Abstract
Aims
The IASLC/ATS/ERS classification of lung adenocarcinoma provides a prognostically significant histologic subclassification. The aim of this study is to investigate the accuracy, limitations, and interobserver agreement of frozen sections (FS) for predicting histologic subtype.
Methods and results
FS and permanent section slides from 361 resected stage I lung adenocarcinomas ≤ 3 cm were reviewed for predominant histologic subtype and presence or absence of lepidic, acinar, papillary, micropapillary, and solid patterns. Fifty cases were additionally reviewed by 3 pathologists to determine interobserver agreement. To test the accuracy of FS in judging degree of invasion, 5 pathologists reviewed FS slides from 35 cases with predominantly lepidic pattern. There was moderate agreement on predominant histologic subtype between FS and final diagnosis (κ = 0.565). FS had high specificity for micropapillary and solid patterns (94% and 96%, respectively), but sensitivity was low (37% and 69%, respectively). The interobserver agreement was satisfactory (κ > 0.6, except for acinar pattern).
Conclusions
FS can provide information on the presence of aggressive histologic patterns – micropapillary and solid – with high specificity but low sensitivity. It was difficult to predict the predominant pattern based on frozen section mostly due to sampling issues.
Keywords: Lung adenocarcinoma, Frozen sections, Histologic subtype, Micropapillary, Solid, Invasion, Limited resection
Introduction
Lung cancer is the leading cause of cancer death worldwide,1 and adenocarcinoma is currently the most common histologic subtype of lung cancer.2, 3 With the widespread use of computed tomography (CT), and with the recent National Cancer Institute National Lung Cancer Screening Trial (NLST) findings 4 on the utility of screening CT scans in high-risk smokers, small early-stage lung adenocarcinomas are expected to be detected more frequently. Surgical resection is the standard of treatment for these patients. However, there is currently no widely accepted criteria for deciding the extent of surgical resection (ie, sublobar vs lobar resection).5, 6
Histologic subtyping is increasingly recognized as a powerful predictor of biological behavior in lung adenocarcinoma, and it would be helpful if this information were available when deciding the extent of surgical resection. The predominant histologic subtype—according to the newly proposed International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) classification7—has been shown to correlate with clinical outcome in multiple independent studies.8–11 In addition, the presence of micropapillary or solid patterns has consistently been shown to correlate with poor prognosis.12–22 Our group has reported that, in tumors with micropapillary histologic pattern, limited resection led to higher rates of locoregional recurrence, compared with anatomic resection.23 These results suggest that limited resection may not be sufficient for these aggressive tumors. On the contrary, adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) have been shown to achieve a 5-year disease-free survival of 100%.8–10 These categories seem to be ideal candidates for sublobar resection, whereas this approach is less optimal for invasive adenocarcinomas with poorly differentiated subtypes, because they carry significant risk of recurrence.8–10, 23
To consider histologic features in the decision to perform limited resection versus anatomic resection, these features need to be accurately identified either preoperatively or intraoperatively. Intraoperative frozen section is a practical option, and it is superior to preoperative biopsy because, with intraoperative frozen section, pathologists usually have a larger amount of tissue available for evaluation. However, whether frozen section can be used to accurately identify histologic subtypes is not known.
In this study, we focused on stage I lung adenocarcinomas ≤ 3 cm, which are potential candidates for limited resection. The primary aim of this study was to clarify the strengths and limitations of frozen section for the identification of histologic features of prognostic significance. We investigated (1) the accuracy of frozen section for predicting the final histology (as determined by permanent section), especially the predominant histologic subtype, and the presence or absence of micropapillary and solid patterns; (2) the ability of frozen section to discriminate between AIS, MIA, and invasive adenocarcinomas; (3) the interobserver agreement between different pathologists; and (4) the reasons for the discrepancy between frozen section diagnoses and permanent section diagnoses.
Materials and methods
Patient Selection
(A)Study Cohort for Evaluation of Predominant Histologic Subtype and Presence or Absence of Major Histologic Patterns
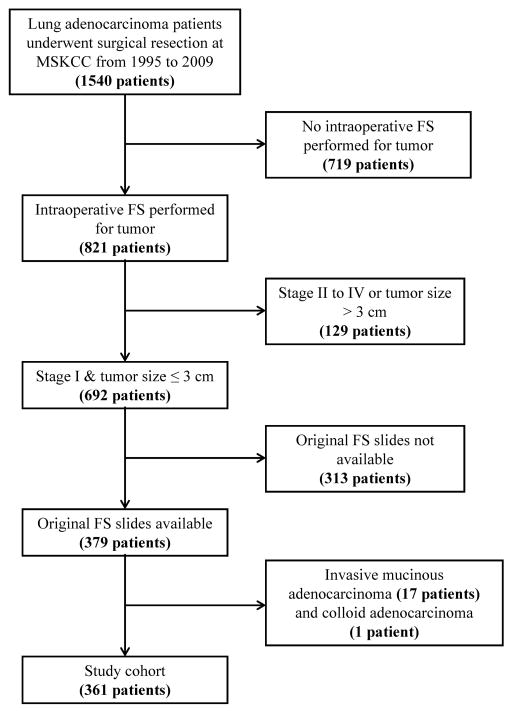
Following approval by the institutional review board at Memorial Sloan-Kettering Cancer Center, we retrospectively identified 361 patients as a study cohort (Figure 1). The clinical data were retrieved from the Memorial Sloan-Kettering Cancer Center Thoracic Service database. Other aspects of some of these cases were previously reported.9, 24
Figure 1.
Patient selection tree. Among the 1540 patients with lung adenocarcinoma who underwent surgical resection from 1995 to 2009, intraoperative frozen section was performed on tumors from 821 patients. Among these patients, 692 had stage I disease with tumor size ≤3 cm. The original frozen section slides were available for 379 patients. Among these patients, we excluded 17 cases of invasive mucinous adenocarcinoma and 1 case of colloid adenocarcinoma. The remaining 361 cases constituted the study cohort. FS, frozen section; MSKCC, Memorial Sloan-Kettering Cancer Center.
(B) Study Cohort for Evaluation of Degree of Invasion
Thirty-five cases with the histologic profile of predominant lepidic growth pattern constituted the study cohort for evaluation of degree of invasion. The 35 cases included 30 cases selected from the aforementioned study cohort of 361 cases and 5 additional cases in which frozen section was performed between 2011 and 2012. In these 5 additional cases, the inflation method was applied during frozen section, using an approach similar to that described elsewhere.25, 26 Before tissue sectioning, the lung specimens were inflated with 1:1 diluted embedding medium (Tissue-Tek OCT; Sakura Finetek, Torrance, CA), using 22-gauge needles through the pleura, until the lung tissue swelled sufficiently. The final diagnoses for these 35 cases were 2 AIS, 15 MIA, and 18 lepidic predominant adenocarcinoma (LPA).
Evaluation of Permanent Section Slides
All available hematoxylin and eosin–stained permanent section slides were reviewed by 3 pathologists (K.K., A.Y., and Y.C.Y.), and problematic cases were additionally reviewed by a senior pathologist (W.D.T.) (Suppl Figure 1). Histologic classification was performed according to the newly proposed IASLC/ATS/ERS lung adenocarcinoma classification.7 Comprehensive histologic subtyping was also performed, with the percentage of each histologic component in each tumor recorded in 5% increments.27 The predominant histologic subtype was determined by the histologic pattern with the highest percentage (this was not necessarily 50% or greater).
Evaluation of Frozen Section Slides for Predominant Histologic Subtype and Presence or Absence of Major Histologic Patterns
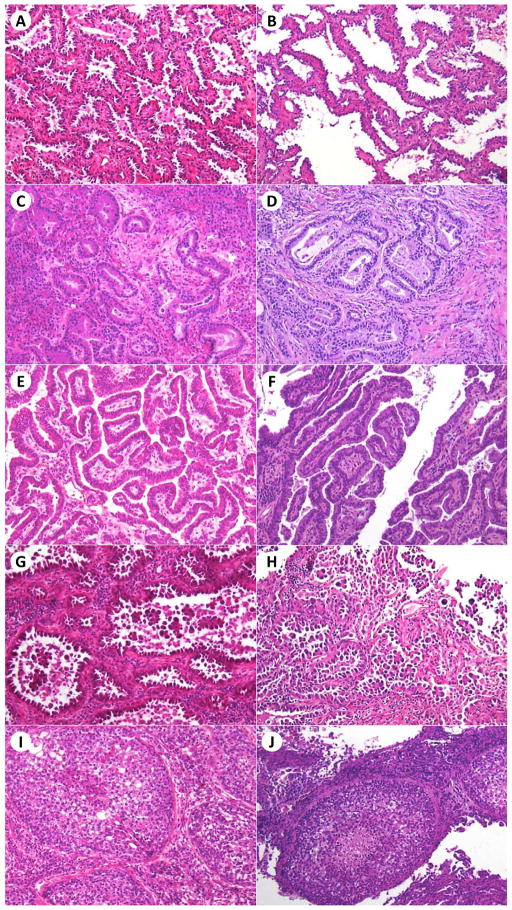
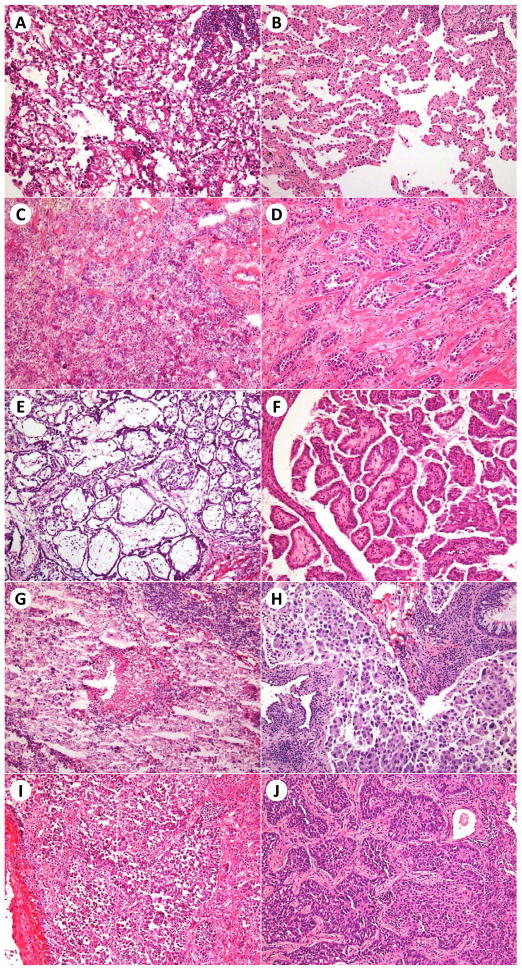
The frozen section slides for all patients were reviewed by a pathologist (Y.C.Y.) to judge the predominant histologic subtype and the presence or absence of lepidic, acinar, papillary, micropapillary, and solid patterns (Figures 2 and 3). Each case had 1 frozen section slide that contained tumor. To assess interobserver agreement, 50 cases were randomly chosen from the total 361 cases. The frozen section slides for these 50 cases were additionally independently reviewed by two other pathologists (K.K. and W.D.T.) to determine the predominant histologic subtype and the presence or absence of lepidic, acinar, papillary, micropapillary, and solid patterns. Interobserver agreement was calculated for each parameter.
Figure 2.
Morphologic profile of histologic patterns in good-quality frozen section slides and the corresponding frozen section control slides. Lepidic pattern (A, B), acinar pattern (C, D), papillary pattern (E, F), micropapillary pattern (G, H), and solid pattern (I, J) in frozen section and frozen section control slides, respectively. Hematoxylin and eosin stain; original magnification × 200.
Figure 3.
Morphologic profile of histologic patterns in poor-quality frozen section slides and the corresponding frozen section control slides. Lepidic pattern (A, B), acinar pattern (C, D), papillary pattern (E, F), micropapillary pattern (G, H), and solid pattern (I, J) in frozen section and frozen section control slides, respectively. Hematoxylin and eosin stain; original magnification × 200.
Evaluation of Frozen Section Slides for Degree of Invasion
The frozen section slides for the 35 cases were independently reviewed by 5 pathologists (Y.C.Y., K.K., N.R., A.L.M., and W.D.T.). Each case had 1 frozen section slide that contained tumor. Clinical information—including tumor size; CT images, including slice thickness; and maximal standardized uptake value on fluorodeoxyglucose positron emission tomography—was provided. The pathologists were asked to choose between “favor AIS,” “favor MIA,” and “favor invasive adenocarcinoma,” on the basis of the frozen section slides and the clinical information. In addition, the pathologists were asked to subjectively evaluate the quality of the frozen section slides as “good,” “average,” or “poor.”
Determination of the Reason for Discrepancy between Frozen Section Diagnoses and Permanent Section Diagnoses
To analyze the reasons for discrepancy, we used frozen section control slides, which were cut from the paraffin blocks of the remaining frozen tissue, as a reference for the original frozen section slides. Frozen section control slides were available for 243 of the 361 cases, and all slides were reviewed (by Y.C.Y.) for predominant histologic subtype and presence or absence of lepidic, acinar, papillary, micropapillary, and solid patterns.
The approach used to determine the reasons for discrepancy is illustrated in Supplemental Figure 2. In brief, if the interpretation was the same for the original frozen section slides and the frozen section control slides but was different from that for the permanent section slides, we considered the reason for discrepancy to be sampling error. If the interpretation was the same for the frozen section control slides and the permanent section slides but was different from that for the original frozen section slides, we considered the reason for discrepancy to be interpretation error. If the interpretations for the original frozen section slides, the frozen section control slides, and the permanent section slides were all different from each other, we considered the reason for discrepancy to be sampling error plus interpretation error.
Clinical Outcome Measurement and Follow-up
In our study cohort, all of the patients had stage I disease, and a high percentage of the deaths were unrelated to lung adenocarcinoma. Therefore, we chose time to recurrence as our primary outcome measurement, which more accurately reflects the biological behavior of the tumors.9 Time to recurrence was defined as the time from the date of operation to the date of disease recurrence. Patients who died of causes other than lung cancer or who were alive on the last follow-up date were censored. Recurrences were classified in accordance with the Society of Thoracic Surgeons Workforce recommendations.28 Local recurrence was defined by tumor recurrence in the same lobe or at the surgical margin of the original tumor. Regional recurrence was defined by tumor recurrence in another ipsilateral lobe, in the ipsilateral hilar lymph nodes, or in the ipsilateral mediastinal lymph nodes. Distant recurrence was defined by tumor recurrence in the ipsilateral supraclavicular lymph nodes, in the contralateral mediastinal nodes, in the contralateral lung, or elsewhere outside the hemithorax.
Statistical Analysis
κ statistics were applied to measure the degree of interobserver agreement, as well as the agreement between frozen section diagnoses and permanent section diagnoses. The degree of agreement was interpreted as follows: slight agreement (κ = 0.00–0.20), fair agreement (κ = 0.21–0.40), moderate agreement (κ = 0.41–0.60), substantial agreement (κ = 0.61–0.80), and almost perfect agreement (κ ≥ 0.81).29
Associations between categorical variables were analyzed using Fisher’s exact test. Survival curves were estimated using the Kaplan-Meier method, and differences between subgroups were compared using the log-rank test. All statistical analyses were performed using two-tailed P values. P < 0.05 was considered to indicate statistical significance. All analyses were performed using SAS statistical software (version 9.2; SAS Institute, Cary, NC).
Results
Clinicopathologic Characteristics of the Patients
The clinicopathologic characteristics of the 361 patients in the study cohort are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of the patients
| Variable | No. (%) |
|---|---|
| All patients | 361 (100) |
| Age, years, mean (range) | 66.80 (23–89) |
| Sex | |
| Female | 235 (65.1) |
| Male | 126 (34.9) |
| Stage | |
| IA | 321 (88.9) |
| IB | 40 (11.1) |
| Tumor size, cm, mean (range) | 1.57 (0.2–3) |
| IASLC/ATS/ERS classification | |
| Adenocarcinoma in situ | 1 (0.3) |
| Minimally invasive adenocarcinoma | 18 (5.0) |
| Lepidic predominant | 53 (14.7) |
| Acinar predominant | 132 (36.6) |
| Papillary predominant | 71 (19.7) |
| Micropapillary predominant | 24 (6.6) |
| Solid predominant | 62 (17.2) |
| Presence of histologic patterns | |
| Lepidic | 248 (68.7) |
| Acinar | 349 (96.7) |
| Papillary | 270 (74.8) |
| Micropapillary | 169 (46.8) |
| Solid | 151 (41.8) |
Interobserver Agreement for Predominant Histologic Subtype and Presence or Absence of Histologic Patterns Using Frozen Sections
Among the 3 pathologists, there was substantial agreement on predominant histologic subtype (κ = 0.662): in 64% of cases, all pathologists made the same diagnosis (Table 2). With regard to the presence or absence of histologic patterns, there was also substantial agreement, with a κ value of > 0.6 for four of the 5 histologic patterns (all but acinar pattern). For acinar pattern, although the κ value was only 0.337, all pathologists agreed on a high percentage of cases (82%).
Table 2.
Interobserver agreement for predominant histologic subtype and presence or absence of histologic patterns in frozen sections
| Parameter | Cases all pathologists agreed on, % (95% CI) | Fleiss κ |
|---|---|---|
| Predominant histologic subtype | 64 (49–77) | 0.662 |
| Presence or absence of histologic pattern | ||
| Lepidic | 72 (59–84) | 0.632 |
| Acinar | 82 (69 –91) | 0.337 |
| Papillary | 76 (62–87) | 0.679 |
| Micropapillary | 76 (62–87) | 0.650 |
| Solid | 84 (71–93) | 0.737 |
Accuracy of Frozen Section for Prediction of Predominant Histologic Subtype and Presence or Absence of Histologic Patterns
The accuracy of frozen section for prediction of predominant histologic subtype was 68% (κ = 0.565 [moderate agreement]; Table 3). With regard to the presence or absence of histologic patterns, the highest accuracy rate was for acinar pattern (89%), followed by solid (84%), lepidic (80%), papillary (72%), and micropapillary (67%). The sensitivity of frozen section for detection of the presence of histologic patterns was highest for acinar pattern (90%), followed by lepidic (75%), papillary (70%), solid (69%), and micropapillary (37%). The specificity of frozen section was highest for solid pattern (96%), followed by micropapillary (94%), lepidic (91%), papillary (79%), and acinar (67%).
Table 3.
Accuracy of frozen section for predicting predominant histologic subtype and presence or absence of histologic patterns in permanent sections
| Parameter | Accuracy, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | κ |
|---|---|---|---|---|
| Predominant histologic subtype | ||||
| Overall | 68 (63–73) | NA | NA | 0.565 |
| Lepidic | 90 (86–92) | 75 (64–84) | 93 (90–96) | 0.681 |
| Acinar | 76 (71–80) | 70 (61–77) | 79 (73–84) | 0.481 |
| Papillary | 85 (81–88) | 62 (50–72) | 91 (87–94) | 0.527 |
| Micropapillary | 94 (91–96) | 21 (9–40) | 99 (97–100) | 0.277 |
| Solid | 91 (88–94) | 79 (67–87) | 94 (90–96) | 0.700 |
| Presence or absence of histologic pattern | ||||
| Lepidic | 80 (76–84) | 75 (69–80) | 91 (84–96) | 0.588 |
| Acinar | 89 (85–92) | 90 (86–93) | 67 (35–90) | 0.252 |
| Papillary | 72 (67–77) | 70 (64–75) | 79 (69–87) | 0.397 |
| Micropapillary | 67 (62–72) | 37 (30–45) | 94 (89–97) | 0.321 |
| Solid | 84 (80–88) | 69 (61–76) | 96 (92–98) | 0.670 |
NA, not applicable
Reasons for the Discrepancy between Frozen Section Diagnoses and Permanent Section Diagnoses
The reasons for the discrepancy between frozen section diagnoses and permanent section diagnoses of predominant histologic subtype are summarized in Table 4. We found that sampling error, which accounted for 62.7% to 74.1% of errors, was the major reason for discrepancy. Interpretation error was the second most common reason for discrepancy, accounting for 20.2% to 37.3% of errors. Sampling error plus interpretation error was rare, accounting for 10.7% of errors.
Table 4.
Reason for discrepancy between frozen section diagnoses and permanent section diagnoses
| Parameters | No. (%) of each type of error
|
Total | ||
|---|---|---|---|---|
| Sampling error | Interpretation error | Sampling +interpretation error | ||
| Predominant histologic subtype | ||||
| Overall | 58 (69.0) | 17 (20.2) | 9 (10.7) | 84 (100) |
| Lepidic | 8 (57.1) | 5 (35.7) | 1 (7.1) | 14 (100) |
| Acinar | 19 (79.2) | 4 (16.7) | 1 (4.2) | 24 (100) |
| Papillary | 18 (78.3) | 4 (17.4) | 1 (4.3) | 23 (100) |
| Micropapillary | 5 (41.7) | 3 (25.0) | 4 (33.3) | 12 (100) |
| Solid | 8 (72.7) | 1 (9.1) | 2 (18.2) | 11 (100) |
| Presence/absence of histologic pattern | ||||
| Lepidic | 32 (64.0) | 18 (36.0) | 0 (0) | 50 (100) |
| Acinar | 20 (74.1) | 7 (25.9) | 0 (0) | 27 (100) |
| Papillary | 44 (67.7) | 21 (32.3) | 0 (0) | 65 (100) |
| Micropapillary | 47 (62.7) | 28 (37.3) | 0 (0) | 75 (100) |
| Solid | 26 (72.2) | 10 (27.8) | 0 (0) | 36 (100) |
Identification of Micropapillary and Solid Patterns in Frozen Sections Was Correlated with Postoperative Disease Recurrence
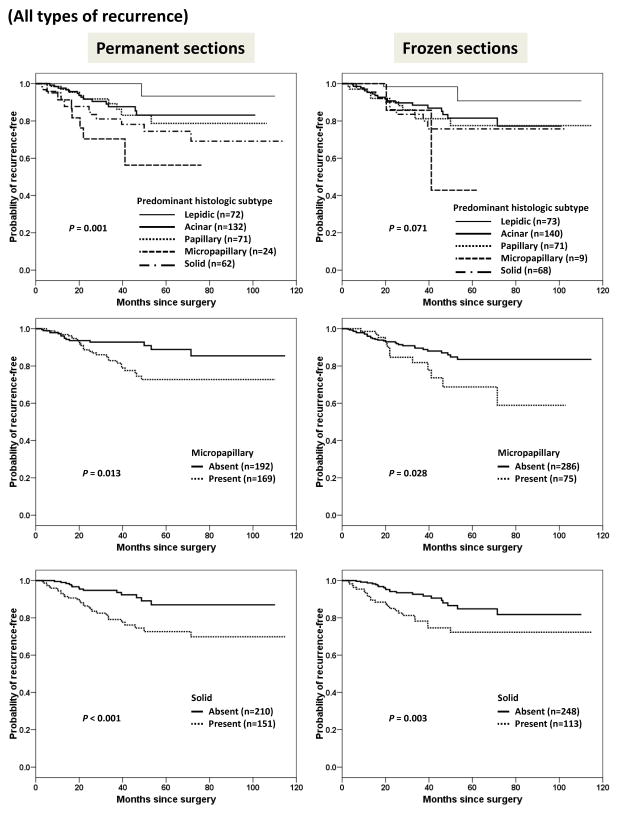
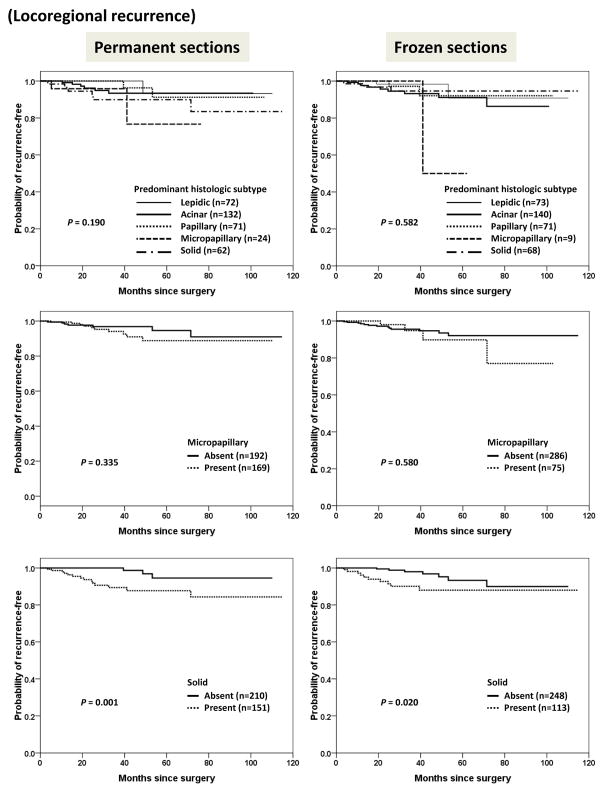
We next attempted to investigate the correlation between histologic features and disease recurrence; the results are shown in Figure 4.
Figure 4.
Kaplan-Meier curves for time to all types of recurrence stratified by predominant histologic subtype, and presence or absence of micropapillary or solid pattern in permanent sections and frozen sections.
As expected, when permanent sections were used, the predominant histologic subtype and the presence or absence of micropapillary and solid pattern were correlated with disease recurrence (P = 0.001 for predominant histologic subtype; P = 0.013 for micropapillary pattern; and P < 0.001 for solid pattern). When frozen sections were used, the predominant histologic subtype did not significantly correlate with disease recurrence (P = 0.071). However, when frozen sections were used, even with the disadvantage of low sensitivity, the presence or absence of micropapillary and solid patterns still helped to stratify patients with respect to their risk of recurrence (P = 0.028 for micropapillary pattern; P = 0.003 for solid pattern).
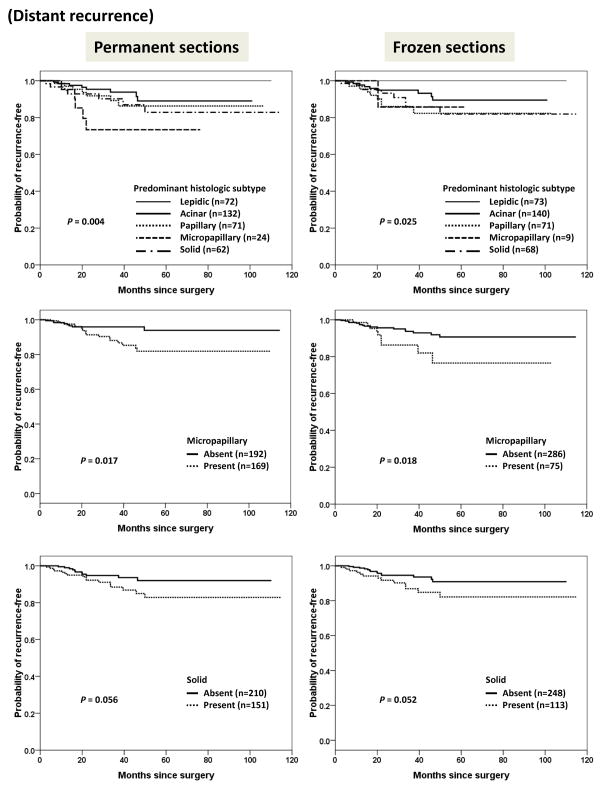
We further analyzed the pattern of recurrence with respect to histologic features. As shown in Figure 5, the presence or absence of solid pattern in permanent sections (P = 0.001) and frozen sections (P = 0.020) were correlated with locoregional recurrence. Distant recurrence was significantly correlated with predominant histologic subtype, and the presence or absence of micropapillary pattern in either permanent or frozen sections (Figure 6).
Figure 5.
Kaplan-Meier curves for time to locoregional recurrence stratified by predominant histologic subtype, and presence or absence of micropapillary or solid pattern in permanent sections and frozen sections.
Figure 6.
Kaplan-Meier curves for time to distant recurrence stratified by predominant histologic subtype, and presence or absence of micropapillary or solid pattern in permanent sections and frozen sections.
Accuracy and Interobserver Agreement for Degree of Invasion Using Frozen Sections
The details of the frozen section diagnoses for the 35 cases evaluated for degree of invasion—to determine the accuracy and interobserver agreement for discriminating between AIS, MIA, and invasive adenocarcinomas by the 5 pathologists—are summarized in Table 5.
Table 5.
Frozen section diagnoses by 5 pathologists in 35 cases of AIS/MIA/LPA
| Permanent section diagnosis | Frozen section interpretation by 5 pathologists, %
|
|||
|---|---|---|---|---|
| AIS | MIA | LPA | Total | |
| AIS (n = 2) | 100 | 0 | 0 | 100 |
| MIA (n = 15) | 6.7 | 41.3 | 52.0 | 100 |
| LPA (n = 18) | 3.3 | 17.7 | 79.0 | 100 |
LPA, lepidic predominant adenocarcinoma; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma.
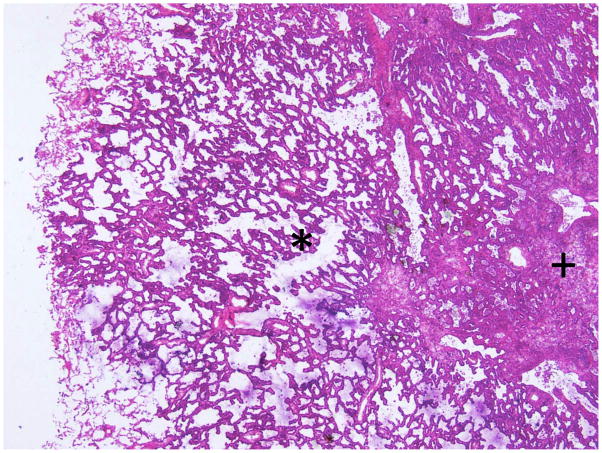
The interobserver agreement for degree of invasion was not satisfactory. The κ value was 0.378 (fair agreement), and only 15 cases (43%) had complete agreement among all pathologists. The average accuracy rate of frozen section diagnosis was 64% (range, 54% to 74%). However, for the 5 cases that underwent specimen inflation at the time of frozen section (Figure 7), the interobserver agreement seemed to be better (κ = 0.578 [moderate agreement]), and the average accuracy rate also increased to 80% (range, 60% to 100%). The quality of frozen section slides for these 5 cases was also better. The evaluation of slide quality for the cases with inflation was as follows: 68% were good, 32% were average, and 0% were poor. This is in contrast to the cases that did not undergo inflation: 24% were good, 44.7% were average, and 31.3% were poor. In the 2 cases of AIS, all pathologists made accurate interpretations using frozen sections. In cases of MIA, 6.7% of frozen section diagnoses were AIS, 41.3% were MIA, and 52% were invasive adenocarcinoma. In cases of LPA, 3.3% of frozen section diagnoses were AIS, 17.7% were MIA, and 79% were invasive adenocarcinoma.
Figure 7.
An example of a case in which the frozen section inflation method was applied. The alveolar spaces are well expanded, and the area of invasive growth (marked by “+”) could be easily distinguished from the surrounding, noninvasive area (marked by “*”).
Discussion
For stage I lung adenocarcinomas, surgical resection is the standard of care in patients who can tolerate the procedure. However, the extent of resection (ie, sublobar vs lobar resection) is an area of controversy.5, 6 A randomized trial by the Lung Cancer Study Group (LCSG) showed that lobar resection was associated with better overall survival than sublobar resection. There was also lower rate of local recurrence in lobar resection, but there was no difference in the rate of distant recurrences in both groups.30 Similar results had also been reported by a few more recent retrospective studies.31, 32 On the other hand, sublobar resection has the advantage of preserving lung function, better perioperative morbidity and mortality,6 and recently there were many studies demonstrating that well-selected use of sublobar resection can yield comparable survival and recurrence rates to lobar resection.33–39
In this study, we evaluated the accuracy of frozen section for predicting the predominant histologic subtype according to the 2011 IASLC/ATS/ERS classification, as well as interobserver agreement for frozen section diagnoses; the presence or absence of the 5 major histologic patterns; and the degree of invasion. These histologic features have been shown to correlate with clinical outcome,8–11 and if pathologists can learn how to accurately recognize them by frozen section, they will be particularly useful for surgeons deciding the extent of surgical resection to perform.
For predominant histologic subtype, we found that the accuracy of frozen section was not satisfactory (68%; κ = 0.565), and the diagnosis of predominant histologic subtype by frozen section did not significantly correlate with clinical outcome. This is in contrast with the observations published by Motoi et al., who reported 98.6% accuracy in predicting the histologic subtype of the 75 adenocarcinomas in their study.40 Although the quality of frozen section slides, tissue sampling from frozen sections, and pathologists’ diagnoses may be variable between different studies, further investigation is required to answer this question.
In terms of the presence or absence of micropapillary and solid patterns, although frozen section suffered from poor sensitivity (37% for micropapillary; 69% for solid), the high specificity was encouraging (94% for micropapillary; 96% for solid). Furthermore, the identification of micropapillary and solid patterns using frozen sections correlated well with postoperative disease recurrence. We have recently shown that the presence of a micropapillary pattern is an independent predictor of recurrence in lung adenocarcinoma patients who undergo limited resection in contrast to those who have lobectomy. This result implied that lobectomy is probably more appropriate than limited resection in tumors with micropapillary pattern. This brings new importance to the issue of recognizing a micropapillary pattern in the frozen section setting, a problem that has received little attention previously.23 For solid pattern, although there is substantial evidence about its impact on poor survival and disease recurrence,20–22, 41 the association between solid pattern and locoregional recurrence in respect to the extent of surgical resection, to our knowledge, had not been demonstrated. It remains to be determined whether there is a benefit to perform lobar resection instead of sublobar resection in tumors with solid pattern.
In the hope of improving the accuracy of frozen section for identification of histologic features, we analyzed the reasons for the discrepancy between frozen section diagnoses and permanent section diagnoses. We found that sampling error, rather than interpretation error, was the major cause of discrepancy. This result is reasonable, since lung adenocarcinoma is notorious for its remarkable intratumoral histologic heterogeneity. Taking only 1 section from the tumor in frozen sections may not be representative of the tumor’s predominant histologic pattern, and minor histologic component in the tumor could be easily missed. To reduce sampling error, taking more sections during frozen section may be a practical approach. However, the costs and benefits of frozen section still require further evaluation.
To our knowledge, the present study is the first study to evaluate the interobserver agreement for diagnoses of histologic patterns using frozen sections. Our results suggest that there is good interobserver agreement in pulmonary pathologists regarding histologic patterns using frozen sections. The paradoxical result we observed of high-percentage agreement (82%) and low κ value (0.337) for acinar pattern was likely attributable to the fact that cases with acinar pattern were far more common than were those without it.29 This is consistent with other reports evaluating interobserver agreement using permanent sections. Warth et al.42 reported that the interobserver agreement for diagnosis of predominant histologic pattern using permanent sections was substantial for pulmonary pathologists (κ = 0.44–0.72) and fair for pathology residents (κ = 0.38–0.47). Thunnissen et al.43 reported that, among 26 international expert pulmonary pathologists, the κ values for classical and difficult selected images of the major lung adenocarcinoma histologic subtypes were 0.77 and 0.38, respectively.
In regard to AIS, MIA, and LPA, 79% of the interpretations using frozen sections were accurate for LPA; however, only 41.3% were accurate for MIA, and more than half (52%) of interpretations were overdiagnosed as invasive adenocarcinoma. Our findings concurred with the recent study by Walts et al.44 In their study, only 46% of MIA was accurately recognized in frozen section, and there were 9% of deferrals and 46% frozen section errors in cases of MIA. The most common frozen section errors were also overcalling MIA as invasive adenocarcinoma. These results indicate that the degree of invasion is often overestimated using frozen sections, and it is also very difficult to distinguish MIA from LPA using frozen sections. Although frozen section is accurate for detection of the presence of invasion, it is not an appropriate method for judging the degree of invasion. In frozen section slides, alveolar spaces are frequently collapsed, which can make evaluation of invasion quite problematic. The inflation method, as described by Myung et al.25 and Xu et al.,26 has been shown to be able to expand the alveolar spaces in frozen section slides. In this study, we included 5 cases in which the inflation method was performed during frozen section, to evaluate the effect of inflation. Although the case numbers were too small to draw any conclusions, this method was promising, since slide quality, interobserver agreement, and accuracy all seemed to improve in the cases with inflation.
From the results of our study, judging degree of invasion in frozen sections appeared to be quite difficult, even for experienced pulmonary pathologists. Fortunately, these difficulties may not lead to major problems in patient management. Multiple studies had demonstrated that patients with AIS and MIA both had excellent prognosis, and hence there is little clinical significance in the distinction between AIS and MIA in frozen sections.8, 10, 45, 46 For LPA, accumulating evidence indicates that it is only associated with minor risk of recurrence, and more importantly, most patients with LPA who experienced a recurrence had one or more potential risk factors for recurrence, such as close margin, lymphatic or vascular invasion, or high grade histologic patterns.45, 46 These results suggest that it might be more important to identify these risk factors in frozen sections than to identify LPA itself. Indeed, in addition to histologic patterns and degree of invasion, there are also many other important histopathological features which may contribute to recurrence, such as lymphovascular invasion, pleural invasion, perineural invasion, necrosis, and resection margin status.47–49 Although we did not evaluate these features in this study, identification of these features in frozen sections may also be potentially of value in the management of small early stage lung adenocarcinomas.
Our study has several limitations. First, this was a retrospective study with a time span of 15 years; therefore, the quality of frozen section and tissue processing could not be uniformly controlled. This is a particular problem for the assessment of invasion as there was no concerted effort at the time of frozen section to obtain high quality sections to address this issue. Second, all 5 pathologists who participated in our study were pulmonary pathologists. It remains to be determined whether our results also apply to nonpulmonary pathologists. Finally, the frozen section diagnoses in this study were performed by pathologists without the pressures of time or diagnostic accuracy, unlike real-life frozen section diagnosis in clinical practice. It remains to be prospectively tested whether pathologists can achieve the same performance in a real-life frozen section diagnosis situation.
In conclusion, our study shows that frozen section can provide useful histologic information in lung adenocarcinoma, and it has high specificity for micropapillary and solid patterns. Identification of micropapillary and solid patterns using frozen sections correlates with postoperative disease recurrence. Therefore if these patterns are reported at the time of frozen section, surgeons may consider using this information when making their decision regarding the extent of surgical resection to perform. On the contrary, the degree of invasion is frequently overestimated by frozen section, and we found that in this retrospective material frozen section is not accurate in distinguishing MIA from LPA. Quality of the frozen section is essential in the differential diagnosis of lepidic predominant tumors and the retrospective material available to review in this study was a limiting factor since over 75% of the slides were of average or poor quality. This emphasizes the need for improved approaches in frozen sections especially for lepidic predominant tumors, such as the inflation method. Correlation with CT findings to guide sampling particularly in those adenocarcinomas with a significant ground glass component could be helpful. Hopefully these could improve the ability of frozen section to judge degree of invasion, and they should be tested in future studies.
Supplementary Material
Supplement Figure 1. Experience in lung pathology of the 5 pathologists participating in reviewing frozen section slides.
Supplement Figure 2. Strategy for determining the reason for discrepancy between frozen section diagnoses and permanent section diagnoses. In brief, if the interpretation was the same for the original frozen section slides and the frozen section control slides but was different from that for the permanent section slides, we considered the reason for discrepancy to be sampling error. If the interpretation was the same for the frozen section control slides and the permanent section slides but was different from that for the original frozen section slides, we considered the reason for discrepancy to be interpretation error. If the interpretations for the original frozen section slides, the frozen section control slides, and the permanent section slides were all different from each other, we considered the reason for discrepancy to be sampling error plus interpretation error. FS, frozen section; PS, permanent section.
Acknowledgments
We thank Joe Dycoco for his help with the lung adenocarcinoma database within the Division of Thoracic Service, Department of Surgery. We also thank David Sewell for his help with manuscript editing.
Funding/Support
This work was supported, in part, by the International Association for the Study of Lung Cancer Young Investigator Award; National Lung Cancer Partnership/LUNGevity Foundation Research Grant; American Association for Thoracic Surgery Third Edward D. Churchill Research Scholarship; William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center; New York State Empire Clinical Research Investigator Program; National Cancer Institute (grants 1R21CA164568-01A1, 1R21CA164585-01A1, U54CA137788, and U54CA132378); and the U.S. Department of Defense (grants PR101053 and LC110202).
Footnotes
Disclosure/Conflicts of Interest
All authors affirm that we have no actual or potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations.
Author contributions: Yi-Chen Yeh, Jun-ichi Nitadori, Kyuichi Kadota, Akihiko Yoshizawa, Natasha Rekhtman, Andre L. Moreira and William D. Travis performed the research. Prasad S. Adusumilli and William D. Travis designed the research study. Valerie W. Rusch and Prasad S. Adusumilli contributed essential clinical data. Yi-Chen Yeh and Camelia S. Sima analyzed the data. Yi-Chen Yeh, Jun-ichi Nitadori, Kyuichi Kadota, Prasad S. Adusumilli and William D. Travis wrote the paper.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: Male:Female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 3.Youlden DR, Cramb SM, Baade PD. The international epidemiology of lung cancer: Geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 4.The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukui T, Sakakura N, Mori S, et al. Controversy about small peripheral lung adenocarcinomas: How should we manage them? J Thorac Oncol. 2007;2:546–552. doi: 10.1097/JTO.0b013e318060d30d. [DOI] [PubMed] [Google Scholar]
- 6.Blasberg JD, Pass HI, Donington JS. Sublobar resection: A movement from the lung cancer study group. J Thorac Oncol. 2010;5:1583–1593. doi: 10.1097/jto.0b013e3181e77604. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new international association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed iaslc/ats/ers classification of lung adenocarcinoma: Prognostic subgroups and implications for further revision of staging based on analysis of 514 stage i cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the iaslc/ats/ers lung adenocarcinoma classification for prognosis and association with egfr and kras gene mutations: Analysis of 440 japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 11.Warth A, Muley T, Meister M, et al. The novel histologic international association for the study of lung cancer/american thoracic society/european respiratory society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 12.Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: A distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–109. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Makimoto Y, Nabeshima K, Iwasaki H, et al. Micropapillary pattern: A distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (</=20 mm) with mixed bronchioloalveolar and invasive subtypes (noguchi’s type c tumours) Histopathology. 2005;46:677–684. doi: 10.1111/j.1365-2559.2005.02126.x. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami T, Nabeshima K, Makimoto Y, et al. Micropapillary pattern and grade of stromal invasion in pt1 adenocarcinoma of the lung: Usefulness as prognostic factors. Mod Pathol. 2007;20:514–521. doi: 10.1038/modpathol.3800765. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein a expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol. 2007;20:638–647. doi: 10.1038/modpathol.3800780. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol. 2008;21:992–1001. doi: 10.1038/modpathol.2008.79. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Mora N, Presmanes MC, Monroy V, et al. Micropapillary lung adenocarcinoma: A distinctive histologic subtype with prognostic significance. Case series. Hum Pathol. 2008;39:324–330. doi: 10.1016/j.humpath.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Liang Z, Gao J, Luo Y, Liu T. Pulmonary adenocarcinoma with a micropapillary pattern: A clinicopathological, immunophenotypic and molecular analysis. Histopathology. 2011;59:1204–1214. doi: 10.1111/j.1365-2559.2011.04050.x. [DOI] [PubMed] [Google Scholar]
- 20.Riquet M, Foucault C, Berna P, Assouad J, Dujon A, Danel C. Prognostic value of histology in resected lung cancer with emphasis on the relevance of the adenocarcinoma subtyping. Ann Thorac Surg. 2006;81:1988–1995. doi: 10.1016/j.athoracsur.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer. 2010;116:659–669. doi: 10.1002/cncr.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohtaki Y, Yoshida J, Ishii G, et al. Prognostic significance of a solid component in pulmonary adenocarcinoma. Ann Thorac Surg. 2011;91:1051–1057. doi: 10.1016/j.athoracsur.2010.11.071. [DOI] [PubMed] [Google Scholar]
- 23.Nitadori J-i, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105:1212–1220. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage i lung adenocarcinoma. Mod Pathol. 2012;25:1117–1127. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myung JK, Choe G, Chung DH, et al. A simple inflation method for frozen section diagnosis of minute precancerous lesions of the lung. Lung Cancer. 2008;59:198–202. doi: 10.1016/j.lungcan.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Chung JH, Jheon S, et al. The accuracy of frozen section diagnosis of pulmonary nodules: Evaluation of inflation method during intraoperative pathology consultation with cryosection. J Thorac Oncol. 2010;5:39–44. doi: 10.1097/JTO.0b013e3181c09f9c. [DOI] [PubMed] [Google Scholar]
- 27.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: Modification of the 2004 who mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, egfr mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 28.Donington J, Ferguson M, Mazzone P, et al. American college of chest physicians and society of thoracic surgeons consensus statement for evaluation and management for high-risk patients with stage i non-small cell lung cancer. Chest. 2012;142:1620–1635. doi: 10.1378/chest.12-0790. [DOI] [PubMed] [Google Scholar]
- 29.Kundel HL, Polansky M. Measurement of observer agreement1. Radiology. 2003;228:303–308. doi: 10.1148/radiol.2282011860. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for t1 n0 non-small cell lung cancer. Lung cancer study group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. discussion 622–613. [DOI] [PubMed] [Google Scholar]
- 31.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage i non-small cell lung cancer: A 13-year analysis. Ann Thorac Surg. 2006;82:408–415. doi: 10.1016/j.athoracsur.2006.02.029. discussion 415–406. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg. 2011;92:1819–1823. doi: 10.1016/j.athoracsur.2011.06.099. discussion 1824–1815. [DOI] [PubMed] [Google Scholar]
- 33.Yamato Y, Tsuchida M, Watanabe T, et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg. 2001;71:971–974. doi: 10.1016/s0003-4975(00)02507-8. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura H, Saji H, Ogata A, Saijo T, Okada S, Kato H. Lung cancer patients showing pure ground-glass opacity on computed tomography are good candidates for wedge resection. Lung Cancer. 2004;44:61–68. doi: 10.1016/j.lungcan.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T, Okada A, Imakiire T, Koike T, Hirono T. Intentional limited resection for small peripheral lung cancer based on intraoperative pathologic exploration. Jpn J Thorac Cardiovasc Surg. 2005;53:29–35. doi: 10.1007/s11748-005-1005-7. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: Fifty-case experience. J Thorac Cardiovasc Surg. 2005;129:991–996. doi: 10.1016/j.jtcvs.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 37.Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg. 2009;88:1106–1111. doi: 10.1016/j.athoracsur.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 38.Yoshioka M, Ichiguchi O. Selection of sublobar resection for c-stage ia non-small cell lung cancer based on a combination of structural imaging by ct and functional imaging by fdg pet. Ann Thorac Cardiovasc Surg. 2009;15:82–88. [PubMed] [Google Scholar]
- 39.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage ia lung cancer. Ann Surg. 2010;251:550–554. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 40.Motoi N, Hamanaka W, Oba T, et al. Evaluation of histologic accuracy on diagnosis and invasion of small-sized lung cancer using intra-operative frozen section. J Thorac Oncol. 2011;6:S566. [Google Scholar]
- 41.Solis LM, Behrens C, Raso MG, et al. Histologic patterns and molecular characteristics of lung adenocarcinoma associated with clinical outcome. Cancer. 2012;118:2889–2899. doi: 10.1002/cncr.26584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warth A, Stenzinger A, von Brünneck A-C, et al. Interobserver variability in the application of the novel iaslc/ats/ers classification for pulmonary adenocarcinomas. Eur Respir J. 2012;40:1221–1227. doi: 10.1183/09031936.00219211. [DOI] [PubMed] [Google Scholar]
- 43.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol. 2012;25:1574–1583. doi: 10.1038/modpathol.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walts AE, Marchevsky AM. Root cause analysis of problems in the frozen section diagnosis of in situ, minimally invasive, and invasive adenocarcinoma of the lung. Arch Pathol Lab Med. 2012;136:1515–1521. doi: 10.5858/arpa.2012-0042-OA. [DOI] [PubMed] [Google Scholar]
- 45.Xu L, Tavora F, Battafarano R, Burke A. Adenocarcinomas with prominent lepidic spread: Retrospective review applying new classification of the american thoracic society. Am J Surg Pathol. 2012;36:273–282. doi: 10.1097/PAS.0b013e31823b3eeb. [DOI] [PubMed] [Google Scholar]
- 46.Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage i disease. Am J Surg Pathol. 2014;38:448–460. doi: 10.1097/PAS.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kilicgun A, Turna A, Sayar A, Solak O, Urer N, Gurses A. Very important histopathological factors in patients with resected non-small cell lung cancer: Necrosis and perineural invasion. Thorac Cardiovasc Surg. 2010;58:93–97. doi: 10.1055/s-0029-1186240. [DOI] [PubMed] [Google Scholar]
- 48.Schuchert MJ, Schumacher L, Kilic A, et al. Impact of angiolymphatic and pleural invasion on surgical outcomes for stage i non-small cell lung cancer. Ann Thorac Surg. 2011;91:1059–1065. doi: 10.1016/j.athoracsur.2010.11.038. discussion 1065. [DOI] [PubMed] [Google Scholar]
- 49.Koike T, Yoshiya K, Tsuchida M, Toyabe S. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage ia non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013;146:372–378. doi: 10.1016/j.jtcvs.2013.02.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. Experience in lung pathology of the 5 pathologists participating in reviewing frozen section slides.
Supplement Figure 2. Strategy for determining the reason for discrepancy between frozen section diagnoses and permanent section diagnoses. In brief, if the interpretation was the same for the original frozen section slides and the frozen section control slides but was different from that for the permanent section slides, we considered the reason for discrepancy to be sampling error. If the interpretation was the same for the frozen section control slides and the permanent section slides but was different from that for the original frozen section slides, we considered the reason for discrepancy to be interpretation error. If the interpretations for the original frozen section slides, the frozen section control slides, and the permanent section slides were all different from each other, we considered the reason for discrepancy to be sampling error plus interpretation error. FS, frozen section; PS, permanent section.