Abstract
The development and exacerbation of depression and anxiety are associated with exposure to repeated psychosocial stress. Stress is known to affect the bidirectional communication between the nervous and immune systems leading to elevated levels of stress mediators including glucocorticoids (GCs) and catecholamines and increased trafficking of proinflammatory immune cells. Animal models, like the repeated social defeat (RSD) paradigm, were developed to explore this connection between stress and affective disorders. RSD induces activation of the sympathetic nervous system (SNS) and hypothalamic-pituitary (HPA) axis activation, increases bone marrow production and egress of primed, GC-insensitive monocytes, and stimulates the trafficking of these cells to tissues including the spleen, lung, and brain. Recently, the observation that these monocytes have the ability to traffic to the brain perivascular spaces and parenchyma have provided mechanisms by which these peripheral cells may contribute to the prolonged anxiety-like behavior associated with RSD. The data that have been amassed from the RSD paradigm and others recapitulate many of the behavioral and immunological phenotypes associated with human anxiety disorders and may serve to elucidate potential avenues of treatment for these disorders. Here, we will discuss novel and key data that will present an overview of the neuroendocrine, immunological and behavioral responses to social stressors.
Keywords: repeated social defeat, GC-insensitive monocytes, macrophages, cell trafficking, sensitization, inflammation, microglia, anxiety-like behavior
1. Introduction
Although the biological mechanisms are not fully understood, the individual response to prolonged or severe stressors contributes to the development and exacerbation of depression and anxiety (Maes et al., 1998; Kalynchuk et al., 2004; Raison et al., 2006; Norman et al., 2010; Capuron & Miller, 2011; Gilman et al., 2013). One potential contributor to the etiology of stress-related mental health disorders involves the bidirectional communication between the immune system and the central nervous system (CNS) (Miller et a., 2009). In humans, the experience of chronic stress is associated with proinflammatory leukocytic phenotypes that are unresponsive to the anti-inflammatory actions of glucocorticoids (GCs) (Cohen et al., 2012) and a transcriptional profile that is consistent with the expansion and priming of myeloid-derived cells (Miller et al., 2008; Powell et al., 2013). The mechanistic association between inflammation and depression is particularly well-established (Raison et al., 2006; Dantzer et al., 2008; Miller et al., 2009; Norman et al., 2010; Capuron & Miller, 2011), while the case continues to build for the mechanistic association between inflammation and anxiety (Maes et al., 1998; Pitsavos et al., 2006; O'Donovan et al., 2010; Pace & Heim, 2012).
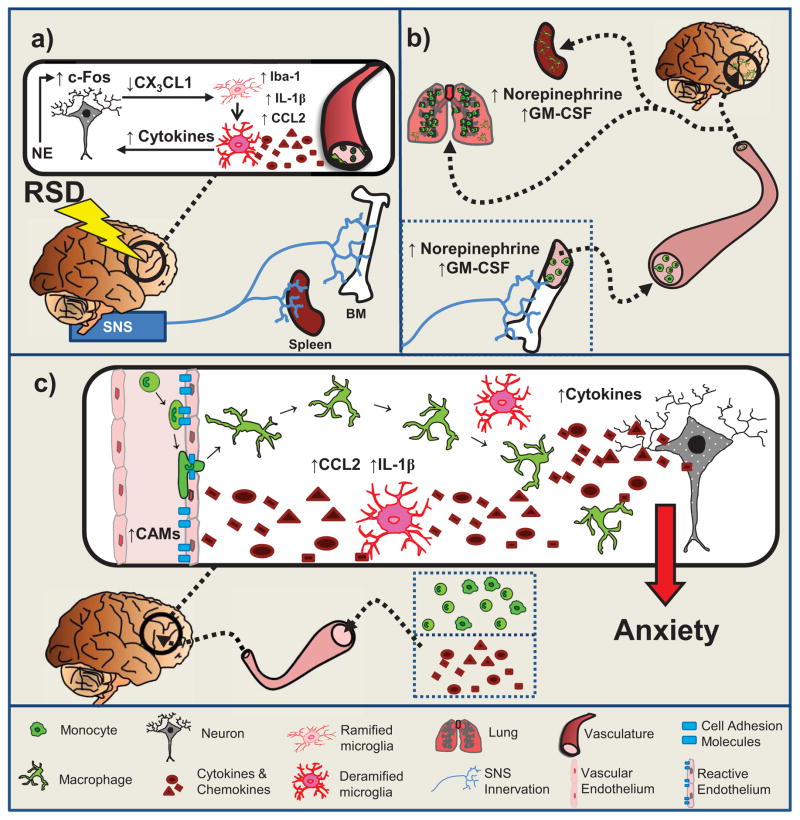
The murine repeated social defeat (RSD) paradigm recapitulates many key immunological and behavioral features associated with psychosocial stress in humans. In the bone marrow, RSD increases production, egress, and trafficking of proinflammatory immune cells that are insensitive to GCs (Avitsur et al., 2002; Engler et al., 2004; Engler et al., 2005; Kinsey et al., 2007; Hanke et al., 2012; Wohleb et al., 2012) (Figure 1a) and traffic to the spleen (Engler et al., 2004), lung (Curry et al., 2010), and brain (Wohleb et al., 2013) (Figure 1b). Furthermore, data from the RSD paradigm support a conserved inflammatory transcriptional response in leukocytes that is comparable to that of chronically-stressed human populations (Powell et al., 2013).
Figure 1. Repeated Social Defeat and Anxiety.
a. Neuronal and microglia activation co-occur in brain regions associated with fear/anxiety and threat appraisal. SNS activation results in increased NE in BM, spleen, & circulation.
b. β-AR signaling promotes release of GC insensitive myeloid-derived cells that traffic to spleen, lungs, and brain and contributes to increased tissue-specific and circulating inflammatory cytokines.
c. Reactive endothelium facilitate monocyte trafficking to the perivascular and extra-vascular spaces. Brain monocyte/macrophage trafficking reinforces fear/anxiety and threat circuitry, promoting the development and maintenance of prolonged anxiety-like behavior.
RSD not only precipitates a proinflammatory environment within the periphery, but also activates brain regions associated with fear, anxiety, and threat appraisal that appear to contribute to behavioral changes. In many of these stress-responsive brain regions, neuronal activation co-occurs with neuroinflammatory events like microglial activation and recruitment of primed monocytes from the periphery (Wohleb et al., 2013). Studies suggest a connection between monocyte trafficking to the brain and RSD-induced anxiety-like behavior (Wohleb et al., 2013; Wohleb et al., 2014a). Moreover, RSD induces long-term stress-sensitization that is related to monocyte trafficking from the spleen to the brain (Wohleb et al., 2014a). In this review, we will introduce the concept of social defeat stress and provide an overview of studies that have used the RSD paradigm to elucidate the peripheral and central effects of social stress. Finally, we will discuss monocyte trafficking to the CNS and its relationship to anxiety-like behavior.
1.1 Stress and Inflammation
Inflammation is an adaptive biological response to tissue injury and other immune challenges and is a key indicator of mental and physical disease. Inflammation is characterized, in part, by an increase in primed and activated immune cells and the subsequent release of proinflammatory immune products (Black & Garbutt, 2002). In particular, stress majorly impacts the expression of cytokine and chemokine genes that are instrumental in mobilizing immune cells to aid in the resolution of an insult and restoration of tissue function (Black & Garbutt, 2002). In response to stress, a milieu of hormones, peptides, and neurotransmitters are released by the nervous and endocrine systems that pleiotropically function to modulate the immune system. Specifically, the response to stress activates the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS) subsequently releasing GCs and catecholamines, respectively. Depending on the nature, intensity, and duration of the stressor and the type of immune challenge, stress effects on the immune system differ. On one hand, chronic, unrelenting stress (e.g., prolonged restraint stress) is able to induce immunosuppression and cause a skew towards an anti-inflammatory immune cell phenotype that increases susceptibility to disease (Cohen et al., 2012). On the other hand, bouts of repeated defeat stress (e.g., RSD) can induce immunoenhancement and cause a skew towards a proinflammatory phenotype by inducing a state of GC-insensitivity to occur in innate immune cells; this insensitivity prevents the GC-induced suppression of inflammation through immune cell apoptosis and inhibition of nuclear factor κ B (NFκB) pathways (Chrousos et al., 1996; Barnes & Adcock, 2009). This enhanced inflammation can be described as a double-edged sword. In the short-term, inflammation beneficially eradicates the immune challenge, but, over time, inflammation can worsen tissue damage and negatively impact disease outcome. Associated with the experience of social stress, it is this prolonged inflammatory state that has the potential to contribute to the etiology of depression and anxiety (Meduri & Yates, 2004; Barnes & Adcock, 2009).
1.2 Social Stressors
Numerous studies indicate that social status modulates the immunological and physiological responses to stress. Some of the first studies in this field examined subordinate non-human primates, who, in response to social reorganization (i.e., a shift in social hierarchy) had reduced body weights, higher levels of circulating GCs (i.e., cortisol), and increased susceptibility to upper respiratory infections (Cohen et al., 1997; Sapolsky, 2005). In humans, longitudinal studies of social defeat involving bullying or loss of social status in the workplace have demonstrated increased incidence of depression, anxiety, loss of self-esteem, increased incidence of illness, and other aberrant physiological and behavioral symptoms (Marmot & Feeney, 1997; Griffin et al., 2002; Stansfeld et al., 2003; Bilgel et al., 2006). The commonality and grave impact of social stress on humans led to the development of several animal models.
In the laboratory, murine social stress is modeled using resident-intruder paradigms whereby the response to social stress involves protecting the resources of the cage from intruders (Tamashiro et al., 2005). Competition for access to resources causes the formation of social hierarchies within the cage consisting of a dominant alpha, co-dominants, and subordinates (Ginsburg and Allee, 1975). These hierarchies are disrupted when an intruder vies to enhance social status by challenging and defeating the dominant alpha male (Ginsburg and Allee, 1975). Social status changes among cage mates often involve episodes of physical defeat occurring through the expression of aggression and acceptance of subordination. This disruption is not only psychologically stressful for the mice involved, but also entails a physical component whereby there is a risk of injury. Defeated subordinate animals receive bite wounds and experience pain that is not life-threatening, but contributes to significant changes in the immune system, cellular activation in the CNS, and expression of prolonged anxiety-like behavior (Bailey et al., 2004).
1.3 Mouse Model of Social Stress: Repeated Social Defeat Paradigm (RSD)
RSD is an ethologically-relevant resident-intruder paradigm involving intermale aggression and loss of social status by the disruption of an established social hierarchy (Avitsur et al., 2001; Stark et al., 2001). Around the beginning of the active cycle (17:00), an aggressive mouse is introduced into a cage of resident mice that have an established social hierarchy. Aggressor mice are older, larger, and, due to retired breeder status, have had previous social experience with being a sole dominant in a cage.
Within minutes of introduction, the aggressive intruder engages the residents in brief, yet consistent, attacks for the length of the 2 h cycle. During these attacks, the residents display classic behavioral signs of fear and submissiveness that are dependent on social status (Blanchard & Blanchard, 1977; Avitsur et al., 2002; Avitsur et al., 2007).During these attacks, the residents display classic behavioral signs of fear and submissiveness that are dependent on social status (Blanchard & Blanchard, 1977; Avitsur et al., 2002; Avitsur et al., 2007). For instance, the threatened resident mouse, especially if it is a dominant alpha or co-dominant, may choose to fight with the intruder in an effort to reestablish dominance over the territory and resources or, alternatively, the resident mouse may attempt to flee (Avitsur et al., 2007). By the end of 6 cycles of RSD, the ultimate behavior displayed by the defeated residents is subordination, which involves huddling in a corner and, upon approach, rearing on hind legs to bare their ventral body surface to the aggressor (Blanchard & Blanchard, 1977; Avitsur et al., 2007). In all, this unique paradigm of social stress has elucidated many mechanisms regarding the effects of stress on the peripheral immune system and inflammatory processes.
2. Peripheral Effects of Repeated Social Defeat Stress
RSD causes gross anatomical changes to peripheral organs that are indicative of substantial immune and endocrine alterations (e.g., thymic involution, adrenal hypertrophy, and splenomegaly) (Engler et al., 2005). Data generated over the last decade has yielded insight into the tissue-specific production, egress, and trafficking of bone marrow-derived, GC-insensitive monocytes/macrophages in mice that have undergone RSD treatment. These cells have a unique phenotype in that they are not only GC-insensitive, but also possess a primed and activated phenotype, i.e., they have elevated levels of Toll-like receptors (TLRs), adhesion molecules, and co-stimulatory receptors on their surface (Avitsur et al., 2003; Engler et al., 2004; Bailey et al., 2007; Powell et al., 2009). When stimulated, these cells produce high levels of proinflammatory cytokines and chemokines, thus promoting an immunoenhancive environment within the periphery.
Additionally, RSD induces the increase of plasma and tissue catecholamines (norepinephrine and epinephrine) and plasma GCs (corticoisterone) (Avitsur et al., 2001; Hanke et al., 2012). RSD-induced levels of GCs increase as the cycle number increases indicating that habituation to RSD as a stressor does not occur (Avitsur et al., 2001; Stark et al., 2001; Bailey et al., 2004; Engler et al., 2005). Furthermore, there is no break in the circadian rhythm of the RSD-induced catecholamine or GC responses. The morning after the last RSD cycle, catecholamine and GC levels return to baseline (Avitsur et al., 2002; Hanke et al., 2012). Additionally, in vivo dexamethasone suppression tests indicate that RSD mice maintain a normal GC negative feedback loop as dexamethasone equivalently suppresses plasma GC levels in RSD and control mice (Avitsur et al., 2002). Overall, the peripheral immunological and endocrine effects of RSD are tissue-specific and many significant changes are seen in the spleen, bone marrow, and circulation.
2.1 Spleen
Six cycles of RSD results in splenomegaly, a hallmark characteristic of the RSD response, which causes a near doubling of the spleen mass (Avitsur et al., 2002). Cycle-dependent increases in spleen mass correspond with increasing accumulation of CD11b+ monocytes and granulocytes (Avitsur et al., 2002). CD11b is a cell adhesion molecule expressed on both granulocytes and monoctyes and is considered to be an indicator of activation. In vitro, monocytes from RSD spleens have enhanced cell viability in the presence of lipopolysaccharides (LPS) and varying concentrations of corticosterone. GC-insensitive monocytes also have enhanced IL-6 responses in the presence of LPS and GCs that is initiated somewhere between 3 and 6 cycles of RSD and persists for at least 10 days past the termination of the stressor (Avitsur et al., 2002). While GCs normally reduce cell viability and suppress the production of proinflammatory cytokines in response to stress, RSD-treated splenocytes are unresponsive to GCs. This phenomenon of GC-insensitivity is also tissue-specific. For example, monocytes from the spleens of RSD mice are GC-insensitive, while peritoneal monocytes from RSD mice are not (Avitsur et al., 2002). Data indicate that CD11b+ monocytes derived from the bone marrow as a consequence of the stressor are responsible for this phenomenon of GC-insensitivity. When CD19+ B-cells and CD11b+ macrophages were selectively depleted from splenocyte cultures, it was the depletion of CD11b+ cells, but not CD19+ cells, which abolished the RSD-induced GC-insensitivity and cytokine responses (Stark et al., 2001). It is important to note that not all RSD mice develop this state of GC-insensitivity in the spleen; it is most robust in mice that exhibited more subordinate behaviors during the stress treatment (Avitsur et al., 2007).
GC-insensitivity presents in diseases and disorders that include asthma, infection, depression, and anxiety. It is possible that cytokines or neuroendocrine mediators induce GC-insensitivity by altering the number or function of GC receptors (Kino et al., 2003). Quan et al., (2003) examined the molecular mechanisms of GC-insensitivity in RSD splenocytes and found that the GC receptor in RSD splenocytes (CD11b+ cells) did not have the ability to suppress NFκB activity due to a failure of the GC receptor to translocate to the nucleus (Quan et al., 2003). This change of GC receptor function could not be detected at the pre-translational level suggesting this change is not permanent (Quan et al., 2003). A previous study demonstrated that GC sensitivity was recovered 30 days after the termination of RSD (Avitsur et al., 2002). Overall, RSD-induced GC-insensitivity in the presence of LPS is, at least in part, due to the inability of the GC receptor to translocate to the nucleus and interact with NFκB.
The question was raised about the origin of the increased number of GC-insensitive CD11b+ leukocytes in the RSD spleen. One hypothesisis that the repeated activation of neuroendocrine systems caused the mobilization of neutrophils and monocytes from the bone marrow to the spleen (Figure 1b). The murine spleen has low myelopoietic activity and most of the granulocytes and monocytes that circulate come from the bone marrow (Ohno et al., 1993). It is well known that stress and severe infection causes a rapid release of neutrophils and monocytes into circulation referred to as neutrophilia and monocytosis, respectively subsequently leading to the increased production of these cells in the bone marrow (Stefanski & Engler, 1998; Stefanski, 2000; Powell et al., 2013). Therefore, attention turned from RSD effects on the spleen toward examination RSD effects on the bone marrow.
2.2 Bone Marrow
The bone marrow is the site from which all circulating blood cells are generated. These cells originate from a common hematopoietic stem cell that commits to and differentiates along specific cell lineages (e.g., erythrocytic, granulocytic, monocytic, or lymphocytic) (Dorshkind, 1990). Stromal cells and macrophages produce colony stimulating factors like granulocyte macrophage colony stimulating factor (GM-CSF) and macrophage colony stimulating factor (M-CSF) that induce the proliferation and maturation of immature bone marrow cells (Lieschke & Burgess, 1992). The stromal cells are organized into hematopoietic microenvironments that are composed of nutrient arteries, capillaries, and emissary veins (Dorshkind, 1990). Influx and efflux of cells and mediators to and from the bone marrow affects the differentiation and release of immune cells (Dorshkind, 1990). In the case of the stress response, activation of the HPA axis and the SNS and the subsequent release of GCs and catecholamines alter the function, timing of the release, and migration of these bone marrow cells (Engler et al., 2004; Engler et al., 2005).
Repeated exposure to RSD is associated with increased cell mobilization and myelopoiesis in the bone marrow and an accumulation of neutrophils and monocytes in circulation and in the spleen (Engler et al., 2004). Furthermore, RSD treatment is associated with the depletion of B-cells and erythrocytes in the bone marrow and blood (Engler et al., 2004). This decrease in cellularity could be due to the continued GC- sensitivity of these cells and resultant GC-induced apoptosis. Interestingly, bone marrow cells from control mice are GC-insensitive, but, after 6 cycles of RSD, these cells disappear from the bone marrow and the remaining cells are GC-sensitive (Engler et al., 2005). This is the opposite for the spleen whose cells, at the start of RSD, begin GC- sensitive and become insensitive after RSD. Data suggest that the unique RSD-induced microenvironment of the bone marrow produces an increase in primed, activated and GC insensitive immune cells. By the 6th cycle of RSD, continued trafficking of immune cells from the bone marrow causes not only a depletion in mature cells, but also the primed, activated, GC insensitive cells as they have been mobilized to organs such as the spleen and brain.
The enhanced myelopoiesis observed in the bone marrow of RSD mice is likely due to combination of increased production of GM-CSF leading to myeloid-derived cell survival in the periphery (Figure 1b). These colony stimulating factors likely act as chemotactic signals for circulating myeloid-derived cells to leave circulation and enter the spleen. GM-CSF, in particular, is a potent chemoattractant, and, by up-regulating CD11b expression, increases the adhesion of blood neutrophils and monocytes to the vascular endothelium (Socinski et al., 1988; Kirby et al., 2007). As the number of RSD cycles increases, GM-CSF expression, but not M-CSF, is increased in the bone marrow (Engler et al., 2005) (Figure 1b). In the spleen, only after the 2nd cycle does GM-CSF and M-CSF increase, and by the 6th cycle the expression returns to baseline (Figure 1b).
Finally, there is a substantial increase in the proportion of immature myeloid-derived cells (defined as CD11b+/Gr-1low) following the 4th cycle in RSD groups that corresponds to the increase in GM-CSF expression (Engler et al., 2005). Neutralizing GM-CSF with an antibody or pre-treatment with propranolol (a non-selective β-adrenergic receptor (βAR) antagonist) before each stress cycle inhibited RSD-induced increases in monocytic and granulocytic bone marrow progenitor cells (Powell et al., 2013). Overall, in the bone marrow, RSD induces GC-insensitivity in immune cells and increases the production of myeloid progenitor cells and GM-CSF, and decreases mature CD11b+ cells. These data point to the bone marrow as the most likely origin of the peripheral and central accumulation of proinflammatory GC-insensitive CD11b+ cells.
2.3 Circulation
RSD stimulates proinflammatory cytokine release into circulation. These cytokines enhance inflammation and mobilize immune cells to enter circulation and tissues. In circulation, several cytokines are increased, e.g., IL-6, TNF-α, KC, MIP-2, and MCP-1 CCL2. The production of many of these cytokines is βAR-mediated in that pretreatment with the βAR antagonist propranolol abrogates RSD-induced increases in IL-6, TNF-α, and CCL2 (Wohleb et al., 2011; Hanke et al., 2012). Additionally, RSD induces an increase in adrenocorticotropic hormone (ACTH), with a peak after the 2nd cycle (Engler et al., 2005) and cycle-dependent decreases after this peak. Conversely, levels of corticosterone begin to elevate at the 2nd cycles and peak at the 6thcycle. This inverse relationship of ACTH and GC suggests that the negative GC feedback mechanism on the pituitary gland is intact indicating that RSD does not result in a central state of GC-insensitivity (Engler et al., 2005).
2.4 IL-1R1 and βAR Signaling Mediate Peripheral RSD Responses
Through use of antagonists and knockout (KO) mice, data indicate that the immunological effects of RSD are mediated by interleukin-1 receptor type 1 (IL-1R1) and βARs. IL-1R1 is a receptor that binds to both IL-1α and IL-1β and is an important mediator involved in the inflammatory response. Comparable to wild-type mice, IL-1R1 knockout (IL-1R1KO) mice develop a stress phenotype that includes adrenal hypertrophy, thymic involution, and elevated serum corticosterone in response to RSD (Engler et al., 2008). However, unlike wild-type RSD mice, CD11b+ cells do not accumulate in spleen, blood, or bone marrow of IL-1R1KO RSD mice and splenocytes from these mice fail to develop GC-insensitivity (Engler et al., 2008). After RSD, there is an increase in IL-1 with IL-1β gene expression increased in the spleen and liver and IL-1α increased in the liver (Engler et al., 2008). Further, IL-1β, but not IL-1α, protein is elevated in the plasma (Engler et al., 2008). The lack of splenic accumulation of immune cells in IL-1R1KO mice could be one reason for the lack of GC-insensitivity in IL-1R1KO mice. Stress-associated release of immune cells from the bone marrow and signaling of IL-1 are likely important components contributing to the induction of GC-insensitivity in RSD immune cells.
In the context of stress, βAR activation appears to primarily mediate the proinflammatory actions of the high surge of stress-induced catecholamines and is able to increase the production of cytokines in immune cells (e.g., Johnson et al., 2013; Kim et al., 2013; Tan et al., 2007; Izeboud et al., 1999). When mice are pretreated with propranolol (βAR antagonist) 1 h before each cycle of RSD, the RSD-induced splenomegaly and plasma increases of IL-6, TNF-α, and CCL2 are prevented (Wohleb et al., 2011; Hanke et al., 2012). Pretreatment with propranolol did not alter GC levels indicating that blockade of βAR signaling does not affect HPA axis activation (Wohleb et al., 2011). Flow cytometric analyses revealed that propranolol prevented the accumulation of CD11b+ cells in RSD spleens and prevented the increases in splenic monocyte expression of TLR2 and 4 and CD86 following RSD (Hanke et al., 2012). TLR2 and 4 are membrane-bound receptors that induce inflammatory responses through the activation of NF-κB. TLR2 recognizes a great variety of bacterial, fungal, viral, and endogenous molecules. TLR4 recognizes components of the Gram-negative cell wall such as LPS and lipid A. CD86 is a cell surface activation marker on monocytes that works with CD80 to prime T cells and provide the costumulatory signals necessary for T cell activation and survival. Additionally, supernatants from 18 h LPS-stimulated ex vivo cultures of splenocytes from propranolol-treated RSD mice contained less IL-6 than vehicle-treated RSD mice (Hanke et al., 2012). Propranolol pretreatment in vivo, eliminated the development of GC-insensitivity of CD11b+ monocytes ex vivo when compared to splenocytes from vehicle-treated RSD mice (Hanke et al., 2012) suggesting that priming of GC-insensitive monocytes by RSD is a consequence of SNS activation. Taken together, RSD induces circulating and tissue-specific increases in catecholamines, GM-CSF, and major changes in the immune system that include increases in primed and activated cells and proinflammatory cytokines in the spleen, bone marrow, and circulation (Figure 1b).
3. RSD-Induced Behavioral and CNS Effects
While many of the previous data using the RSD paradigm had an intense focus on the stress-induced modulation of peripheral immune compartments (Avitsur et al., 2001; Bailey et al., 2004; Engler et al., 2004; Engler et al., 2008; Hanke et al., 2012), recent focus has shifted toward the effects of social defeat on behavior and the brain. These studies demonstrate powerful connections between neuroinflammatory signaling, peripheral myelopoietic activity, and anxiety-like behavior.
One response to RSD is the development of anxiety-like behavior, which is evident in tests like open field, novel object, and light/dark assays (Kinsey et al., 2007; Bailey et al., 2009; Wohleb et al., 2011; Hanke et al., 2012). It is important to note that behavioral observations in this paradigm are measured the morning after the final cycle of social defeat (around 09:00h which is ~14h post RSD) when the neuroendocrine mediators (e.g., corticosterone, epinephrine, norepinephrine, etc. ) of the arousal systems have returned to baseline (Kinsey et al., 2007; Wohleb et al., 2013; Hanke et al., 2012). Anxiety-like behavior is most robustly observed following the 6 cycles of RSD, but also occurs in a cycle-dependent manner persisting to at least 8 days past the termination of the stressor (Wohleb et al., 2013; Kinsey et al., 2007). The development of RSD-induced anxiety strongly corresponds to immunological events in the periphery. For instance, the RSD-induced establishment and resolution of GC-insensitivity (Avitsur et al., 2002) and increased myelopoiesis (Engler et al., 2004; Wohleb et al., 2013) follow similar kinetics to the development of anxiety-like behavior (Wohleb et al., 2013). These strong temporal relationships between RSD-induced peripheral immunomodulation and anxiety support the hypothesis that prolonged behavioral changes following RSD are dependent on both peripheral and neuroinflammatory signaling.
3.1 Neuronal and Microglial Activation Co-Occur in Stress-Responsive Brain Regions
Initial studies regarding RSD-induced anxiety-like behavior examined the pattern of neuronal activation in response to RSD. Neuronal c-Fos expression, as determined by immunohistochemistry, was used as an indicator of regional neuronal activation (Wohleb et al., 2011). This study revealed distinct patterns of increased neuronal c-Fos expression within specific regions of the brain. Following RSD, c-Fos+ cells were increased in the prefrontal cortex (PFC), lateral septum (LS), bed nucleus of the stria terminalis (BNST), paraventricular nucleus (PVN), and medial amygdala (Me AMYG) (Wohleb et al., 2011), which are brain regions associated with fear, anxiety, and threat appraisal (LeDoux, 2000). Interestingly, increased c-Fos expression was observed following a single cycle of RSD indicating that substantial activation of fear, anxiety, and threat appraisal pathways preceded the major immunological events associated with additional cycles of RSD (i.e., GC-insensitivity, splenomegaly, thymic involution, increases in proinflammatory cytokines occur between 3 and 6 cycles) (Wohleb et al., 2011). Furthermore, 6 cycles of RSD enhanced microglial activation as seen through Iba1 immunohistochemistry (Wohleb et al., 2011). Pretreatment with propranolol prevented both RSD-induced neuronal and microglial activation as assessed by c-Fos and Iba1 labeling (Wohleb et al., 2011). Overall, data indicate that brain region-specific neuronal activation is dependent on β-AR signaling and this activation appears to precede central and peripheral immune events associated with RSD. Thus, these data suggestthat neuronal activation is an upstream event that contributed to RSD-induced immunomodulation (Figure 1a).
Region-specific microglial and neuronal activation associated with chronic stress is observed in multiple models of repeated stress. Resembling work by Hinwood et al., (2012), neuronal activity in RSD mice was regionally associated with local alterations in microglial morphology including increased cell size, and thickening and shortening of cell processes, i.e., deramification (Wohleb et al., 2011) (Figure 1a). These microglia appear to be of an activated phenotype that are more inflammatory and phagocytic (Walker et al., 2014). Consistent with this observation, enriched microglia isolated from the brains of RSD mice have increased proinflammatory cytokine gene expression and increased protein expression of inflammatory markers (Wohleb et al., 2011) (Figure 1a). These findings also strongly resemble work by Tynan et al., (2010) that revealed region-specific microglial activation following chronic restraint stress in rats.
There is a spatial coincidence of the activation of neurons and microglia that suggests a relation between these cell types. And the temporal kinectics of activation suggests a causative role of stress-induced neuronal activity in the local activation of microglia. This interpretation is supported by the fact that neuronal activation kinetically precedes microglial activation and cytokine production (Wohleb et al., 2011; Wohleb et al., 2013) (Figure 1a). Although it is possible that these two events may act independently of each other, it is well recognized that neuronal activity can regulate local microglial activation through specific chemokine signaling pathways (Kettenmann et al., 2013). One canonical pathway involves neuronal fractalkine (CX3CL1) and microglial fractalkine receptor (CX3CR1) (Biber et al., 2007).
Under homeostatic conditions within the CNS, fractalkine is highly and primarily expressed by neurons (Bazan et al., 1997; Kim et al., 2011), while the fractalkine receptor is highly expressed by microglia (Jung et al., 2000). Fractalkine receptor stimulation of brain microglia is anti-inflammatory (Cardona et al., 2006; Corona et al., 2010) and promotes microglial quiescence under homeostatic conditions (Wolf et al., 2013). Consistent with this observation, microglial activation following RSD is associated with reduced expression of brain fractalkine (Wohleb et al., 2013) and microglial fractalkine receptor (Wohleb et al., 2014b). These findings demonstrate that reduced neuronal fractalkine expression in response to RSD contributes to increased microglial activation (Figure 1a). Neuronal fractalkine expression, or lack thereof, is involved in microglial chemotaxis and calcium regulation and plays a fundamental role within neuronal and microglial communication (Harrison et al., 1998). Our data suggest that neuronal activity may cause regional reductions in fractalkine expression that result in region-specific microglial activation (Figure 1a). This hypothesized relationship remains to be fully tested.
3.2 RSD Induces Distinct Patterns of Monocyte/Macrophage Trafficking to the Brain
Using flow cytometric assessment of CD11b+/CD45hi monocyte/macrophages, RSD was found to induce monocyte trafficking to the brain (Wohleb et al., 2011). Despite vascular perfusion, the increase in this CD11b+/CD45hi population persisted suggesting that these CD11b+/CD45hi monocyte/macrophages were perivascular or vascular-adherent monocyte/macrophages (Wohleb et al., 2012). Functional studies from other labs identified this CD11b+/CD45hi population as brain macrophages (Ford et al., 1995; Sedgwick et al., 1991). Brain macrophages can be defined as peripherally-derived monocytes that either accumulate in the perivascular space (CD11b+/CD45hi) or cross the blood brain barrier into the brain parenchyma (CD11b+/CD45lo). If these cells enter the brain parenchyma, CD45 expression is reduced and these cells morphologically and phenotypically become indistinguishable from resident microglia (Mildner et al., 2007). Based on data from flow cytometric scatter properties and Ly6C expression, the CD11b+/CD45hi brain macrophages were likely derived from circulating Ly6Chi monocytes (Wohleb et al., 2011).
These findings were further corroborated using lysozyme M-green fluorescent protein (LysM-GFP) knock-in mice (Wohleb et al., 2013). These mice express GFP under the LsyM promoter that drive myeloid-derived cells to strongly express GFP (e.g., monocytes and granulocytes) (Rieger et al., 2009). In this study, RSD induced an increase in CD11b+/CD45hi/LysM-GFP+ cells as detected by flow cytometry. Immmunofluorescent histology demonstrated increased rod/circular GFP+ cells that co-localized with the vasculature throughout the brain (Wohleb et al., 2013). It should be noted that as with CD45 expression, LysM expression is also reduced in monocytes once they extravasate into the brain parenchyma (Wohleb et al., 2013). Because GFP is driven by the LysM promoter, extravasated monocytes lose GFP expression (i.e., become GFP−) and are indistinguishable from resident microglia. Taken together, RSD robustly increased monocyte recruitment to the brain, but the specific sub-anatomical localization of these cells was not yet verified.
Monocyte trafficking to the brain is usually observed in association with substantial CNS-related tissue pathology and inflammation, e.g., multiple sclerosis, traumatic injury, stroke, Alzheimer’s disease, or infection (Hafler et al., 2005; Gate et al., 2010; Donnelly et al., 2011; Hawthorne & Popovich, 2011; McGavern & Kang, 2011; de Vries et al., 2012). Therefore, the possibility that RSD could induce monocyte trafficking to the brain was intriguing because it would occur in the absence of CNS tissue pathology (Wohleb et al., 2013). To better assess this possibility of monocyte trafficking, green fluorescent protein positive (GFP+) bone marrow chimeric mice were created. Wild-type bone marrow was reconstituted with bone marrow-derived donor cells that ubiquitously expressed GFP (Wohleb et al., 2013). In this study, the resident microglia were identified as negative for GFP (GFP−), while bone marrow-derived cells were GFP+. It is important to note that when Evan’s blue dye was intravenously injected, vascular permeability of the blood brain barrier was not observed in these chimeric mice demonstrating that the drug-induced bone marrow ablation did not compromise the blood brain barrier (Wohleb et al., 2013). Additionally, the control chimeric mice in these studies displayed little or no GFP+ cells within the CNS parenchyma, which is consistent with previous studies demonstrating that microglia are not bone marrow-derived under homeostatic conditions (Kierdorf et al., 2013). However, when mice underwent RSD, GFP+ bone marrow-derived monocytes extravasated into the brain parenchyma in a region- and cycle-dependent manner (Wohleb et al., 2013). Visually, these GFP+ parenchymal macrophages demonstrated a ramified, microglia-like morphology with elevated expression of Iba1 (Wohleb et al., 2013). This substantial influx of parenchymal ramified GFP+ macrophages was only observed following 6 cycles of RSD (Wohleb et al., 2013). Similar to the pattern of c-Fos and Iba1 expression (Wohleb et al., 2011), increased ramified GFP+ cells were specifically observed in brain regions associated with fear, anxiety, and threat appraisal including the PFC, PVN, LS, BNST, and AMYG, but were not observed in other brain regions like the motor cortex, striatum, somatosensory cortex, or cerebellum (Wohleb et al., 2013). This regional specificity of RSD-induced trafficking reinforced the patterns of neuronal and microglial activation that were observed in other studies (Wohleb et al., 2011; Wohleb et al., 2013). Overall, in the absence of tissue pathology or global permeability of the blood brain barrier, RSD caused selectively permissible and region-specific extravasation of monocytes into brain regions associated with fear, anxiety, and threat appraisal.
Other murine models of chronic stress have reported monocyte trafficking to the brain. In particular, the Fujimiya lab demonstrated that brain macrophage infiltration occurs under other stressors (Ataka et al., 2013; Brevet et al., 2010; Sawada et al., 2014). For example, following five days of foot shock stress, bone marrow-derived monocytes trafficked to the ventral hippocampus and demonstrated a ramified, microglia-like morphological phenotype (Brevet et al., 2010). Interestingly, the stress associated with simply observing other mice undergoing foot shock was sufficient to recruit monocytes to the brain parenchyma (Ataka et al., 2013). Additionally, both CCR2 and βAR signaling contributed to the trafficking of bone marrow-derived monocytes to the brain (Ataka et al., 2013; Sawada et al., 2014). In a more recent chronic neuropathic pain study, partial sciatic nerve ligation induced anxiety-like behavior and the infiltration of bone marrow-derived monocytes into the AMYG (Sawada et al., 2014). Oral administration of a CCR2 antagonist or microinjections of IL-1 receptor antagonist to the AMYG prevented monocyte trafficking (Sawada et al., 2014).
Stress tends to cause distinct patterns of monocyte recruitment to the brain: global and region-specific. Globally, RSD increased diffuse perivascular monocyte recruitment that was observed through flow cytometry and immunohistochemistry in both wild-type and LysM-GFP mice. On the other hand, in a region-specific manner, monocytes extravasated into the brain parenchyma following RSD in GFP+ bone marrow chimeric mice. It is important to note the distinction between these trafficking patterns, as they are likely caused by distinct mechanisms that lead to differential effects on brain and behavior. For instance, global increases in perivascular monocytes are likely more peripherally regulated (e.g., circulating cytokines) while region-specific monocyte infiltration are likely more causally connected to regional neuronal and microglial activation. In fact, global increases in perivascular brain macrophages are proportionally linked to increases in circulating Ly6Chi inflammatory monocytes, and the trafficking pattern of these cells occurs throughout the brain even in areas that are unrelated to the expression of fear, anxiety, and threat appraisal (Wohleb et al., 2013). Taken together, research from multiple labs demonstrates that repeated psychological stress causes distinct patterns of monocyte trafficking to the brain.
3.3 Stress-induced Brain Macrophage Trafficking Promotes Anxiety-like Behavior
One key finding is that the establishment of anxiety-like behavior following RSD is promoted by myeloid-derived cell trafficking to the brain (Wohleb et al., 2013). All circulating monocytes express CX3CR1 (Yona et al., 2012), but not all monocytes express CCR2. Additionally, brain macrophage trafficking and anxiety-like behavior following RSD was absent in both CCR2KO (CCL2 receptor KO) and CX3CR1KO (fractalkine receptor KO) mice (Wohleb et al., 2013). More specifically, CCR2KO and CX3CR1KO mice lacked both global perivascular-monocyte trafficking and region-specific extravascular trafficking following RSD (Wohleb et al., 2013). However, these KO mice did demonstrate peripheral immunomodulatory effects of RSD comparable to wild-type mice including increased circulating Ly6Chi monocytes. Therefore, it was concluded that monocyte trafficking to the brain was likely responsible for the promotion of anxiety-like behavior following RSD. This is consistent with a previous finding regarding βAR- and IL-1R1-dependent trafficking and anxiety-like behavior. Specifically, propranolol (βAR antagonist) or using IL-1R1KO prevented both RSD-induced anxiety-like behavior and brain monocyte trafficking (Wohleb et al., 2011; Wohleb et al., 2013). Together, these data support the idea that RSD-induced anxiety-like behavior is dependent upon monocyte trafficking to the brain and is mediated by SNS and IL-1 signaling (Figure 1a,c).
The hypothesis that monocyte trafficking to the brain is a factor that precipitates the behavioral responses to stress is relatively novel, and thus other reports of similar phenomena are relatively sparse. Despite this, there are a few examples of brain macrophage trafficking affecting behavior in the literature. For instance, in an inflammatory liver disease model, the Swain laboratory demonstrated an important role for brain macrophage trafficking in the development of fatigue and depressive-like behavior (D'Mello et al., 2009). Additionally, in a neuropathic pain model, region-specific trafficking of monocytes to the AMYG was CCR2/CCL2-dependent and this trafficking was found to promote pain-induced anxiety-like behavior (Sawada et al., 2014). Thus, we contend that monocyte trafficking to the brain is a unique pathway in which the immune system communicates with the brain and has important implications in the context of stress and inflammation-related mental health complications.
The specific molecular mechanisms connecting monocyte trafficking to stress-induced changes in behavior are not fully elucidated, but it is likely that brain macrophages contribute to RSD-induced anxiety through the propagation of cytokine signaling from the periphery to the brain. For example, even though they do not physically cross the blood brain barrier, it is well established that both peripheral and central cytokine signaling potently influences behavior through receptors located on either side of endothelial cells (Dantzer et al., 2008; Capuron & Miller, 2011). Because the brain is highly sensitive to increases in proinflammatory cytokines, microglia tend to be highly regulated by anti-inflammatory ligands and receptors (e.g., CD200/CD200R (Lyons et al., 2007), CX3CR1/CX3CL1 (Cardona et al., 2006), or TGFβ/TGFβR (Butovsky et al., 2014)). Although similar in morphology and phenotype to microglia, peripherally-derived brain macrophages tend to be more inflammatory and less subject to immune regulation (Perry & Teeling, 2013).Along these same lines, RSD primes and activates peripherally-derived monocytes inducing increased cytokine expression and resistance to the immunosuppressive effects of GCs (Engler et al., 2005; Wohleb et al., 2011; Powell et al., 2013; Wohleb et al., 2014a). Moreover, Sawada et al., (2014) demonstrated that pain-induced anxiety-like behavior associated with monocyte trafficking to the AMYG was prevented by local microinjection of IL-1R1 antagonist. Likewise, in a model of inflammatory liver disease, Kerfoot et al., (2005) demonstrated that recruitment of TNF-α-expressing monocytes to the brain promoted depressive-like behavior and fatigue. These reports further suggest that monocyte trafficking to the brain promotes changes in behavior through central cytokine signaling. Nonetheless, the contribution of perivascular trafficking relative to parenchymal extravasation in the promotion of anxiety is yet unclear.
3.4 Neuron-Microglia-Endothelia Dynamics In The Context Of Psychological Stress: Evidence for An Immunological Neurovascular Unit
The region-specific trafficking patterns observed using bone marrow chimeric mice (Brevet et al., 2010; Ataka et al., 2013; Wohleb et al., 2013) provide insight into the dynamic relationships among neurons, microglia, cerebral vasculature (i.e., endothelial cells) and the recruitment of monocytes to the brain in the context of psychological stress. For instance, increases in neuronal and microglial activation are region-specific and co-occur in fear, anxiety, and threat appraisal brain regions (Wohleb et al., 2011). Furthermore, parenchymal infiltration of peripherally-derived monocytes is also region-specific and co-occurs within many of these same areas (Wohleb et al., 2013). Accumulation of perivascular brain macrophages tends to be global and is not coupled to region-specific neuronal and microglial activation. Moreover, data suggest that RSD-induced microglial activation is likely an upstream event of monocyte trafficking to the brain and this is dependent on receptors such as CCR2 and CX3CR1 in that increased microglia cytokine expression was still observed in CCR2KO and CX3CR1KO mice that lacked both perivascular and parenchymal trafficking (Wohleb et al., 2013). In all, data suggest that the infiltration of peripherally-derived monocytes is regionally coupled to neuronal and microglial activation giving cause to speculate that these events may be causally linked.
Microglial activation that is spatially coupled to local neuronal activation results in increased cytokines and chemokines, e.g., IL-1β, IL-6, TNF, and CCL2 (Wohleb et al., 2011; Wohleb et al., 2013; Wohleb et al., 2014b) (Figure 1c). In other contexts, these same microglia-derived cytokines and chemokines are capable of increasing cell adhesion molecule (CAM) expression on the luminal surface of vascular endothelial cells and increasing the respective receptors on vascular monocytes (Kim et al., 1996; Wang et al., 2007; Shi et al., 2011). CAMs on endothelial cells and their associated receptors on leukocytes (e.g., CD11b) are required for extravasation across the blood brain barrier into the parenchyma. Therefore, brain region-specific trafficking following RSD would require increased expression of these CAMs in the same regions. Indeed, RSD caused an exposure- and brain region-dependent increases in both gene and protein expression of vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1) (Sawicki, in press, 2014). Furthermore, vascular ICAM/VCAM expression was increased in stress-responsive brain regions where neuronal and microglial activation co-occurred with macrophage trafficking (Sawicki, in press, 2014). Therefore, region-specific trafficking is likely related to region-specific activation of brain vascular endothelium and increases in CAMs through local stimulation by microglia-derived cytokines and/or chemokines that facilitate monocyte adhesion and extravasation (Figure 1c).
This idea that neuronal activity locally influences brain vasculature is reminiscent of the concept of a neurovascular unit (Hawkins & Davis, 2005). The concept of the neurovascular unit was initially used as a framework to explain the coupling of neuronal activity to alterations in local cerebral blood flow. However, this concept has expanded to explain vascular-neuronal interactions in the context of inflammatory CNS pathology, e.g., Alzheimer’s disease and stroke (Iadecola & Gorelick, 2004). Arguments presented regarding RSD suggest a top-down association among neuronal activity, microglial activation, and local vascular-facilitation of leukocyte diapadesis in the context of psychological stress. For instance, reports from both Wohleb and Hanke et al., (2011) and Hinwood et al., (2012) indicate a region-specific coupling between neuronal and microglial activation in the context of two different stress paradigms. Related to this, reports from our lab and the Fujimiya lab demonstrated that psychological stressors caused monocytes to extravasate into stress-responsive brain regions (Brevet et al., 2010; Ataka et al., 2013; Wohleb et al., 2013; Sawada et al., 2014). Futhermore, following RSD, substantial overlap occurs between monocyte extravasation and microglial activation within stress responsive brain regions (e.g., AMYG, PFC, and PVN) (Wohleb et al., 2011; Wohleb et al., 2013). Thus,the spatial co-occurrence of region-specific neuronal activity with microglial activation and monocyte extravasation and the kinetic timing of these events during RSD treatment provide evidence that there may exist a top-down causative link among these events (Figure 1c). More interventional studies must be performed before causation can be definitively determined.
3.5 Endothelial IL-1R1 Expression Contributes to RSD-Induced Anxiety
Mounting evidence implicates an important role for IL-1R1 signaling in the propagation of inflammatory signaling into, and within, the brain following RSD. Initial studies demonstrated that IL-1R1KO mice do not exhibit RSD-induced anxiety-like behavior or microglial activation despite having increased c-Fos+ neurons (Wohleb et al., 2011). These data indicate that although fear, anxiety, and threat appraisal-associated brain regions were activated, manifestation of RSD-induced anxiety-like behavior and the activation of microglia were dependent on intact IL-1R1 signaling (Wohleb et al., 2011). The interpretation of these data is complicated by the fact that IL-1R1 contributes to inflammatory signaling in both the periphery and the CNS. For example, IL-1R1KO mice do not develop tissue-specific GC-insensitivity of myeloid-derived cells nor do they exhibit RSD-induced brain monocyte trafficking (Wohleb et al., 2014b) suggesting that abrogation of anxiety-like behavior in IL-1R1KO mice was due to the lack of priming and trafficking of peripheral monocytes. However, subsequent experiments in an endothelial-specific knock down model (eIL-1R1kd) indicated that when IL-1R1 expression on vascular cells of the blood brain barrier were knocked down RSD continued to induce monocyte trafficking, but not anxiety-like behavior, suggesting that IL-1R1 plays a different role specifically within the CNS.
eIL-1R1kd mice exhibited intact peripheral immune responses to RSD in that there were increased circulating monocytes, primed splenic macrophages, and increased monocyte trafficking to the brain (Wohleb et al., 2014b). Despite the monocyte trafficking and the immunoenhanced environment within the periphery, RSD-treated eIL-1R1kd mice did not exhibit increased microglial cytokine expression nor did they exhibit anxiety-like behavior. These data suggest that endothelial IL-1R1 plays a role in microglial cytokine expression and the precipitation of anxiety-like behavior.
3.6 Temporal Kinetics and Clinical Relevance Of RSD-Induced Anxiety-Like Behavior And Monocyte Trafficking
Thus far, findings from the RSD murine model implicate microglial activation and monocyte trafficking to the brain in the establishment of anxiety-like behavior (Wohleb et al., 2011; Wohleb et al., 2013). This work was further extended to reveal strong temporal associations in the resolution and recurrence of anxiety-like behavior and brain monocyte infiltration (Wohleb et al., 2013; Wohleb et al., 2014a; Wohleb et al., 2014b). Initial work demonstrated a cycle dependent increase in both anxiety-like behavior and brain monocytes (Wohleb et al., 2011). Further work revealed that both anxiety-like behavior and recruited monocytes remained in the parenchyma for at least 8 days following the last cycle of RSD and that both of these parameters were resolved by 24 days (Wohleb et al., 2014a) further reinforcing that the establishment and resolution of both anxiety-like behavior and brain monocyte trafficking were temporally linked. Despite the resolution of these parameters by 24 days, RSD-exposed mice remained sensitized to the behavior and immunomodulary effects of the stressor. At 24 days post RSD, sub-threshold stress exposure (i.e., one cycle of RSD) re-established monocyte trafficking and anxiety-like behavior in the stress-sensitized mice (Wohleb et al., 2014a). One cycle of RSD was considered sub-threshold because it did not affect the behavioral or immunological parameters in control mice that were not exposed to the stressor (i.e., naïve mice). Therefore, comparable to the establishment and resolution, the recurrence of anxiety-like behaivor and monocyte trafficking was also temporally coupled.
One interesting finding was that myeloid cell redistribution in stress-sensitized mice after sub-threshold RSD exposure was not associated with alterations in myeloid cell production, egress, or trafficking from the bone marrow, but, instead, the redistribution of these cells was dependent upon a splenic myeloid cell reservoir (Wohleb et al., 2014a). These data supported the hypothesis that myeloid-derived cell trafficking in the stress-sensitized animal originated not from the bone marrow, but from an alternative source. The idea that the spleen serves as an alternative source of monocyte trafficking is seen in several studies involving inflammatory challenges including myocardial infarction (Swirski et al., 2009) and stroke (Seifert et al., 2012).
The hypothesis that spleen-to-brain monocyte trafficking contributed to the reestablishment of anxiety-like behavior was tested. Control and RSD-sensitized mice were splenectomized prior to exposure to sub-threshold stress (Wohleb et al., 2014a). Splenectomy prevented monocyte trafficking to the brain as well as the re-establishment of anxiety-like behavior in stress-sensitized mice (Wohleb et al., 2014a). These data indicated that trafficking of a splenic monocyte population was necessary for promoting the recurrence of anxiety in sensitized mice. Additionally, sub-threshold stress augmented microglial cytokine expression in stress-sensitized mice, which was prevented by splenectomy. Thus, it was concluded that re-establishment of anxiety-like behavior and augmented microglial cytokine expression were dependent upon monocyte trafficking from the spleen to the brain (Wohleb et al., 2014a).
These findings have exciting implications for mental health conditions involving recurring anxiety, yet several important points remain unclear. First, the mechanism by which RSD induces sensitization of splenic myeloid cell trafficking is unknown. However, previously published research sheds insight into this phenomenon. Although the literature regarding myeloid-derived cell trafficking from the spleen is relatively sparse, in the context of cardiovascular disease and stroke, substantial gains have been made (Leuschner et al., 2012; Seifert et al., 2012). In a myocardial infarct model, monocytes recruited to damaged tissues were replenished by extramedullary monocytopoiesis that occurred in the spleen (Leuschner et al., 2012). The Pennypacker laboratory has elucidated regulatory mechanisms in spleen-to-brain myeloid trafficking within the context of cerebral artery occlusion. In their model, substantial splenic myeloid cell egress is observed following stroke (Seifert et al., 2012). This increased egress was associated with more severe pathology (Ajmo et al., 2008) that was dependent upon peripheral noradrenergic signaling (Ajmo et al., 2009). Egress of splenic myeloid-derived cells was not dependent upon direct SNS innervation of the spleen, but rather circulating norepinephrine signaling through adrenergic receptors within the spleen.. This is a relevant finding because even after just one cycle, RSD induces an increase in norepinephrine in both circulation and the spleen (Hanke et al., 2012). When this catecholaminergic signaling is blocked by propranolol pretreatment, myeloid-derived cell production and trafficking is abrogated Thus, it is clear from our data and others that norepinephrine appears to play a critical role in spleen-to-brain myeloid-derived cell trafficking.
One last point of discussion is the clinical relevance of RSD as a model of stress-sensitization and recurring anxiety. Although RSD-sensitization is not considered a model of post-traumatic stress disorder (PTSD), studying stress-sensitization within the RSD paradigm may have relevance. For example, both RSD and PTSD involve a sensitizing event that is often both physical and psychological in nature (Bailey et al., 2004; American Psychiatric Association, 2013; Freeman et al., 2013). Analogous to RSD, the sensitizing event, or trauma, predisposes the individual to recapitulate the behavioral and physiological responses following exposure to either generalized cues or subsequent stressful events (American Psychiatric Association, 2013; Wohleb et al., 2014a). Furthermore, both PTSD and RSD involve chronic maintenance of psychosocial deficits (American Psychiatric Association, 2013). Despite these commonalities, PTSD remains a complex and distinctly human disorder that likely cannot be fully modeled by RSD-sensitization in mice. However, it is increasingly appreciated that neuroimmune signaling contributes to the development and maintenance of PTSD and other chronic anxiety disorders. As reviewed by Pace and Heim (2012), strong clinical associations exist between recurring anxiety and inflammatory signaling. With the RSD paradigm recapitulating many of the behavioral and immunological phenomena associated with PTSD, mechanisms associated with RSD-sensitization (e.g., spleen-to-brain myeloid cell trafficking) may have clinical relevance to recurring stress and anxiety in PTSD and other human anxiety disorders.
4. Conclusion
Following prolonged and repeated stimulation of relevant pathways, psychological stress leads to negative physical and mental health outcomes. In summary, Figure 1 defines the proposed mechanisms by which RSD alters immune functioning and behavior ultimately leading to brain region-specific trafficking of monocytes that are associated with the development of anxiety-like behavior. First, in the CNS, Figure 1a depicts the scenario whereby RSD may induce neuronal activation through the release of norepinephrine and resultant βAR signaling in brain regions associated with fear, anxiety, and threat appraisal. This βAR signaling is hypothesized to result in reduced neuronal fractalkine (CX3CL1) production (a chemokine involved in communicating with microglia and regulating microglial activation) leading to microglial activation as shown by increases in Iba1. These activated microglia would now have the potential to increase production of proinflammatory cytokines and chemokines like IL-1β and CCL2. RSD then stimulates the peripheral SNS activation resulting in increases of epinephrine and norepinephrine in the bone marrow, spleen, and circulation. We propose that GM-CSF expression and βAR signaling promotes the production, release, and trafficking of these primed, GC-insensitive, immune cells from the bone marrow into circulation and various tissues including the spleen and brain (Figure 1b).
In the CNS, cells that comprise the neurovascular unit, which includes neurons, microglia, and endothelial cells, facilitate the bi-directional communication between the brain and the periphery. Figure 1c depicts the relationship among the members of the neurovascular unit in aiding the region-specific extravasation of monocytes following RSD. Microglia communicate with endothelial cells lining the blood brain barrier and this communication increases brain region-specific expression of CAMs that work to recruit monocytes. Proinflammatory cytokines and chemokines, specifically IL-1β and CCL2, interact with endothelial cells inducing selective permissibility of peripheral monocytes to the brain. In all, the complex communication among the members of the neurovascular unit directs psychosocial stress-induced monocyte trafficking and anxiety-like behavior. It is important that future studies place an emphasis on exploring the components of the neurovascular unit in order to further elucidate the roles of the numerous cellular and molecular components and pathways involved.
Recent findings from long-term studies suggest that RSD promotes the accumulation and longevity of primed splenic myeloid cells such that a exposure to a single cycle of RSD in stress-sensitized mice is sufficient to re-establish anxiety-like behavior and myeloid-derived cell redistribution. This finding is of great importance in that numerous mental health complications are linked to or exacerbated by stress even years after the initial stressor. Further research is needed to determine how long sensitization persists and to identify the mechanism(s) inducing sensitization and the reestablishment of monocyte trafficking and anxiety-like behavior. Furthermore, determining whether microglial priming contributes to stress sensitization in the periphery could also be beneficial. Clinically, these issues could be tested by assessing the association of stressful life events and proinflammatory markers in PTSD patients as well as those with other anxiety-related disorders. Overall, data from the RSD paradigm have contributed insight into mechanisms by which stress impacts behavior. These insights may be useful in generating new therapeutic approaches for those suffering from mental health complications and neurological diseases and disorders.
Highlights.
Social stress induces bidirectional responses between the brain and the immune system.
Activation of the sympathetic nervous system alters hematopoiesis towards myelopoiesis.
Primed myeloid cells (PMC) are proinflammatory and traffic to the brain in response to social stress.
Trafficking of PMC to the brain is associated with prolonged anxiety-like behavior.
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) Grants R01-MH-093473 and R01-MH093472 to J.F.S. and J.P.G.
B.L.J., C.M.S. and D.B.M. were supported by an NIH/NIDCR Training Grant T32-DE014320.
We thank Caroline Sawicki for her assistance in editing this review.
Abbreviations
- ACTH
adrenocorticotropic hormone
- αAR
alpha adrenergic receptor
- AMYG
amygdala
- βAR
beta adrenergic receptor
- BNST
bed nucleus of the stria terminalis
- CAM
cell adhesion molecule
- CCL2
chemokine (C-C motif) ligand 2
- CCR2
chemokine (C-C motif) receptor 2
- CD11b
cluster of differentiation molecule 11b
- CD19
cluster of differentiation 19
- CD45
cluster of differentiation 45
- CD86
cluster of differentiation 86
- CNS
Central Nervous System
- CX3CL1
Chemokine (C-X3-C motif) ligand 1
- CX3CR1
chemokine (C-X3-C motif) receptor 1
- eIL-1R1kd
endothelial-specific knock down
- GC
glucocorticoid
- GM-CSF
granulocyte macrophage-colony stimulating factor
- HPA
hypothalamic-pituitary-adrenal
- Iba1
ionized calcium-binding adapter molecule 1
- ICAM-1
intracellular adhesion molecule-1
- IL-1
Interleukin 1
- IL-1R1
interleukin-1 receptor type 1
- IL-1α
Interleukin-1 alpha
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin-6
- KC
keratinocyte chemoattractant
- KO
knockout
- LPS
lipopolysaccharide
- LS
lateral sulcus
- LsyM
lysozyme M
- Ly6C
lymphocyte antigen 6 C
- GFP
green fluorescent protein
- MCP-1
monocyte chemoattractant protein-1
- M-CSF
macrophage-colony stimulating factor
- MeAMYG
medial amygdala
- MIP-2
macrophage inflammatory protein-2
- NFκB
nuclear factor κ B
- NGF
nerve growth factor
- PFC
prefrontal cortex
- PTSD
post-traumatic stress disorder
- PVN
paraventricular nucleus
- RSD
repeated social defeat
- SNS
sympathetic nervous system
- TLR2
toll like receptor 2
- TLR4
toll like receptor 4
- TLRs
Toll-like receptors
- TNF-α
tumor necrosis factor alpha
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajmo CT, Jr, Collier LA, Leonardo CC, Hall AA, Green SM, Womble TA, Cuevas J, Willing AE, Pennypacker KR. Blockade of adrenoreceptors inhibits the splenic response to stroke. Experimental neurology. 2009;218:47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, Hayakawa Y, Tatezawa R, Inui A, Fujimiya M. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PloS one. 2013;8:e81744. doi: 10.1371/journal.pone.0081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Kinsey SG, Bidor K, Bailey MT, Padgett DA, Sheridan JF. Subordinate social status modulates the vulnerability to the immunological effects of social stress. Psychoneuroendocrinology. 2007;32:1097–1105. doi: 10.1016/j.psyneuen.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Padgett DA, Dhabhar FS, Stark JL, Kramer KA, Engler H, Sheridan JF. Expression of glucocorticoid resistance following social stress requires a second signal. Journal of leukocyte biology. 2003;74:507–513. doi: 10.1189/jlb.0303090. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J Neuroimmunol. 2002;124:54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Avitsur R, Engler H, Padgett DA, Sheridan JF. Physical defeat reduces the sensitivity of murine splenocytes to the suppressive effects of corticosterone. Brain Behav Immun. 2004;18:416–424. doi: 10.1016/j.bbi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Engler H, Powell ND, Padgett DA, Sheridan JF. Repeated social defeat increases the bactericidal activity of splenic macrophages through a Toll-like receptor-dependent pathway. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1180–1190. doi: 10.1152/ajpregu.00307.2007. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B. Social stress enhances IL-1beta and TNF-alpha production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiology & behavior. 2009;98:351–358. doi: 10.1016/j.physbeh.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bilgel N, Aytac S, Bayram N. Bullying in Turkish white-collar workers. Occupational medicine. 2006;56:226–231. doi: 10.1093/occmed/kqj041. [DOI] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. Journal of psychosomatic research. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Aggressive behavior in the rat. Behavioral biology. 1977;21:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- Brevet M, Kojima H, Asakawa A, Atsuchi K, Ushikai M, Ataka K, Inui A, Kimura H, Sevestre H, Fujimiya M. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. 2010;88:1890–1897. doi: 10.1002/jnr.22362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Castro M, Leung DY, Webster E, Kino T, Bamberger C, Elliot S, Stratakis C, Karl M. Molecular mechanisms of glucocorticoid resistance/hypersensitivity. Potential clinical implications. American journal of respiratory and critical care medicine. 1996;154:S39–43. doi: 10.1164/ajrccm/154.2_Pt_2.S39. discussion S43–34. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA : the journal of the American Medical Association. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, Marsh CB. Social disruption induces lung inflammation. Brain Behav Immun. 2010;24:394–402. doi: 10.1016/j.bbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Kooij G, Frenkel D, Georgopoulos S, Monsonego A, Janigro D. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia. 2012;53(Suppl 6):45–52. doi: 10.1111/j.1528-1167.2012.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, Ransohoff RM, Popovich PG. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci. 2011;31:9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annual review of immunology. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–117. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Engler A, Bailey MT, Sheridan JF. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J Neuroimmunol. 2005;163:110–119. doi: 10.1016/j.jneuroim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. Journal of immunology. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Freeman D, Thompson C, Vorontsova N, Dunn G, Carter LA, Garety P, Kuipers E, Slater M, Antley A, Glucksman E, Ehlers A. Paranoia and post-traumatic stress disorder in the months after a physical assault: a longitudinal study examining shared and differential predictors. Psychological medicine. 2013;43:2673–2684. doi: 10.1017/S003329171300038X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer's disease: the blood-borne identity. Journal of neural transmission. 2010;117:961–970. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Trinh NH, Smoller JW, Fava M, Murphy JM, Breslau J. Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychological medicine. 2013;43:303–316. doi: 10.1017/S0033291712001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg B, Alee WC. Some effects of conditionaing on social dominance and subordination in inbred strains of mice. In: Schein MW, editor. Social hierarchy and dominance. Halsted Press; New York: 1975. pp. 282–303. [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Fuhrer R, Stansfeld SA, Marmot M. The importance of low control at work and home on depression and anxiety: do these effects vary by gender and social class? Social science & medicine. 2002;54:783–798. doi: 10.1016/s0277-9536(01)00109-5. [DOI] [PubMed] [Google Scholar]
- Hafler DA, Slavik JM, Anderson DE, O'Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunological reviews. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. β-adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological reviews. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawthorne AL, Popovich PG. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2011;8:252–261. doi: 10.1007/s13311-011-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012;22:1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Gorelick PB. Hypertension, angiotensin, and stroke: beyond blood pressure. Stroke; a journal of cerebral circulation. 2004;35:348–350. doi: 10.1161/01.STR.0000115162.16321.AA. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalynchuk LE, Gregus A, Boudreau D, Perrot-Sinal TS. Corticosterone increases depression-like behavior, with some effects on predator odor-induced defensive behavior, in male and female rats. Behavioral neuroscience. 2004;118:1365–1377. doi: 10.1037/0735-7044.118.6.1365. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: New Roles for the Synaptic Stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Kerfoot SM, Kubes P. Local coordination verses systemic disregulation: complexities in leukocyte recruitment revealed by local and systemic activation of TLR4 in vivo. Journal of leukocyte biology. 2005;77:862–867. doi: 10.1189/jlb.1004607. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Katzmarski N, Haas CA, Prinz M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PloS one. 2013;8:e58544. doi: 10.1371/journal.pone.0058544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Berliner JA, Nadler JL. Angiotensin II increases monocyte binding to endothelial cells. Biochem Biophys Res Commun. 1996;226(3):862–868. doi: 10.1006/bbrc.1996.1441. [DOI] [PubMed] [Google Scholar]
- Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, Ludwig A, Lira SA, Jung S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118:e156–167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. The Journal of steroid biochemistry and molecular biology. 2003;85:457–467. doi: 10.1016/s0960-0760(03)00218-8. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. The Journal of physiology. 2007;583:861–874. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2) The New England journal of medicine. 1992;327:99–106. doi: 10.1056/NEJM199207093270207. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Marmot M, Feeney A. General explanations for social inequalities in health. IARC scientific publications. 1997:207–228. [PubMed] [Google Scholar]
- McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nature reviews Immunology. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meduri GU, Yates CR. Systemic inflammation-associated glucocorticoid resistance and outcome of ARDS. Annals of the New York Academy of Sciences. 2004;1024:24–53. doi: 10.1196/annals.1321.004. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010;15:404–414. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O'Farrelly C, Malone KM. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Ogawa M, Nishikawa S, Hayashi S, Kunisada T, Nishikawa S. Conditions required for myelopoiesis in murine spleen. Immunology letters. 1993;35:197–204. doi: 10.1016/0165-2478(93)90091-f. [DOI] [PubMed] [Google Scholar]
- Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2012;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Seminars in immunopathology. 2013;35:601–612. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185 :320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Powell ND, Bailey MT, Mays JW, Stiner-Jones LM, Hanke ML, Padgett DA, Sheridan JF. Repeated social defeat activates dendritic cells and enhances Toll-like receptor dependent cytokine secretion. Brain Behav Immun. 2009;23:225–231. doi: 10.1016/j.bbi.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sawada A, Niiyama Y, Ataka K, Nagaishi K, Yamakage M, Fujimiya M. Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain. 2014 doi: 10.1016/j.pain.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Schein MW, Schein MW, editors. Dowden, Hutchinson & Ross Publishing; 1975. [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenete PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski MA, Cannistra SA, Sullivan R, Elias A, Antman K, Schnipper L, Griffin JD. Granulocyte-macrophage colony-stimulating factor induces the expression of the CD11b surface adhesion molecule on human granulocytes in vivo. Blood. 1988;72:691–697. [PubMed] [Google Scholar]
- Stansfeld SA, Head J, Fuhrer R, Wardle J, Cattell V. Social inequalities in depressive symptoms and physical functioning in the Whitehall II study: exploring a common cause explanation. Journal of epidemiology and community health. 2003;57:361–367. doi: 10.1136/jech.57.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1799–1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Stefanski V. Social stress in laboratory rats: hormonal responses and immune cell distribution. Psychoneuroendocrinology. 2000;25:389–406. doi: 10.1016/s0306-4530(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Engler H. Effects of acute and chronic social stress on blood cellular immunity in rats. Physiology & behavior. 1998;64:733–741. doi: 10.1016/s0031-9384(98)00127-9. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Frontiers in neuroendocrinology. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Walker FR, Beynon SB, Jones KA, Zhao Z, Kongsui R, Cairns M, Nilsson M. Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain Behav Immun. 2014;37:1–14. doi: 10.1016/j.bbi.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. Journal of Neuroimmunology. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014a;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014b;34:2583–2591. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y, Yona S, Kim KW, Jung S. Microglia, seen from the CX3CR1 angle. Frontiers in cellular neuroscience. 2013;7:26. doi: 10.3389/fncel.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]