Abstract
Purpose of review
To present recent findings on the pathogenesis of coxsackievirus B3 (CVB3) myocarditis based on animal models, with a focus on the role of T helper (Th) immune responses in disease progression.
Recent findings
Acute CVB3 myocarditis is known to be increased by Th1 immune responses, but recent findings indicate that Th1-type immunity protects against acute myocarditis by reducing viral replication and prevents the progression to chronic myocarditis and dilated cardiomyopathy (DCM) by inhibiting Th2 responses. Th2 responses reduce acute myocarditis by inhibiting Th1 responses via regulatory T cells and anti-inflammatory cytokines, but can be deleterious when they induce acute cardiac remodeling leading to chronic myocarditis/DCM. Th2-skewed immune responses allow resistant strains of mice to progress from myocarditis to DCM. In contrast, Th17 responses are elevated during acute and chronic myocarditis and have been found to contribute to cardiac remodeling and DCM.
Summary
Recent data indicate that elevated Th2 and Th17 responses during acute CVB3 myocarditis are critical for the progression from myocarditis to DCM and heart failure because of their ability to induce cardiac remodeling. Th1 responses protect against CVB3 myocarditis by inhibiting Th2 responses and viral replication, but increase acute inflammation.
Keywords: myocarditis, autoimmune disease, coxsackievirus, T helper response
INTRODUCTION
Myocarditis is an autoimmune disease that leads to a significant minority of dilated cardiomyopathy (DCM) cases in the US [1–3]. From 4–20% of sudden cardiovascular deaths among young adults, the military, and athletes are due to myocarditis [4]. However, the true incidence and prevalence of myocarditis are unknown due to the lack of widely available, safe and accurate noninvasive diagnostic tests [5,6**]. Although most cases of suspected myocarditis are not linked to a specific cause [6**], viral infections like coxsackievirus B3 (CVB3) are the most commonly identified cause of myocarditis in developed countries [2,4]. Antiviral treatments with interferon (IFN)-β have been shown to reduce inflammation and DCM in animal models and patients [7,8] suggesting that viral infections are an important cause of myocarditis cases in patients.
Infections are also believed to induce or trigger autoimmunity [9–11]. CVB3 induces autoimmune myocarditis that progresses to DCM in susceptible strains of mice like A/J and BALB/c [12,13]. Resistant strains of mice like C57BL/6 develop acute CVB3 myocarditis, but do not progress to DCM. Myocarditis and DCM occur more frequently in men than women and in male than female mice [2,14**,15**]. A recent study of myocarditis/acute DCM patients found that myocardial recovery and transplant-free survival were significantly worse in men [14**]. Similar to findings in clinical biopsies of myocarditis patients [2], the primary infiltrate in mouse models of myocarditis consists of macrophages and neutrophils with lower levels of T cells, B cells, mast cells and dendritic cells [16–18]. Natural killer cells, CD8 T cells and γδT cells, important in antiviral defense, are present in the heart during the early cellular response in viral animal models of myocarditis [12,19]. Acute myocarditis is characterized by a predominantly T helper (Th)1 and Th17 response [19,20**,21,22]. However, only mice that respond to infection and/or self-antigen (i.e. damaged self) with a Th2 response, such as BALB/c and A/J strains, develop the chronic stage of myocarditis with fibrosis and DCM [23,24,25**].
This review focuses on recent findings in animal models of CVB3 myocarditis. There are essentially three animal models of myocarditis: an experimental autoimmune myocarditis (EAM) model induced by adjuvant and cardiac myosin, a hybrid-CVB3 model that closely resembles EAM that is induced using heart-passaged CVB3 and damaged heart proteins, and the classic CVB3 model that uses tissue culture-derived or purified virus. Each of these models contributes uniquely to our understanding of the pathogenesis of disease.
ANIMAL MODELS OF MYOCARDITIS
In order to appreciate recent findings, it is important to understand similarities and differences between the three primary models of autoimmune and CVB3 myocarditis in mice.
CVB3-only model
In 1974 Woodruff et al. originally described CVB3-induced myocarditis in mice [26]. CVB3 purified from tissue culture is the most common method used to induce acute myocarditis. In this model mice are infected intraperitoneally (ip) with purified virus or RNA from various CVB3 strains (i.e. Woodruff, H3, Nancy) after passage through HeLa cells. Viral replication peaks in the heart from day 5–7 post infection (pi) (107–109 plaque forming units/PFU/g heart) and causes severe necrosis but low levels of inflammation (5–10% of heart tissue inflamed) followed by heart failure with only around 30% of mice surviving to day 7 pi (Table 1) [13]. Disease is more severe in male than female mice [27–29], γδT cells are critical for disease induction [19,30**], and increased acute inflammation is associated with a predominant Th1 response- particularly in male mice [19]. The importance of autoreactive T and B cells in this model indicates that autoimmunity is involved in disease pathology [9].
Table 1.
Animal models of myocarditisa
| CVB3-only model | Hybrid-CVB3 model | EAM | |
|---|---|---|---|
| Survival | 20–30% by day 7 pi | 100% to day 90 pi | 100% to day 90 pi |
| Viral replication d7 pi | 107–109 PFU/g heart | 105 PFU/g heart | 0 |
| Acute myocarditis | Peak @ day 7 pi | Peak @ day 10 pi | Peak @ day 21 |
| Severity of myocarditis | 5–10% inflammation | 30–60% inflammation | 30–60% inflammation |
| Key cell mediators | γδT & CD8+ T cells | Macrophages | Macrophages |
| DCM | Few survive | Yes | Yes |
| Sex differences | Males > females | Males > females | Males > females |
Abbreviations: CVB3, coxsackievirus B3; DCM, dilated cardiomyopathy; EAM, experimental autoimmune myocarditis; PFU, plaque forming units; pi, post infection or post inoculation;
EAM
EAM is induced using cardiac myosin/cardiac peptides and adjuvants (i.e. inactivated Mycobacterium and/or Pertussis toxin) administered at day 0 and 7 [31]. Peak inflammation (30–60% of heart tissue inflamed) occurs around 10–14 days later at day 21 post inoculation (Table 1) [32**,33**]. Acute inflammation consists predominantly of macrophages and neutrophils with lower amounts of T and B cells, mast cells and dendritic cells [18,32**]. Disease develops in susceptible strains of mice (e.g. Th2 responders like BALB/c and A/J mice) and progresses to DCM around day 42 post inoculation when fibrosis and necrosis is observed [23,34]. A Th1 immune response has been shown to prevent acute and chronic EAM [34,35] while a Th17 response increases chronic fibrosis and DCM [22]. Thus, only Th2 responding strains develop EAM and progress to DCM [23]. 100% of susceptible wild type (WT) mouse strains survive to develop DCM.
Hybrid-CVB3 model
This model is induced by passaging CVB3 originally isolated from a patient (i.e. Nancy strain) through Vero cells and then through the heart [10]. Infectious virus obtained from the heart (i.e. heart-passaged) along with damaged self-tissue is injected ip into mice at day 0. All mouse strains (e.g. BALB/c, C57BL/6) develop acute myocarditis that peaks at day 10 pi (30–60% of heart tissue inflamed) and is comprised predominantly of macrophages and neutrophils with lower numbers of T and B cells, mast cells and dendritic cells (Table 1) [16], similar to EAM. Viral replication peaks around day 7 pi (105 PFU/g heart), declines by day 10 pi (103 PFU/g heart) and is cleared from the heart by day 14 pi [10,12]. 100% of WT mice survive acute myocarditis, but only susceptible Th2 responding strains of mice like BALB/c develop chronic DCM and fibrosis from day 35 pi [10,24,25**]. Necrosis is observed histologically in the heart during chronic myocarditis/DCM, but not during acute myocarditis. This hybrid-CVB3 model more closely resembles EAM than CVB3-only models (Table 1) [18,24,35]. Myocarditis and DCM are more severe in male mice [15**,16,36*].
RECENT FINDINGS
Several review articles summarize current knowledge on the role of cytokines and Th responses in the pathogenesis of myocarditis (see 30**,32**,33**,37**,38**). This review adds understanding by highlighting the most recent findings on the role of Th responses in the pathogenesis of CVB3 myocarditis.
Th1 response
Recently Wiltshire et al. raised two very important questions: 1) does uncontrolled viral replication drive inflammation during CVB3 myocarditis, or 2) does virus trigger an inflammatory response via TLR activation, for example [39*]? The answer to these questions may lie in the model and/or viral strain being used to induce myocarditis. EAM is a clear example of inflammation being triggered by activation of TLR via cardiac myosin and the inactivated Mycobacterium component of complete Freund’s adjuvant. Viral replication in CVB3-only models, however, is closely associated with elevated cardiac inflammation (Table 2) [13,37**,40**]. In contrast, inflammation usually does not correlate with viral replication in the hybrid-CVB3 model [16,25**,41]. These differences in the role of virus as a “driver” vs. “trigger” of inflammation are highlighted by a number of recent studies that examined the role of Th1 responses in CVB3 myocarditis.
Table 2.
Role of Th immune responses in animal models of myocarditisa
| CVB3-only model | Hybrid-CVB3 model | EAM | |
|---|---|---|---|
| Role of IFN-α (Th1) | Inhibits viral replication | ND | ND |
| Role of IFN-β (Th1) | Inhibits viral replication | Inhibits viral replication Prevents DCM | ND |
| Role of IFN-γ (Th1) | Inhibits viral replication | Inhibits viral replication Prevents DCM | Prevents DCM |
| Role of IL-4 (Th2) | Prevents myocarditis | Increases DCM | Increases DCM |
| Role of IL-33 (Th2) | ND | Increases DCM | ND |
| Role of IL-23 (p40) | Increases myocarditis | No effect on myocarditis | Increases EAM |
| Role of IL-17 (Th17) | Increases myocarditis | ND | No effect on EAM Increases DCM |
Abbreviations: CVB3, coxsackievirus B3; DCM, dilated cardiomyopathy; EAM, experimental autoimmune myocarditis; IL, interleukin; ND, not done; Th, T helper type immune response; IFN, interferon
Wiltshire et al. identified a viral myocarditis susceptibility gene (Vms1) locus on murine Chromosome 3 by comparing susceptible strains of mice such as A/J to resistant strains like B10.A using a CVB3-only model [39*]. They found several IFN-related genes that determined susceptibility to infection (e.g. Fpgt, H28, Tnni3k). IFN-β has been shown previously to protect against viral myocarditis in animal models and patients [7,8], and recently several groups showed that protection by IFN-β is mediated via the transcription factor TRIF (25**,40**,42,43]. CVB3 limits a host antiviral response by repressing mRNA translation. Interestingly, activation of the translational suppressor eukaryotic initiation factor 4E-binding protein-1 (4E-BP1) was found to inhibit IFN-β production by host cells [42]. Additionally, low-dose oral IFN-α administration in a CVB3-only model was found to reduce viral replication in the heart by increasing a Th1 response resulting in reduced acute myocarditis (Table 2) [44]. Although most studies using CVB3-only models find a strong association between the level of cardiac viral replication and myocarditis, studies by Yue et al. found that inhibiting the chemokines IFN-γ-induced protein-10 (IP-10/CXCL10) or monocyte chemotactive protein-1 (MCP-1/CCL2) reduced Th1/IFN-γ responses and myocarditis but had no significant effect on cardiac viral replication [45**,46]. These findings suggest that type I IFNs like IFN-α/β may be more important in preventing viral replication, at least in CVB3-only models, while IFN-γ increases inflammation. Interestingly, investigators using the Nancy strain of CVB3 observed 100% survival at day 7 pi in WT mice compared to around 20–30% survival using other CVB3 strains (25**,40**,43,44,45**,46). Although TLR3 and TRIF deficient mice develop increased myocarditis and cardiac viral replication, only TRIF deficiency results in severe chronic myocarditis/DCM using the hybrid-CVB3 model [25**]. TRIF deficiency decreases IFN-β levels in the heart but not IFN-γ in contrast to TLR3 deficiency, which decreases IFN-γ but not IFN-β. This results in an IL-4-mediated Th2-driven response in TLR3 deficient mice and an IL-33-mediated Th2-driven response in TRIF deficient mice [25**]. An IL-33-skewed response causes more severe cardiac dysfunction than an IL-4 response, yet a Th2-predominant IL-4 or IL-33 response reverses resistance to DCM in C57BL/6 mice [25**]. Th2 cytokines are critical for the development of DCM because of their role in cardiac remodeling. These findings indicate that in the hybrid-CVB3 model both IFN-β and IFN-γ prevent viral replication. In all models, Th1-type immune responses protect against CVB3 myocarditis by reducing viral replication and inhibiting Th2 responses. Interestingly, the protective effect of mesenchymal stem cell (MSC) treatment on CVB3 myocarditis was found to require IFN-γ [47,48**], providing further evidence of a protective role for Th1 responses in myocarditis.
Th2 response
Although Th1 responses protect against viral replication and prevent chronic myocarditis/DCM, they increase acute inflammation- particularly in males [16,19,28,29]. Th2 responses, as transcriptional regulators of IFN-γ, inhibit acute inflammation. Likewise, Treg and IL-10 reduce CVB3 myocarditis [16,30**]. IFN-γ-producing γδT cells, important drivers of disease in CVB3-only models of myocarditis, have been found to inhibit Treg [30**]. Histamine receptor-1, important for mast cell activation, was shown to be protective in this myocarditis model by decreasing pathogenic Vγ4+ γδT cells and Th1 responses in the heart [49**]. In contrast, MSC-type cells inhibit myocarditis by increasing Treg, IL-10 and IFN-γ [47,48**]. Induction of tolerance by nasal cardiac myosin peptide treatment of mice with CVB3 myocarditis reduced disease by elevating IL-10 and Treg [50]. Administration of recombinant galectin-9, a ligand for the inhibitory receptor Tim-3 [16,51], was found to inhibit acute CVB3 myocarditis by increasing IL-4/Th2 cells, Tim-3+Gr1+CD11b+ macrophages and IL-10 in the heart [52**]. And finally, administration of astragaloside IV (obtained from the therapeutic root Astragalus membranaceus) to mice with CVB3 myocarditis was found to reduce chronic myocarditis and DCM by downregulating TGF-β1 and Smad signaling [53]. In summary, researchers have recently identified several agents/pathways that reduce acute CVB3 myocarditis by increasing Th2 and/or regulatory responses, such as nasal administration of cardiac myosin, histamine receptor-1 signaling, galectin-9 and astragaloside IV, but more research is needed to determine whether increasing these Th2-like responses protects or contributes to chronic myocarditis/DCM. If these Th2-type responses activate cardiac remodeling genes during acute CVB3 myocarditis, there is evidence that these gene changes could promote progression to chronic myocarditis and DCM (Table 2) [25**,36*].
Th17 response
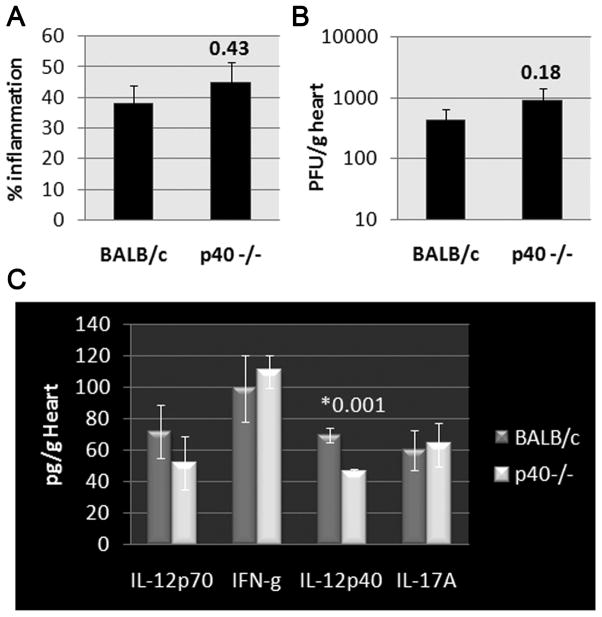
In the past few years the role of IL-17 (i.e. IL-17A) in the pathogenesis of CVB3 myocarditis has been more clearly elucidated. IL-17 levels increase in the circulation and heart during the peak of CVB3 myocarditis (day 7–10 pi) [54,55]. Recently, IL-23 and STAT3, both important in IL-17 responses, as well as IL-17 were found to be significantly elevated in the heart during acute and chronic CVB3 myocarditis (day 7 to 42 pi) [56], suggesting a role for IL-17 in mediating disease. Th17-producing cells are also elevated in the spleen during CVB3 myocarditis (day 7 to 42 pi) compared to controls, representing around 5–14% of splenic CD4+T cells [57,58]. Blocking IL-17 using neutralizing antibody in CVB3-only models improved survival, reduced acute myocarditis and viral replication in the heart, and increased COX-2, prostaglandin E2 and Treg [58–60]. In the hybrid-CVB3 model, treatment of mice for 2 weeks with low-dose inorganic mercury prior to administering CVB3/heart proteins did not alter the severity of acute myocarditis, but increased chronic fibrosis and DCM [61*]. This response was associated with elevated cardiac IL-17 levels during acute CVB3 myocarditis. Similar to this study, elevated IL-17 during the acute phase of myocarditis has been shown to increase chronic fibrosis and DCM in the EAM model [22]. Interestingly, blocking the tissue damage-associated protein high-mobility group box 1 (HMGB1) reduced EAM and suppressed Th17 responses [62], suggesting the possibility that damaged heart proteins released during viral infection may increase IL-17 levels in the heart. Recently, we examined whether IL-23, a cytokine important in promoting IL-17-mediated immunity, was required for acute inflammation using the hybrid-CVB3 model. We found that acute myocarditis was not altered in IL-12p40/IL-23 deficient mice (Figure 1). Viral replication and Th1/Th17-related cytokines were also unchanged in knockout mice compared to WT controls (Figure 1), demonstrating that IL-23 is not critical for the induction of acute myocarditis in the hybrid-CVB3 model. More research is needed to examine the role of IL-23/IL-17 in the progression to chronic myocarditis/DCM using the various CVB3 models.
Figure 1. Role of IL-23/p40 in CVB3 myocarditis.
Male WT BALB/c and IL-12p40 deficient (p40−/−) mice were inoculated ip with 103 PFU of heart-passaged CVB3+heart proteins on day 0 and A) myocarditis, B) viral replication, and C) cytokines assessed at day 10 pi. IL-23/p40 deficiency did not alter myocarditis, viral replication or cytokine levels in the heart, except for reducing IL-12/IL-23p40 levels. Data show the mean ±SEM from one of three experiments using 7–10 mice/group. P values were evaluated using the Student’s t and/or Mann-Whitney rank tests.
CONCLUSION
In this review we have examined recent data on the role of Th responses on the pathogenesis of CVB3 myocarditis. Th1 responses increase acute inflammation, yet this response reduces viral replication and protects against CVB3 myocarditis by increasing survival (in CVB3-only models) and by preventing progression to DCM (in the hybrid-CVB3 model). In contrast, Th2 responses reduce acute myocarditis by elevating regulatory T cells and anti-inflammatory cytokines, but can be detrimental by inducing acute cardiac remodeling leading to chronic myocarditis/DCM. If the immune response is deviated toward a Th2 response in resistant strains of mice, as occurs in TLR3 and TRIF deficient mice, resistance is overcome leading to DCM and heart failure. Th17 responses are also able to increase acute CVB3 myocarditis, but unlike Th1 responses, Th17 immunity contributes to cardiac remodeling leading to DCM and heart failure.
KEY POINTS.
Myocarditis is an autoimmune disease that leads to DCM in susceptible individuals and mice
Viral infections like CVB3 are the most commonly identified cause of myocarditis in developed countries and induce myocarditis and DCM in animal models
Th1-type immune responses reduce CVB3 myocarditis and DCM by inhibiting viral replication and preventing Th2 responses
Th2 responses reduce CVB3 myocarditis by inhibiting inflammation, but can promote progression to DCM by stimulating cardiac remodeling
Th17 responses promote DCM and cardiac remodeling
Acknowledgments
Funding sources: Funding for the work described in this article was obtained from the National Institutes of Health (NIH) grant R01 HL087033 to Dr. Fairweather.
Footnotes
Conflicts of interest
Authors have nothing to disclose.
REFERENCES AND RECOMMENDED READING
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;104:276–308. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wexler RK, Elton T, Pleister A, Feldman D. Cardiomyopathy: an overview. Am Fam Physician. 2009;79:778–784. [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Markham DW, Drazner MH, Mammen PPA. Fulminant myocarditis. Nat Clin Practice. 2008;5:693–706. doi: 10.1038/ncpcardio1331. [DOI] [PubMed] [Google Scholar]
- 5.Schultheiss HP, Kuhl U, Cooper LT. The management of myocarditis. Eur Heart J. 2011;32:2616–2625. doi: 10.1093/eurheartj/ehr165. [DOI] [PubMed] [Google Scholar]
- 6**.Schultheiss HP, Kuhl U. Why is diagnosis of infectious myocarditis such a challenge? Expert Rev Anti Infect Ther. 2011;9:1093–1095. doi: 10.1586/eri.11.135. An important review describing challenges currently faced by clinicians in diagnosing myocarditis. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl U, Pauschinger M, Schwimmbeck PL, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y-X, da Cunha V, Vincelette J, et al. Antiviral and myocyte protective effects of murine interferon-β and –α2 in coxsackievirus B3-induced myocarditis and epicarditis in BALB/c mice. Am J Physiol Heart Circ Physiol. 2007;293:H69–H76. doi: 10.1152/ajpheart.00154.2007. [DOI] [PubMed] [Google Scholar]
- 9.Huber SA. Autoimmunity in coxsackievirus B3 induced myocarditis. Autoimm. 2006;39:55–61. doi: 10.1080/08916930500484906. [DOI] [PubMed] [Google Scholar]
- 10.Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007;41:118–122. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis NM, Kurahara DK, Vohra H, et al. Priming the immune system for heart disease: a perspective on group A streptococci. J Infect Dis. 2010;202:1059–1067. doi: 10.1086/656214. [DOI] [PubMed] [Google Scholar]
- 12.Fairweather D, Kaya Z, Shellam GR, et al. From infection to autoimmunity. J Autoimm. 2001;16:175–186. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- 13.Gauntt C, Huber S. Coxsackievirus experimental heart diseases. Front Biosci. 2003;8:e23–e35. doi: 10.2741/928. [DOI] [PubMed] [Google Scholar]
- 14**.McNamara DM, Starling RC, Cooper LT, et al. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011;58:1112–1118. doi: 10.1016/j.jacc.2011.05.033. This is the first clinical study to show that men with myocarditis/acute idiopathic DCM have a worse outcome than women. Patients were followed for 4 years and women were found to have significantly better transplant-free survival than men. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Onyimba JA, Coronado MJ, Garton AE, et al. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol Sex Differ. 2011;2:2. doi: 10.1186/2042-6410-2-2. A surprising finding that the major gene changes during the innate immune response in the spleen following CVB3 infection in male mice include genes important in promoting cardiac inflammation, remodeling and heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frisancho-Kiss S, Davis SE, Nyland JF, et al. Cutting Edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 17.Huber SA, Job LP. Cellular immune mechanisms in coxsackievirus group B, type 3 induced myocarditis in BALB/c mice. Adv Exp Med Biol. 1983;161:491–508. doi: 10.1007/978-1-4684-4472-8_29. [DOI] [PubMed] [Google Scholar]
- 18.Cihakova D, Barin JG, Afanasyeva M, et al. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am J Pathol. 2008;172:1195–1208. doi: 10.2353/ajpath.2008.070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber SA, Sartini D, Exley M. Vgamma4(+) T cells promote autoimmune CD8(+) cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4(+) Th1 cells. J Virol. 2002;76:10785–10790. doi: 10.1128/JVI.76.21.10785-10790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Noutsias M, Rohde M, Goldner K, et al. Expression of functional T-cell markers and T-cell receptor Vbeta repertoire in endomyocardial biopsies from patients presenting with acute myocarditis and dilated cardiomyopathy. Eur J Heart Fail. 2011;13:611–618. doi: 10.1093/eurjhf/hfr014. This study examines genes that characterize T cells in myocardial biopsies from patients with myocarditis and DCM and found that Th1 and CD8+ T cells were predominant, along with other indicators of viral infection. They did not find evidence of a major role for Th17 cells. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J, Cao AL, Yu M, et al. Th17 cells facilitate the humoral immune response in patients with acute viral myocarditis. J Clin Immunol. 2010;30:226–234. doi: 10.1007/s10875-009-9355-z. [DOI] [PubMed] [Google Scholar]
- 22.Baldeviano GC, Barin JG, Talor MV, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 23.Afanasyeva M, Wang Y, Kaya Z, et al. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am J Pathol. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. IFN-γ protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Abston ED, Coronado MJ, Bucek A, et al. Th2 regulation of viral myocarditis in mice: different roles for TLR3 vs. TRIF in progression to chronic disease. Clin Dev Immunol. 2012;2012:129486. doi: 10.1155/2012/129486. This study in mice shows for the first time that Th2-skewed responses overcome resistance to DCM following CVB3 myocarditis and characterize differences in IL-4 vs. IL-33-driven pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodruff JF, Woodruff JJ. Involvement of T lymphocytes in the pathogenesis of coxsackievirus B3 heart disease. J Immunol. 1974;113:1726–1734. [PubMed] [Google Scholar]
- 27.Huber SA, Job LP, Woodruff JF. Sex-related differences in the pattern of coxsackievirus B3-induced immune spleen cell cytotoxicity against virus-infected myofibers. Infect Immun. 1981;32:68–73. doi: 10.1128/iai.32.1.68-73.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Med Microbiol Immunol. 2005;194:121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- 29.Li K, Xu W, Guo Q, et al. Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ Res. 2009;105:353–364. doi: 10.1161/CIRCRESAHA.109.195230. [DOI] [PubMed] [Google Scholar]
- 30**.Liu W, Huber S. Cross-talk between CD1d-restricted NKT cells and γδ cells in T regulatory cell response. Virol J. 2011;8:32. doi: 10.1186/1743-422X-8-32. This review article highlights recent findings that distinguish the role of NKT vs. γδT cells in regulating myocardial inflammation via their influence on regulatory T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cihakova D, Sharma R, Fairweather D, Afanasyeva M, Rose NR. Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol Med Autoimmunity: Methods and Protocols. 2004;102:175–194. doi: 10.1385/1-59259-805-6:175. [DOI] [PubMed] [Google Scholar]
- 32**.Barin JG, Rose NR, Cihakova D. Macrophage diversity in cardiac inflammation: a review. Immunobiol. 2011 Jun 30; doi: 10.1016/j.imbio.2011.06.009. Epub ahead of print. This is a comprehensive review article describing macrophage diversity and the role of macrophages in the pathogenesis of myocarditis and DCM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Rose NR. Critical cytokine pathways to cardiac inflammation. J Interferon Cytokine Res. 2011;31:705–710. doi: 10.1089/jir.2011.0057. This review provides a broad historical survey of the discovery of the role for key cytokines in the pathogenesis of disease, bringing us to our current understanding of the role of Th1, Th2 and Th17 cells in disease progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afanasyeva M, Georgakopoulos D, Belardi DF, et al. Impaired up-regulation of CD25 on CD4+ T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc Natl Acad Sci USA. 2004;102:180–185. doi: 10.1073/pnas.0408241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afanasyeva M, Georgakopoulos D, Fairweather D, et al. A novel model of constrictive pericarditis associated with autoimmune heart disease in interferon-γ knockout mice. Circulation. 2004;110:2910–2917. doi: 10.1161/01.CIR.0000147538.92263.3A. [DOI] [PubMed] [Google Scholar]
- 36*.Coronado MJ, Brandt JE, Kim E, et al. Testosterone and interleukin-1β increase cardiac remodeling during acute coxsackievirus B3 myocarditis via serpin A 3n. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00783.2011. This study in mice shows that remodeling changes that occur during acute CVB3 myocarditis are important for progression to DCM, and that testosterone is driving those changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Yajima T. Viral myocarditis: potential defense mechanisms within the cardiomyocyte against virus infection. Future Microbiol. 2011;6:551–566. doi: 10.2217/fmb.11.40. A comprehensive review on the role of innate defense mechanisms against CVB3 infection and the consequences for myocarditis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Marchant DJ, Boyd JH, Lin DC, et al. Inflammation in myocardial diseases. Circ Res. 2012;110:126–144. doi: 10.1161/CIRCRESAHA.111.243170. An excellent and thorough review of the role of innate factors and Th responses in the pathogenesis of various inflammatory heart diseases including myocarditis, DCM and cardiac allograft rejection. [DOI] [PubMed] [Google Scholar]
- 39*.Wiltshire S, Leiva-Torres G, Vidal S. Quantitative trait locus analysis, pathway analysis, and consomic mapping show genetic variants of Tnni3k, Fpgt, or H28 control susceptibility to viral myocarditis. J Immunol. 2011;186:6398–6405. doi: 10.4049/jimmunol.1100159. Identification of a gene conferring susceptibility to CVB3 infection and comparing susceptible A/J to resistant B.10 or C57BL mice. [DOI] [PubMed] [Google Scholar]
- 40**.Riad A, Westermann D, Zietsch C, et al. TRIF is a critical survival factor in viral cardiomyopathy. J Immunol. 2011;186:2561–2570. doi: 10.4049/jimmunol.1002029. The first report of the important role for TRIF signaling in protecting against heart failure following CVB3 myocarditis by regulating IFN-β levels following infection. [DOI] [PubMed] [Google Scholar]
- 41.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-γ and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 42.Burke J, Sonenberg N, Platanias L, Fish E. Antiviral effects of interferon-β are enhanced in the absence of the translational suppressor 4E-BP1 in myocarditis induced by coxackievirus B3. Antivir Ther. 2011;16:577–584. doi: 10.3851/IMP1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Z, Desai M, Philip J, et al. Conditional transgenic expression of TIR-domain-containing adaptor-inducing interferon-β (TRIF) in the adult mouse heart is protective in acute viral myocarditis. Basic Res Cardiol. 2011;106:1159–1171. doi: 10.1007/s00395-011-0226-4. [DOI] [PubMed] [Google Scholar]
- 44.Yu Z, Huang Z, Shao C, et al. Oral administration of interferon-α2b-transformed Bifidobacterium longum protects BALB/c mice against coxsackievirus B3-induced myocarditis. Virol Journal. 2011;8:525. doi: 10.1186/1743-422X-8-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Yue Y, Gui J, Ai W, et al. Direct gene transfer with IP-10 mutant ameliorates mouse CVB3-induced myocarditis by blunting Th1 immune responses. PLoS ONE. 2011;6:e18186. doi: 10.1371/journal.pone.0018186. Reports that the chemokine interferon-inducible protein 10 (IP-10) reduces myocardial inflammation without affecting viral replication by reducing a Th1 response, providing further evidence of the importance of Th1 responses in increasing acute inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yue Y, Gui J, Xu W, Xiong S. Gene therapy with CCL2 (MCP-1) mutant protects CVB3-induced myocarditis by compromising Th1 polarization. Mol Immunol. 2011;48:706–713. doi: 10.1016/j.molimm.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Miteva K, Haag M, Peng J, et al. Human cardiac-derived adherent proliferating cells reduce murine acute coxsackievirus B3-induced myocarditis. PLoS ONE. 2011;6:e28513. doi: 10.1371/journal.pone.0028513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Van Linthout S, Savvatis K, Miteva K, et al. Mesenchymal stem cells improve murine acute coxsackievirus B3-induced myocarditis. Eur Heart J. 2011;32:2168–2178. doi: 10.1093/eurheartj/ehq467. An important study demonstrating that administration of mesenchymal stem cells to mice with CVB3 myocarditis was able to improve cardiac function and required IFN-γ to exert their protective effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Case L, Moussawi M, Roberts B, et al. Histamine H(1) receptor signaling regulates effector T cell responses and susceptibility to coxsackievirus B3-induced myocarditis. Cell Immunol. 2012;272:269–274. doi: 10.1016/j.cellimm.2011.10.004. This study using histamine receptor-1 deficient mice showed that this receptor protects mice from CVB3 myocarditis by inhibiting Th1 and γδT cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fousteri G, Dave A, Morin B, et al. Nasal cardiac myosin peptide treatment and OX40 blockade protect mice from acute and chronic virally-induced myocarditis. J Autoimm. 2011;36:210–220. doi: 10.1016/j.jaut.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frisancho-Kiss S, Nyland JF, Davis SE, et al. Cutting Edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J Immunol. 2006;176:6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 52**.Lv K, Xu W, Wang C, et al. Galectin-9 administration ameliorates CVB3 induced myocarditis by promoting the proliferation of regulatory T cells and alternatively activated Th2 cells. Clin Immunol. 2011;140:92–101. doi: 10.1016/j.clim.2011.03.017. Galectin-9, a ligand for the inhibitory receptor Tim-3, treatment of mice with CVB3 myocarditis was found to reduce inflammation in the heart by increasing regulatory T cell populations that express Tim-3 and IL-4-secreting Th2 cells. [DOI] [PubMed] [Google Scholar]
- 53.Chen P, Xie Y, Shen E, et al. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol. 2011;658:168–174. doi: 10.1016/j.ejphar.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 54.Yuan J, Yu M, Lin QW, et al. Neutralization of IL-17 inhibits the production of anti-ANT autoantibodies in CVB3-induced acute viral myocarditis. Int Immunopharmacol. 2010;10:272–276. doi: 10.1016/j.intimp.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Yuan J, Yu M, Lin QW, et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol. 2010;185:4004–4010. doi: 10.4049/jimmunol.1001718. [DOI] [PubMed] [Google Scholar]
- 56.Yang F, Wu W-F, Yan Y-L, et al. Expression of IL-23/Th17 pathway in a murine model of coxsackie virus B3-induced viral myocarditis. Virology Journal. 2011;8:301. doi: 10.1186/1743-422X-8-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qing K, Weifeng W, Fan Y, et al. Distinct different expression of Th17 and Th9 cells in coxsackievirus B3-induced mice viral myocarditis. Virology Journal. 2011;8:267. doi: 10.1186/1743-422X-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie Y, Chen R, Zhang X, et al. The role of Th17 cells and regulatory T cells in coxsackievirus B3-induced myocarditis. Virology. 2011;421:78–84. doi: 10.1016/j.virol.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Xie Y, Chen R, Zhang X, et al. Blockade of interleukin-17A protects against coxsackievirus B3-induced myocarditis by increasing COX-2/PGE2 production in the heart. FEMS Immunol Med Microbiol. 2011 Dec 5; doi: 10.1111/j.1574-695X.2011.00918.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60.Fan Y, Weifeng W, Yuluan Y, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of coxsackievirus B3-induced viral myocarditis reduces myocardium inflammation. Virology Journal. 2011;8:17. doi: 10.1186/1743-422X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Nyland JF, Fairweather D, Shirley DL, et al. Low dose inorganic mercury increases severity and frequency of chronic coxsackievirus-induced autoimmune myocarditis in mice. Toxicol Sci. 2011;125:134–143. doi: 10.1093/toxsci/kfr264. The first study to show that low-dose mercury can increase DCM by altering cardiac remodeling and Th17 responses following CVB3 myocarditis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su Z, Sun C, Zhou C, et al. HMGB 1 blockade attenuates experimental autoimmune myocarditis possibly by suppressing Th17-cell expansion. Eur J Immunol. 2011;9:3586–3595. doi: 10.1002/eji.201141879. [DOI] [PubMed] [Google Scholar]