned to fundamental functions, such as cellular and organelle structure, binding and catalytic activity, metabolic process and biological regulation, in agreement with a previous report of the mangrove species S. alba [9]. However, these results are inconsistent with reports of two other mangrove species, R. mangle and Heritiera littoralis, which have been suggested to possess mangrove-specific genomic characteristics [27]. The discrepancy between our results and previous study may due to the species specificity and the differences in the tissue types for RNA sequencing. In Acanthus, we found that the GO classifications between the mangrove and terrestrial species were similar, suggesting few genetic components specific to the mangrove transcriptomes.
Positively selected genes in A. ilicifolius
In the past decade, more and more studies have suggested that the differential adaptation of populations is the primary force driving population divergence and speciation [28–30]. Identifying and characterizing the pattern of selective pressures across a genome, such as sequence divergence, may provide insights into the processes and mechanisms of speciation [31–33]. In this study, although no genetic components specific to the mangrove transcriptomes were identified, we identified signals of natural selection in a few genes. According to the Ka/Ks ratios, 13 of the 6634 pairs of orthologs between two Acanthus species were found to be under positive selection (Additional file 5). Of them, one ortholog (Ail_c48842_g1_i2) was annotated as TIP1;2, a gene that is involved in response to salt stress. The expression of this gene in NaCl stress-sensitive yeast mutant strains has been shown to highly increase the resistance to NaCl treatment [34]. The ortholog Ail_c53470_g4_i1 was found to be a homolog of GF14 omega, which is considered to be involved in rescuing defects in DNA-damage and DNA-replication checkpoints and may play an important role in the oxidative stress response [35]. Another two orthologs, Ail_c48391_g1_i2 and Ail_c52685_g1_i1, function in response to attack by microbes [36, 37].
Furthermore, to investigate the genetic bases of the adaptive traits of A. ilicifolius, we employed the improved branch-site likelihood method and identified 99 candidate PSGs out of the 2245 orthologous genes in A. ilicifolius. In contrast with its terrestrial relative A. leucostachyus, A. ilicifolius is faced with three major abiotic stresses in the intertidal environment, high salinity, high temperatures and ultraviolet radiation, and periodic inundation. The annotations indicated that a total of 23 PSGs were involved in the responses to these three stress conditions (Fig. 5; bold in Additional file 7). Four genes that function in response to salt stress were assigned to the first group (blue shading in Fig. 5). Of them, Ail_c57143_g1_i2, annotated as ornithine aminotransferase, may play a key role in responding to salt stress in A. ilicifolius. The product of this gene transfers the amine group from ornithine to glutamate, a precursor of proline [38]. Proline is known to be the major osmolyte involved in response to salt stress in A. ilicifolius, as well as many other mangroves and terrestrial plants [39–41]. Another gene, Ail_CL2465Contig1, is homologous to the gene encoding ubiquitin-specific protease 16 (UBP16) of Arabidopsis thaliana, which could increase salt tolerance by positively regulating plasma membrane Na(+)/H(+) antiport activity under salt stress condition [42].
The second group consisted of 12 genes related to scavenging and the repairing of damage due to oxidative and superoxide stress and UV radiation under high temperature and light conditions (orange shading in Fig. 5). In the intertidal zone, high temperatures and ultraviolet radiation may result in the dramatic accumulation of ROS, leading to severe damage of DNA and cell structures [43]. Two of the ten genes, Ail_c48553_g1_i1 and Ail_c56385_g1_i1, are involved in the last step of the synthesis of glutathione, which is an important antioxidant in plants that protects vital cellular components from damage caused by ROS [44]. Another gene Ail_c51326_g2_i1 showed homology to the gene encoding the chloroplastic drought-induced stress protein of 32 kD (CDSP32) of Solanum tuberosum. CDSP32 is a thioredoxin that participates in defense against oxidative damage in photosynthetic membranes [45–47].
Seven genes involved in seed germination and embryo development were assigned into the third group. Zhang et al. [48] have revealed that short-term tide inundation of <15 h per day has no impact on the time of seed germination; however, long-term inundation of ≥15 h per day delays seed germination and decreases the germination rate in A. ilicifolius. Genes Ail_CL5647Contig1 is homologous to BRIZ2 of Arabidopsis thaliana. BRIZ2 is involved in forming a heteromeric E3 ligase complex, which is required for seed germination and post-germination growth [49]. Another three genes Ail_CL3016Contig1, Ail_CL3870Contig1 and Ail_c54714_g1_i1 were found to play important role in embryo development [50–52]. Thus, these genes may greatly improve the reproductive success of A. ilicifolius in harsh coastal environments. These three groups of resistance related PSGs are crucial to the survival and adaptation of A. ilicifolius to extreme habitats. Adaptation to the differential environments might be the primary force driving species diversification in Acanthus.
The FPKM values of the most of the 23 genes were low for both A. ilicifolius and A. leucostachyus, suggesting that there was no apparent increase in the expression of these genes in response to salt stress in A. ilicifolius under natural conditions (Additional file 6b). In contrast to terrestrial glycophytes, mangrove trees are more adaptive to moderate levels of salt than to freshwater environments [53]. Transcription analysis of Ceriops tagal has also revealed that most salt-induced genes are involved in long-term strategies in response to short-term high-salinity stress [54]. A total of three genes were more highly expressed in A. ilicifolius than in A. leucostachyus. Two of the three were homologous to the gene encoding gamma-hydroxybutyrate dehydrogenase (GHBDH), which functions in oxidative stress tolerance in Arabidopsis thaliana [55–57]. In contrast with A. leucostachyus, which was collected under rainforest canopies, A. ilicifolius was sampled from frontal thickets on the stream edges of recently accreting estuarine banks of Zhujiang River. Direct sunlight may cause the higher expression of these two oxidative stress-related genes in A. ilicifolius. The third gene, Ail_c52163_g1_i1, is homologous to the gene encoding chloroplast stem-loop binding protein of 41 kDa, which is involved in oxidative stress tolerance and chloroplast RNA metabolism in seedlings. In contrast, the expression of Ail_c51326_g2_i1, the product of which is CDSP32, was two-fold higher in A. leucostachyus compared with A. ilicifolius. This result is in agreement with Parida [58], who has reported that polypeptides with a molecular weight of 32 kDa are decreased in B. parviflora as a result of salt stress. However, in this study, only one individual was collected for each species with no biological replicates; therefore, the comparison of expression levels between the two species are not that reliable and only represent preliminary findings that may be expanded upon in a further investigation based on more samples.
Conclusion
Acanthus is the only genus consisting of both mangrove and terrestrial species. In this study, we characterized the transcriptomes of one mangrove species in Acanthus, A. ilicifolius, and its terrestrial relative A. leucostachyus. This comparative transcriptome analysis has greatly enriched the current knowledge of the origin of the mangrove species in Acanthus and their adaptive evolution to abiotic stresses in the intertidal environment. Phylogenetic analysis indicated that the initial entry of the mangrove species in Acanthus into the intertidal environment might have occurred during the transgression in the Early to Middle Miocene. However, our study found that positive selection plays an important role in the process of adaption to harsh intertidal zones.
Methods
RNA extraction and transcriptome sequencing
One individual was collected under natural conditions for each of the two species, A. ilicifolius and A. leucostachyus. The sample of A. ilicifolius was collected near an estuary in Nansha, Guangzhou, while that of A. leucostachyus was sampled in from the Xishuangbanna Tropical Botanical Garden in Xishuangbanna. Both samples were of good health and no disease, and collected at the natural growth conditions without any artificial stress treatment. Fresh leaves and roots tissues from each sample were collected in the morning and pooled with approximately equal quantities for RNA extraction. Total RNA was extracted from each pooled sample using a modified CTAB method [59]. RNA samples were sequenced using an Illumina HiSeq 2000 platform.
De novo assembly and functional annotation
After obtaining clean reads without adaptor-ligated regions by Illumina sequencing, we first filtered out reads of low quality. For each read, the longest contiguous segments with base qualities of 20 or greater and lengths of 50 bp or greater were extracted using the Dynamic Trim and LengthSort script in SolexaQA_v.2.2, respectively [60]. A de novo transcriptome assembly was carried out with the latest version of the Trinity program (trinityrnaseq_r20140413), with a minimum k-mer coverage of 2 [14]. To remove redundancy, two re-assembly programs, TGICL-2.1 [15] and CD-HIT [16], were employed to reassemble highly similar transcripts with identity thresholds of 0.94 and 0.90, respectively. Due to likelihood of the misassembly of contigs with low coverage and depth, we remapped the reads to the assembled transcripts using Bowtie2 [61] and SAMtools [62] and removed those contigs with less than 75 % of sites with at least 2× from the datasets. Open reading frames (ORFs) for each contig were determined by TransDecoder, a downstream software of Trinity, with the criteria that only coding regions containing at least 100 amino acids were retained for subsequent analysis. To identify the most descriptive annotations for the datasets, all of the assembled unigenes were searched against Swiss-Prot database in AgBase [17] with a cutoff e-value of 1e-6. Gene Ontology (GO) [63] classifications were performed with WEGO [64].
Identification of orthologous contigs
The genomic data sets of Sesamum indicum and Mimulus guttatus were download from the Sinbase (http://www.ocri-genomics.org/Sinbase/index.html) and JGI databases (http://genome.jgi-psf.org/mimulus/mimulus.home.html), respectively. Putative orthologs between each pair of the three species, the two Acanthus species and Av. marina, were retrieved from the proteome sequences using OrthoMCL [65]. For each pair, we performed an all-versus-all protein sequence similarity search using BLASTP, with an e-value cutoff of 1e-10 and an identity threshold of 50 %. Similar sequences were subsequently clustered with the Markov Clustering Algorithm and grouped into species families and single-copy gene families. For each pair of orthologs, a parallel tool, ParaAT (Parallel Alignment and back-Translation), was applied to perform protein-coding DNA alignments [66] and multiple alignments of protein sequences with ClustalW2 using the default parameters [67]. All of the gaps generated during the alignment were deleted, and the amino acid alignments were back-translated to the corresponding codon sequences by PAL2NAL [68].
Phylogenetic analysis and estimations of divergent time between different species
The synonymous substitution rate (Ks) and non-synonymous rates (Ka) of the orthologs between each pair of A. ilicifolius, A. leucostachyus and Av. marina was estimated by using KaKs_Calculator [69] with the YN00 model [70]. The non-synonymous to synonymous substitution ratio (Ka/Ks) was calculated for the orthologs between A. ilicifolius and A. leucostachyus. To infer the phylogenetic relationship of the genus Acanthus, we constructed phylogenetic trees for A. ilicifolius, A. ebracteatus and A. volubilis and three terrestrial species A. leucostachyus, A. mollis and A. montanus. Primers corresponding to three nuclear loci with a Ks value of approximately 0.145 and low heterozygosity that could accurately determine the phylogenetic relationship of A. ilicifolius and A. leucostachyus, were designed from the transcriptome data and screened for further polymerase chain reaction (PCR) amplification (Additional file 8). The nrITS (including ITS-1, the 5.8S rRNA gene, and ITS-2) region and three chloroplast fragments (trnV-trnM, rpl-rps and trnL-trnF) were also included and amplified with universal primers (Additional file 8). All of the sequences used in this study were deposited in GenBank with the accession numbers were [GenBank: KM652490-KM652552] and [GenBank: KM888781-KM888791] (Additional file 9). These sequences were aligned with CLUSTALX version 1.7 [71] and manually adjusted using SeqMan (version 7.10; DNAStar, London, UK). All the sequences were combined in series as one dataset. The phylogenetic trees were constructed with the combined dataset by Maximum likelihood (ML) method in PAUP* 4.0b [72]. Av. marina was used as an outgroup based on previous taxonomy (McDade, 1999 [73]; Schäferhoff, 2010 [74]). Before the tree construction, appropriate nucleotide substitution models were selected from the 56 models of nucleotide substitution for the combined dataset with Modeltest 3.7 (Posada, 2004 [75]). TVM + G was identified as the best-fit models for the combined dataset based on the Akaike Information Criterion. Bootstrap analyses were performed with 1000 replicates for the heuristic search with tree bisection–reconnection branch swapping and the maxtrees was set to 500. Furthermore, we calculated Kimura two-parameter distances [76] for the nrITS and chloroplast fragments, respectively, and the pairwise Ks value by Li-Wu-Luo method [77] for the three nuclear genes in Mega 5.0 [78].
Based on the 2104 pairs of orthologs among the five species A. ilicifolius, A. leucostachyus, Av. marina, S. indicum and M. guttatus, we estimated the divergence times between the mangrove and non-mangrove species of Acanthus, as well as between Acanthus and Avicennia, using the mcmctree program of PAML4.8 [18], using the independent rates model with the HKY85 substitution model. The first and second codons of each sequence were combined into two datasets, respectively. The likelihood was calculated using the pruning algorithm of Felsenstein. The program of the same parameters was run independently twice with different seeds to ensure convergence. Tripp and McDade [79] have used the record of a fossil taxon of Avicennia (38 MYA) to identify the most recent common ancestor (MRCA) of the extant Avicennia species and have estimated the divergent time of the Avicennia clade and Acanthaceae s.s. as 82 MYA, which is quite larger than the estimations of Bremer et al. (54 MYA) [80] and Bell et al. (41 MYA) [81]. Their approximation may have been an overestimation of the origin time of the genus Avicennia, leading to an overestimation of the divergence time of the Avicennia clade and Acanthaceae s.s.. In this study, we employed the divergence time of Scrophulariaceae (approximately 68–75 MYA) as fossil calibration information based on the results of the phylogenetic dating of Asterid Flowering Plants [80]. The appearance time of a fossil taxon A. rugatus Reid and Chandler (approximately 33.7–28.8 MYA) [82], the most recent common ancestor of Acanthus, was also considered as an indirect calibration.
Detecting genes under positive selection in A. ilicifolius
The branch-site model implemented in the codeml module of PAML was used to identify candidate genes under positive selection along the branch of A. ilicifolius from 2245 pairs of orthologs among four species, including A. ilicifolius, A. leucostachyus, Av. marina and S. indicum [18–20]. The branch-site model A1 in combination with the BEB procedure was specified by setting the branch of A. ilicifolius as the “foreground branch” and all other branches in the tree as “background” branches. We compared the model A1 with and without ω fixed to 1 to determine whether the branch of A. ilicifolius was under positive selection. We applied the likelihood ratio test (LTR) to assess the reliability of the results. A p-value of less than 0.05 was considered significant to ensure for the power and accuracy of the LRT for detecting the signals of positive selection. In addition, Bonferroni’s multiple testing correction was applied to control the false discovery rate, with a threshold of lower than 0.05. Retained candidate genes were annotated with the GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) database [21] using the automatic annotation tool Blast2GO [83] with a cutoff e-value of 1e − 6. Transcript abundances were calculated using TopHat program [84], which outputs read counts and the number of fragments per kilobase of exon per million fragments mapped (FPKM) [22], and significance of the difference between the two species was tested using the Fisher’s exact test.
Availability of supporting data
The data sets supporting the results of this article are included within the article and additional files.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 91331202, 41130208, 41276107, 31170213, and 91231106), the State Key Laboratory of Biocontrol (Grant Nos. 13A03, 12 K04, and 2011 K01), the Scientific Research Foundation for Returned Overseas Chinese Scholars, State Education Ministry, and the Chang Hungta Science Foundation of Sun Yat-sen University.
Abbreviations
- BS
Bootstrap support
- CI
credibility interval
- FPKM
Fragments per kilobase of exon region in a given gene per million mapped fragments
- GO
Gene ontology
- Ka
Non-synonymous rates
- Ka/Ks
Non-synonymous to synonymous substitution ratio
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- Ks
Synonymous substitution rate
- MRCA
Most recent common ancestor
- MYA
Million year ago
- PSGs
Positively selected genes
- RSO
Reactive oxygen species
- UV
Ultraviolet
Additional files
Length distribution of the contigs after removing redundancy in Acanthus ilicifolius (red) and A. leucostachyus (blue). (PDF 261 kb)
Pairwise synonymous substitution rate (Ks) of the three nuclear loci for the six Acanthus species. The abbreviations were listed in Additional file 9. (XLSX 9 kb)
Pairwise Kimura two-parameter distances of nrITS for the six Acanthus species. The abbreviations were listed in Additional file 9. (XLSX 9 kb)
Pairwise Kimura two-parameter distances of the combined chloroplast fragments for the six Acanthus species. The abbreviations were listed in Additional file 9. (XLSX 9 kb)
List of the 13 pairs of orthologs between Acanthus ilicifolius and A. leucostachyus with Ka/Ks ratios significantly larger than 1. – indicates that the orthologs cannot find their orthologous genes in Arabidopsis. (XLSX 10 kb)
GO distribution of the 99 candidate positively selected genes (PSGs) and FPKM of 23 stress-responsive genes in Acanthus ilicifolius. a GO distribution of the 99 candidate PSGs in Acanthus ilicifolius. b FPKM of the 23 PSGs involved in salt, high temperature and UV stress tolerance and seedling germination and embryo development for A. ilicifolius (red) and A. leucostachyus (blue). Red double asterisks indicate the transcript expression in A. ilicifolius is higher than that in A. leucostachyus with p-value less than 0.01, while blue double asterisks indicate the transcript expression in A. ilicifolius is less than A. leucostachyus with p-value less than 0.01. (PDF 483 kb)
List of the 99 candidate positively selected genes (PSGs) in Acanthus ilicifolius . – indicates that the gene cannot find their orthologous genes in Arabidopsis. Gene ID of the 23 PSGs involved in salt, high temperature and UV stress tolerance, seedling germination and embryo development is shown in bold. (XLSX 14 kb)
Gene IDs, sequence lengths, gene descriptions and primer sequences used in this study. (XLSX 9 kb)
Sampling information and GenBank accession numbers of the six Acanthus species and the outgroup Avicennia marina used in this study. The sequences of the three nuclear genes of Av. marina were obtained from the unpublished genomic data set of Av. marina and were not deposited in Genbank. (XLSX 9 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RCZ and SHS designed the study. YCY, CRZ and YFD collected materials. YCY and JFL performed experiments. YCY, SHY, ZZ, SHX and WXG analyzed and interpreted the data. YCY, SHY, JFL, RCZ and SHS wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Yuchen Yang, Email: yangyuchensysu@gmail.com.
Shuhuan Yang, Email: yangshuhuan8909@hotmail.com.
Jianfang Li, Email: lijf0711@163.com.
Yunfei Deng, Email: yfdeng@scbg.ac.cn.
Zhang Zhang, Email: lylazhang@foxmail.com.
Shaohua Xu, Email: xushaohua08@163.com.
Wuxia Guo, Email: qingting7906@126.com.
Cairong Zhong, Email: crzhong169@163.com.
Renchao Zhou, Email: zhrench@mail.sysu.edu.cn.
Suhua Shi, Email: lssssh@mail.sysu.edu.cn.
References
- 1.Tomlinson P. The botany of mangroves. Cambridge tropical biology series. Cambridge, New York, USA: Cambridge University Press; 1986. [Google Scholar]
- 2.Duke NC. Mangrove floristics and biogeography. Tropical mangrove ecosystems. 1992. pp. 63–100. [Google Scholar]
- 3.Duke N, Ball M, Ellison J. Factors influencing biodiversity and distributional gradients in mangroves. Glob Ecol Biogeogr Lett. 1998;7(1):27–47. doi: 10.2307/2997695. [DOI] [Google Scholar]
- 4.Ricklefs RE, Schwarzbach AE, Renner SS. Rate of lineage origin explains the diversity anomaly in the world’s mangrove vegetation. Am Nat. 2006;168(6):805–10. doi: 10.1086/508711. [DOI] [PubMed] [Google Scholar]
- 5.Shi S, Huang Y, Zeng K, Tan F, He H, Huang J, et al. Molecular phylogenetic analysis of mangroves: independent evolutionary origins of vivipary and salt secretion. Mol Phylogenet Evol. 2005;34(1):159–66. doi: 10.1016/j.ympev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Fu X, Huang Y, Deng S, Zhou R, Yang G, Ni X, et al. Construction of a SSH library of Aegiceras corniculatum under salt stress and expression analysis of four transcripts. Plant Sci. 2005;169(1):147–54. doi: 10.1016/j.plantsci.2005.03.009. [DOI] [Google Scholar]
- 7.Ezawa S, Tada Y. Identification of salt tolerance genes from the mangrove plant Bruguiera gymnorhiza using Agrobacterium functional screening. Plant Sci. 2009;176(2):272–8. doi: 10.1016/j.plantsci.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Miyama M, Tada Y. Transcriptional and physiological study of the response of Burma mangrove (Bruguiera gymnorhiza) to salt and osmotic stress. Plant Mol Biol. 2008;68(1–2):119–29. doi: 10.1007/s11103-008-9356-y. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Zhou R, Huang Y, Zhang M, Yang G, Zhong C, et al. Transcriptome sequencing of a highly salt tolerant mangrove species Sonneratia alba using Illumina platform. Mar Genomics. 2011;4(2):129–36. doi: 10.1016/j.margen.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Feng J, Lu J, Yang Y, Zhang X, Wan D, et al. Transcriptome differences between two sister desert poplar species under salt stress. BMC Genomics. 2014;15(1):337. doi: 10.1186/1471-2164-15-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Deng YF, Wood JRI, Daniel TF. Acanthaceae. Flora of China. 2011;19:369–477. [Google Scholar]
- 12.Nguyen P, Ho C-L, Harikrishna JA, Wong M-L, Rahim RA. Functional screening for salinity tolerant genes from Acanthus ebracteatus Vahl using Escherichia coli as a host. Trees. 2007;21(5):515–20. doi: 10.1007/s00468-007-0144-0. [DOI] [Google Scholar]
- 13.Nguyen PD, Ho C-L, Harikrishna JA, Wong MC, Rahim RA. Generation and analysis of expressed sequence tags from the mangrove plant, Acanthus ebracteatus Vahl. Tree Genet Genomes. 2006;2(4):196–201. doi: 10.1007/s11295-006-0044-2. [DOI] [Google Scholar]
- 14.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, et al. TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19(5):651–2. doi: 10.1093/bioinformatics/btg034. [DOI] [PubMed] [Google Scholar]
- 16.Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–2. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy FM, Wang N, Magee GB, Nanduri B, Lawrence ML, Camon EB, et al. AgBase: a functional genomics resource for agriculture. BMC Genomics. 2006;7(1):229. doi: 10.1186/1471-2164-7-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24(8):1586–91. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Nielsen R. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol Biol Evol. 2002;19(6):908–17. doi: 10.1093/oxfordjournals.molbev.a004148. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Nielsen R, Yang Z. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol Biol Evol. 2005;22(12):2472–9. doi: 10.1093/molbev/msi237. [DOI] [PubMed] [Google Scholar]
- 21.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic acids research. 2011. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed]
- 22.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller KG, Kominz MA, Browning JV, Wright JD, Mountain GS, Katz ME, et al. The Phanerozoic record of global sea-level change. Science. 2005;310(5752):1293–8. doi: 10.1126/science.1116412. [DOI] [PubMed] [Google Scholar]
- 24.Duke NC. Australia’s mangroves: the authoritative guide to Australia’s mangrove plants. Brisbane: MER; 2006. [Google Scholar]
- 25.Percival M. Floristics and ecology of the mangrove vegetation of Papua New Guinea. 1975. [Google Scholar]
- 26.Zhou R, Zeng K, Wu W, Chen X, Yang Z, Shi S, et al. Population genetics of speciation in nonmodel organisms: I. Ancestral polymorphism in mangroves. Mol Biol Evol. 2007;24(12):2746–54. doi: 10.1093/molbev/msm209. [DOI] [PubMed] [Google Scholar]
- 27.Dassanayake M, Haas J, Bohnert H, Cheeseman J. Shedding light on an extremophile lifestyle through transcriptomics. New Phytol. 2009;183(3):764–75. doi: 10.1111/j.1469-8137.2009.02913.x. [DOI] [PubMed] [Google Scholar]
- 28.Coyne JA, Orr HA. Speciation. MA: Sinauer Associates Sunderland; 2004. [Google Scholar]
- 29.Schemske DW. Adaptation and the origin of species. Am Nat. 2010;176(S1):S4–25. doi: 10.1086/657060. [DOI] [PubMed] [Google Scholar]
- 30.Wu CI. The genic view of the process of speciation. J Evol Biol. 2001;14(6):851–65. doi: 10.1046/j.1420-9101.2001.00335.x. [DOI] [Google Scholar]
- 31.Rieseberg LH, Blackman BK. Speciation genes in plants. Annals of Botany. 2010. doi: 10.1093/aob/mcq126. [DOI] [PMC free article] [PubMed]
- 32.Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317(5840):910–4. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siol M, Wright SI, Barrett SC. The population genomics of plant adaptation. New Phytol. 2010;188(2):313–32. doi: 10.1111/j.1469-8137.2010.03401.x. [DOI] [PubMed] [Google Scholar]
- 34.Pih KT, Kabilan V, Lim JH, Kang SG, Piao HL, Jin JB, et al. Characterization of two new channel protein genes in Arabidopsis. Mol Cells. 1999;9(1):84–90. [PubMed] [Google Scholar]
- 35.Sorrell DA, Marchbank AM, Chrimes DA, Dickinson JR, Rogers HJ, Francis D, et al. The Arabidopsis 14-3-3 protein, GF14ω, binds to the Schizosaccharomyces pombe Cdc25 phosphatase and rescues checkpoint defects in the rad24− mutant. Planta. 2003;218(1):50–7. doi: 10.1007/s00425-003-1083-7. [DOI] [PubMed] [Google Scholar]
- 36.Raffaele S, Rivas S. Regulate and be regulated: integration of defense and other signals by the AtMYB30 transcription factor. Front Plant Sci. 2013;4:98. doi: 10.3389/fpls.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taji T, Seki M, Satou M, Sakurai T, Kobayashi M, Ishiyama K, et al. Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol. 2004;135(3):1697–709. doi: 10.1104/pp.104.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Ruiter H, Kollöffel C. Arginine catabolism in the cotyledons of developing and germinating pea seeds. Plant Physiol. 1983;73(3):525–8. doi: 10.1104/pp.73.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Datta P, Ghose M. Estimation of osmotic potential and free amino acids in some mangroves of the Sundarbans, India. Acta Botanica Croatica. 2003;62(1):37–45. [Google Scholar]
- 40.Parida AK, Jha B. Salt tolerance mechanisms in mangroves: a review. Trees. 2010;24(2):199–217. doi: 10.1007/s00468-010-0417-x. [DOI] [Google Scholar]
- 41.Stránská J, Kopečný D, Tylichová M, Snégaroff J, Šebela M. Ornithine δ-aminotransferase: an enzyme implicated in salt tolerance in higher plants. Plant Signal Behav. 2008;3(11):929–35. doi: 10.4161/psb.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou H, Zhao J, Yang Y, Chen C, Liu Y, Jin X, et al. Ubiquitin-specific protease16 modulates salt tolerance in Arabidopsis by regulating Na+/H+ antiport activity and serine hydroxymethyltransferase stability. Plant Cell. 2012;24(12):5106–22. doi: 10.1105/tpc.112.106393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kipp E, Boyle M. The effects of heat stress on reactive oxygen species production and chlorophyll concentration in Arabidopsis Thaliana. Res Plant Sci. 2013;1(2):20–3. [Google Scholar]
- 44.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66(8):1499–503. doi: 10.1016/S0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 45.Rey P, Pruvot G, Becuwe N, Eymery F, Rumeau D, Peltier G. A novel thioredoxin‐like protein located in the chloroplast is induced by water deficit in Solanum tuberosum L. plants. Plant J. 1998;13(1):97–107. doi: 10.1046/j.1365-313X.1998.00015.x. [DOI] [PubMed] [Google Scholar]
- 46.Broin M, Rey P. Potato plants lacking the CDSP32 plastidic thioredoxin exhibit overoxidation of the BAS1 2-cysteine peroxiredoxin and increased lipid peroxidation in thylakoids under photooxidative stress. Plant Physiol. 2003;132(3):1335–43. doi: 10.1104/pp.103.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rey P, Cuiné S, Eymery F, Garin J, Court M, Jacquot JP, et al. Analysis of the proteins targeted by CDSP32, a plastidic thioredoxin participating in oxidative stress responses. Plant J. 2005;41(1):31–42. doi: 10.1111/j.1365-313X.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- 48.L-e ZHANG, B-w LIAO, GUAN W. Effects of simulated tide inundation on seed germination and seedling growth of mangrove species Acanthus ilicifolius. Chin J Ecol. 2011;10:009. [Google Scholar]
- 49.Hsia MM, Callis J. BRIZ1 and BRIZ2 proteins form a heteromeric E3 ligase complex required for seed germination and post-germination growth in Arabidopsis thaliana. J Biol Chem. 2010;285(47):37070–81. doi: 10.1074/jbc.M110.168021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleine T. Arabidopsis thaliana mTERF proteins: evolution and functional classification. Front Plant Sci. 2012;3:233. doi: 10.3389/fpls.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, et al. Identification of genes required for embryo development in Arabidopsis. Plant Physiol. 2004;135(3):1206–20. doi: 10.1104/pp.104.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ni DA, Sozzani R, Blanchet S, Domenichini S, Reuzeau C, Cella R, et al. The Arabidopsis MCM2 gene is essential to embryo development and its over‐expression alters root meristem function. New Phytol. 2009;184(2):311–22. doi: 10.1111/j.1469-8137.2009.02961.x. [DOI] [PubMed] [Google Scholar]
- 53.Ball M, Pidsley S. Growth responses to salinity in relation to distribution of two mangrove species, Sonneratia alba and S. lanceolata, in northern Australia. Funct Ecol. 1995;9:77–85. doi: 10.2307/2390093. [DOI] [Google Scholar]
- 54.Liang S, Fang L, Zhou R, Tang T, Deng S, Dong S, et al. Transcriptional homeostasis of a mangrove species, Ceriops tagal, in saline environments, as revealed by microarray analysis. PLoS One. 2012;7(5):e36499. doi: 10.1371/journal.pone.0036499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allan WL, Simpson JP, Clark SM, Shelp BJ. γ-Hydroxybutyrate accumulation in Arabidopsis and tobacco plants is a general response to abiotic stress: putative regulation by redox balance and glyoxylate reductase isoforms. J Exp Bot. 2008;59(9):2555–64. doi: 10.1093/jxb/ern122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breitkreuz K, Allan W, Van Cauwenberghe O, Jakobs C, Talibi D, Andre B, et al. A novel gamma-hydroxybutyrate dehydrogenase: identification and expression of an Arabidopsis cDNA and potential role under oxygen deficiency. J Biol Chem. 2003;278:41552–6. doi: 10.1074/jbc.M305717200. [DOI] [PubMed] [Google Scholar]
- 57.Dixon DP, Skipsey M, Grundy NM, Edwards R. Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol. 2005;138(4):2233–44. doi: 10.1104/pp.104.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parida AK, Das AB, Mittra B, Mohanty P. Salt-stress induced alterations in protein profile and protease activity in the mangrove Bruguiera parviflora. Z Naturforsch C Bio Sci. 2004;59(5–6):408–14. doi: 10.1515/znc-2004-5-622. [DOI] [PubMed] [Google Scholar]
- 59.Fu X, Deng S, Su G, Zeng Q, Shi S. Isolating high-quality RNA from mangroves without liquid nitrogen. Plant Mol Biol Report. 2004;22(2):197. doi: 10.1007/BF02772728. [DOI] [Google Scholar]
- 60.Cox MP, Peterson DA, Biggs PJ. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinf. 2010;11(1):485. doi: 10.1186/1471-2105-11-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, et al. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34(suppl 2):W293–7. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer S, Brunk BP, Chen F, Gao X, Harb OS, Iodice JB et al. Using OrthoMCL to Assign Proteins to OrthoMCL‐DB Groups or to Cluster Proteomes Into New Ortholog Groups. Current Protocols in Bioinformatics. 2011:6.12.1-19. [DOI] [PMC free article] [PubMed]
- 66.Zhang Z, Xiao J, Wu J, Zhang H, Liu G, Wang X, et al. ParaAT: a parallel tool for constructing multiple protein-coding DNA alignments. Biochem Biophys Res Commun. 2012;419(4):779–81. doi: 10.1016/j.bbrc.2012.02.101. [DOI] [PubMed] [Google Scholar]
- 67.Larkin MA, Blackshields G, Brown N, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 68.Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34(suppl 2):W609–12. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z, Li J, Zhao X-Q, Wang J, Wong GK-S, Yu J. KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genomics Proteomics Bioinformatics. 2006;4(4):259–63. doi: 10.1016/S1672-0229(07)60007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Z, Nielsen R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol. 2000;17(1):32–43. doi: 10.1093/oxfordjournals.molbev.a026236. [DOI] [PubMed] [Google Scholar]
- 71.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swofford DL. Paup*: Phylogenetic analysis using parsimony (and other methods) 4.0. Beta. 2001. [Google Scholar]
- 73.McDade LA, Moody ML. Phylogenetic relationships among Acanthaceae: evidence from noncoding trnL-trnF chloroplast DNA sequences. Am J Bot. 1999;86(1):70–80. doi: 10.2307/2656956. [DOI] [PubMed] [Google Scholar]
- 74.Schäferhoff B, Fleischmann A, Fischer E, Albach DC, Borsch T, Heubl G, et al. Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences. BMC Evol Biol. 2010;10(1):352. doi: 10.1186/1471-2148-10-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53(5):793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- 76.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–20. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 77.Li W-H, Wu C-I, Luo C-C. A new method for estimating synonymous and nonsynonymous rates of nucleotide substitution considering the relative likelihood of nucleotide and codon changes. Mol Biol Evol. 1985;2(2):150–74. doi: 10.1093/oxfordjournals.molbev.a040343. [DOI] [PubMed] [Google Scholar]
- 78.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tripp EA, McDade LA. A rich fossil record yields calibrated phylogeny for Acanthaceae (Lamiales) and evidence for marked biases in timing and directionality of intercontinental disjunctions. Syst Biol. 2014;63(5):660–84. doi: 10.1093/sysbio/syu029. [DOI] [PubMed] [Google Scholar]
- 80.Bremer K. Molecular phylogenetic dating of asterid flowering plants shows early Cretaceous diversification. Syst Biol. 2004;53(3):496–505. doi: 10.1080/10635150490445913. [DOI] [PubMed] [Google Scholar]
- 81.Bell CD, Soltis DE, Soltis PS. The age and diversification of the angiosperms re-revisited. Am J Bot. 2010;97(8):1296–303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- 82.Martínez-Millán M. Fossil record and age of the Asteridae. Bot Rev. 2010;76(1):83–135. doi: 10.1007/s12229-010-9040-1. [DOI] [Google Scholar]
- 83.Conesa A, Götz S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics. 2008;2008:619832. doi: 10.1155/2008/619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–78. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


