Abstract
The genus Astronium (Anacardiaceae) includes species, such as Astronium fraxinifolium, Astronium graveolens, and Astronium urundeuva, which possess anti-inflammatory, anti-ulcerogenic, healing, and antimicrobial properties. Nanostructured lipid systems are able to potentiate the action of plant extracts, reducing the required dose and side effects and improving antimicrobial activity. This work aims to evaluate a nanostructured lipid system that was developed as a strategy to improve the anti-Candida albicans activity of hydroethanolic extracts of stems and leaves from Astronium sp. The antifungal activity against C. albicans (ATCC 18804) was evaluated in vitro by a microdilution technique. In addition to the in vitro assays, the Astronium sp. that showed the best antifungal activity and selectivity index was submitted to an in vivo assay using a model of vulvovaginal candidiasis infection. In these assays, the extracts were either used alone or were incorporated into the nanostructured lipid system (comprising 10% oil phase, 10% surfactant, and 80% aqueous phase). The results indicated a minimal inhibitory concentration of 125.00 µg/mL before incorporation into the nanostructured system; this activity was even more enhanced when this extract presented a minimal inhibitory concentration of 15.62 µg/mL after its incorporation. In vivo assay dates showed that the nanostructure-incorporated extract of A. urundeuva leaves was more effective than both the unincorporated extract and the antifungal positive control (amphotericin B). These results suggest that this nanostructured lipid system can be used in a strategy to improve the in vitro and in vivo anti-C. albicans activity of hydroethanolic extracts of Astronium sp.
Keywords: plant extract, anticandidal activity, microdilution, microemulsion, vulvovaginal candidiasis
Introduction
Certain species of Candida are classified as some of the most important pathogenic and fungal microorganisms involved in opportunistic diseases. They are responsible for many pathological processes that are capable of triggering highly complex injuries and creating risks to human health, such as vulvovaginal candidiasis (VVC), which is characterized as an inflammation of the vulva and vagina routinely associated with the presence of Candida species. This genus consists of approximately 200 different species, occurring in various body sites, such as the oropharynx, oral cavity, skin folds, bronchial secretions, vagina, urine, and feces.1,2
The yeast fungus Candida albicans, especially prevalent in cases of vaginal infections, is found in 80%–95% of symptomatic cases of vulvovaginitis as well as in asymptomatic cases of the disease or cases with no clinical signs of the disease in question. C. albicans is a dimorphic fungus and a commensal of the skin, gastrointestinal tract, and genitalia and is classified as an opportunistic pathogen (ie, a pathogen that only causes disease in conditions of immunosuppression with signs and symptoms being most critical to patients with underlying diseases such as diabetes and patients with the acquired immunodeficiency syndrome [HIV] and neutropenia).3
According to the World Health Organization, VVC is estimated to comprise approximately 95% of female genital tract infections, characterizing it as a condition of great importance and whose diagnosis is essential to better match the treatment.4
A number of clinically relevant models of systemic and mucosal candidiasis in rodents were established predominantly to study the host–pathogen interactions, efficacy, and pharmacokinetics of antifungal drugs and vaccines. In vivo experimental models of VVC have been extremely useful in identifying hormonal factors that influence the infection, the virulence of the yeasts, the susceptibility of the yeasts to treatment, and treatment of infection. Unlike its ability to colonize humans, C. albicans is not a natural colonizer of the mucosal surfaces of rodents; therefore, it is necessary to effectively and safely establish an infection model that simulates all the patterns commonly observed in women with VVC. However, such rodent models involve both advantages and limitations. The advantage is that any host response to C. albicans is not affected by preexisting innate or adaptive immune responses to the fungus, but the establishment of colonization or infection in the vaginal mucosa usually requires the use of immunosuppressants or antibiotics, treatment with estrogen, or the use of germ-free or transgenic animals.5
Most Candida infections can be treated with azoles that are administered orally or topically. It is also important to exclude predisposing factors and to treat the partner, when the Candida is sexually transmitted.6
Despite all the scientific advances in drug therapy, multi-drug resistance by some fungal strains is also increasing and is worrisome. Recently, several genital isolates of Candida have demonstrated drug resistance in cases of recurrent candidiasis. This, in addition to the negative environmental and social impacts caused by chemical residues from synthetic medicines2 and the increased cost and mortality rates caused by multidrug resistant strains in intensive care units,6,7 has stimulated a search for new substances with anti-Candida activity.
The Astronium genus, a member of the Anacardiaceae family, includes approximately 13 species found from Mexico to Argentina, with 12 species found in Brazil, especially in the Caatinga, Amazon, and Atlantic forest regions.8 Some species are of great economic importance because they are used as ornamental plants, produce edible fruits and, especially, can be used as timber for construction.9 Astronium fraxinifolium, Astronium graveolens, and Astronium urundeuva are some species that are commonly used to treat several disorders, including microbial infections. Among these species, A. urundeuva is the most well-known due to its biological properties as some studies have described the presence of important components, including flavonoids, essential oils, and hydrolyzable and condensed tannins.10 Some of the antiulcer effects of aqueous and alcoholic extracts of plants from the Astronium genus against experimental ulcers were observed in rats. More recently, other studies have shown antioxidant, antidiarrheal, anti-inflammatory, and analgesic effects.11
The chemical complexity of extracts is an extremely important consideration for their incorporation into vehicles that enhance the release of the active ingredient. Vehicles must concurrently improve the solubility of the drug, minimize the degradation process, reduce any toxicity, and mask any bad taste while controlling active absorption and the biological response.
Technological strategies, such as nanostructured lipid systems, that have the ability to efficiently compartmentalize several active groups and modify their properties and behavior in a biological environment are promising vehicles for plant extracts.12,13 Among the nanostructured lipid systems, we highlight microemulsions (MEs), which are transparent, fluid, optically isotropic, and thermodynamically stable dispersions and are composed of two immiscible liquids containing appropriate amounts of surfactant and sometimes a cosur-factant.14 They are optically transparent due to the reduced size of the microdomains of water and oil (10–200 nm), which do not scatter visible light.15
MEs have been broadly studied for their ability to enhance the bioavailability of poorly soluble drugs as they offer a cost-effective approach in such cases. MEs have a very low surface tension and are the size of a small droplet, which results in high absorption and permeation. Interest in these versatile carriers is increasing, and their applications have been diversified to various administration routes in addition to the conventional oral route.16–18
MEs have great potential as intravaginal/rectal drug delivery vehicles for lipophilic drugs, such as microbicides, steroids, and hormones because of their high drug solubilization capacity, increased absorption, and improved clinical potency. Nevertheless, the use of MEs for intravaginal or intrarectal administration is constrained by rigorous demands related to the nontoxicity of the formulation and its bioavailability.19 Some authors developed MEs containing cholesterol as oil phase that were useful for topical application of several drugs.
Oliveira et al20 evaluated the potential of a fluconazole-loaded ME for the treatment of cutaneous leishmaniases. The formulation also contained cholesterol (oil phase), phosphate buffer at pH 7.4 (aqueous phase), and mixture of surfactants soybean phosphatidylcholine (SPC), polyoxyl-60 hydrogenated castor oil, and sodium oleate in a proportion of 3:8:6.20
Franzini et al18 investigated the structural properties induced by the composition of biocompatible phospholipid-based ME and amphotericin B association. An anionic ME containing a mixture of SPC, Tween-20 and sodium oleate (surfactant), and cholesterol (oil phase) was investigated as drug carriers for amphotericin B, and the results showed that several structures can be formed depending on the composition of the ME.18 Silveira et al21 published a review that mentioned some studies that employed the use of MEs as delivery systems for amphotericin B in topical eye treatment.
Silva et al22 developed and characterized a biocompatible isotropic and anisotropic oil-in-water colloidal dispersion containing a mixture of surfactants (SPC, polyoxyethylene glycerol tri-hydroxy stearate and sodium oleate, 7:7:3), purified water (aqueous phase), and cholesterol (oil phase). The ME had appropriate diameter size for intravenous route administration and was used to load methyl dihydrojasmonate, a jasmonate-derived compound, which is very poorly water-soluble.22 Assumpção et al23 also used this formulation to load doxorubicin, an anticancer drug. They evaluated the pharmacokinetic profile and cardiotoxicity and the results showed that the ME increased the concentrations of the drug in plasma and decreased the distribution volume when compared to the conventional doxorubicin.23
Some methods for structural characterization include rheology and electric conductivity, viscosity, light scattering, electric birefringence, sedimentation, X-ray diffraction (XRD), neutron diffraction, transmission electron microscopy (TEM), high-performance liquid chromatography, and resonance nuclear magnetic resonance spectroscopy.24
The objective of this study was to evaluate a nanostructured lipid system composed of SPC, cholesterol (CHO), polyoxyethylene (20) cetyl ether (Brij® 58), and phosphate-buffered saline (PBS; pH 7.4) as a strategy to improve the in vitro and in vivo anti-C. albicans activity of hydroethanolic extract of Astronium sp.
Materials and methods
Plant material and extraction
A. fraxinifolium stems and leaves were collected in December 2007 in Porto Nacional, Tocantins, Brazil, and authenticated by Eduardo R dos Santos, PhD. A voucher specimen (SANO N° 333) was deposited at the Herbarium of the University of Tocantins in Palmas/TO.
A. graveolens stems and A. graveolens leaves were collected in October 2007 in Campinas, São Paulo, Brazil. A. urundeuva stems and A. urundeuva leaves (AUl) were collected in November 2007 in Bálsamo and Votuporanga, São Paulo, Brazil. Both were authenticated by Jorge Tamashiro, PhD, from the Institute of Biosciences, State University of Campinas (IB – UNICAMP), in the city of Campinas. A voucher specimen of A. graveolens (SANO N° 148133) and another of A. urundeuva (SANO N° 1446) were deposited at the Herbarium of the IB – UNICAMP.
Extracts were obtained using an exhaustive percolation method according to Simões et al.25
After the extraction process, the leachates were evaporated under reduced pressure at 50°C on a rotary evaporator. The extracts were transferred to a tared glass and left in the container until the solvent was completely removed. When necessary, the extracts were freeze-dried to remove water.
In vitro antifungal activity
The C. albicans samples used in this study were all obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Minimal inhibitory concentration (MIC) was determined using the microdilution technique according to the standard reference method M27-A3 CLSI26 and Duarte et al27 for yeast (ATCC C. albicans [18804]) with modifications. The extracts were dissolved in 20% dimethyl sulfoxide (DMSO) and Mueller-Hinton broth for an initial concentration of the extract of 2,000 µg/mL. Then, a two-fold serial dilution was performed to obtain concentrations ranging from 7.8 µg/mL to 1,000 µg/mL. For each concentration, 100 µL/well was added to a 96-well microplate containing 80 µL/well of RPMI-1640, and the yeast inocula were standardized at 2.5×103 CFU/mL. The positive controls were amphotericin B and fluconazole; 20% DMSO was used as negative control. The microplates were incubated at 37°C for 48 hours for the yeast. The MIC of the samples was determined after the addition (30 µL) of 2% of 2,3,5-triphenyltetrazolium chloride and incubation at 37°C for 2 hours. Yeast growth changes the colorless triphenyltetrazolium chloride to red color. MIC was defined as the lowest sample concentration that prevented this change and exhibited inhibition of microorganism growth. In all assays, the samples were processed in triplicate.
Nanostructured lipid system preparation
Pseudoternary phase diagram
MEs containing CHO (10%) as an oil phase, PBS (pH 7.4) as an aqueous phase (80%), and a surfactant mixture SPC/Brij® 58 (10%) were used to obtain the optimal hydrophilic–lipophilic balance (HLB) value for the stabilization of the clear ME system.28 The HLB value describes the simultaneous attraction of the surfactant mixture for the oil and aqueous phases; when the HLB value is similar to the required HLB of the oil phase of the ME, the system provides the minimum energy condition for ME formation. The composition of the surfactant system SPC/Brij® 58 was established to obtain an HLB value of 12.47.
For this purpose, SPC and Brij® 58 were added at a 1:2 ratio. To this semisolid mixture of SPC/Brij® 58 (S), CHO (O) was added. To obtain the phase diagram, adequate S/O weight ratios were used in the range of 1:9–9:1. The phase diagram was constructed by fixing the proportion of the surfactant and oil phase and titrating with the aqueous phase (PBS 50 mmol/L pH 7.4). The mixture was sonicated using a rod sonicator (Q700 of Qsonica®) with a potency of 700 W in discontinuous mode for 10 minutes with an interval of 30 seconds in an ice bath every 2 minutes during the sonication process. After sonication, the MEs were centrifuged at 11,180× g for 15 minutes to eliminate the waste released by the titanium rod sonicator. Each region of the system was visually classified as a viscous system; as a liquid that was optically transparent, translucent, or opaque; or as a phase separation. Thus, it was possible to delimit the different regions of the phase diagram. From these data, the region for the incorporation of the extract fractions was selected.
MEs were prepared 24 hours before the experiments and maintained at 25°C±0.1°C to complete the equilibration of the system.
Preparation of vegetable extract-loaded nanostructured lipid system
After obtaining the ME, the extracts of Astronium sp. were loaded into the nanostructured lipid system. Then, 0.004 g of extract was added to 2 mL of ME followed by the mixture being homogenized and sonicated for 3 minutes at the room temperature in a discontinuous mode to facilitate incorporation of the nanostructured material into the lipid system at a concentration of 2,000 µg/mL. Vegetable extract-loaded nanostructured lipid system was characterized by mean diameter and polydispersity in a Zetasizer Nano NS using the Zetasizer Software.
Nanostructured lipid system characterization
Diameter mean and polydispersity index
ME droplet diameters were determined with and without vegetable extracts. All samples were diluted (100 µL of sample in 900 µL of deionized water). The ME droplet size distribution was determined by dynamic light scattering in a Zetasizer Nano model (Malvern Instruments, Malvern, UK). Samples were oriented in the analysis chamber so that the laser beam could cross throughout dispersion. The temperature of the system was maintained at 20°C, and the laser wavelength was 532 nm. Ten determinations of the diameter and polydispersity index (PDI) of the drops in each sample were made (n=3).
Transmission electron microscopy
TEM was applied to determine the microstructure of ME. The ME sample was first lyophilized and resuspended in water and then placed on a carbon-coated copper grid. The TEM images were provided by JEM-2100 (JEOL, Tokyo, Japan) with an operating voltage of 120 kV.
X-ray diffraction
XRD characterization of ME and AUl loaded into the ME was carried out using a Bruker D8 Advance diffractometer. A rotating X-ray generator (40 kW and 40 mA) with Cu Kα radiation (1.5418 Å) was used. θ–2θ scans were made for all samples; the ranges of XRD measurements were usually from 2.4° to 7° 2θ with a scanning rate of 0.01°/sec. The samples were transferred to a spinner stage in a thermally controlled sample holder centered in the X-radiation beam; all X-ray scatterings were done at 25°C.
Bioadhesion assay
The bioadhesive force between the pig vaginal mucosa and the samples was assessed by detachment test using a TA.XTplus Texture Analyser (Stable Micro Systems, Surrey, UK). The porcine vaginal mucosa was obtained from a local slaughterhouse and cleaned. The samples were packed into shallow cylindrical vessels, and the test started lowering the analytical probe, which contained the vaginal mucosa, at a constant speed (1 mm⋅s−1) onto the surface of the sample. The vaginal mucosa and the sample were kept in contact during 60 seconds, and no force was applied during this interval. After 60 seconds, the skin was drawn upward (0.5 mm⋅s−1) until the contact between the surfaces was broken. The bioadhesive force of the samples was measured in the maximum detachment force as the resistance to the withdrawal of the probe, what reflects the bioadhesion characteristic. Seven replicates were analyzed at 37°C±0.5°C.29–31
In vitro cytotoxic activity
Vero line (ATCC® CCL-81™) was used to determine cytotoxicity (IC50). The cells were maintained in flasks with a 12.50 cm2 surface area containing 10 mL of culture medium incubated at 37°C in 5% CO2. The culture medium consisted of DMEM (Vitrocell®) medium supplemented with 10% fetal bovine serum, gentamicin sulfate (50 mg/L), and amphotericin B (2 mg/L).
The cytotoxicity assay was performed as standardized in our lab.32 This technique consists of collecting the cells using a solution of trypsin/EDTA (Vitrocell®), centrifuging (2,000 rpm for 5 minutes) and counting the number of cells in a Neubauer chamber followed by adjustment of the cell concentration to 3.4×105 cells/mL in DMEM. Then, 200 µL of this suspension was deposited in each well of a 96-well microplate to obtain a concentration of 6.8×104 cells/well, and the microplates were then incubated at 37°C with 5% CO2 for 24 hours to facilitate cell attachment to the plate. The following dilutions of test compounds were prepared to obtain concentrations from 1,000 to 3.90 µg/mL. These dilutions were added to the cells after the removal of the medium and the non-adherent cells. Then, the cells were incubated for an additional 24 hours. The cytotoxicity of the compounds was determined by adding 30 µL of resazurin and reading on a Spectrafluor Plus (TECAN®) reader after 6 hours of incubation using a microplate and excitation and emission filters at wavelengths of 530 nm and 590 nm, respectively. The IC50 was defined as the highest concentration of compound that allowed the viability of at least 50% of the cells.
Selectivity index
The selectivity index (SI) of the extracts alone or incorporated into the nanostructured system was calculated from the ratio of the IC50 to the MIC values. A higher SI value means that the analyzed extract is more active against the yeast and less cytotoxic to the host with an SI >10 being considered a promising value for the substances tested.33
Experimental VVC
Female Wistar rats (body weight 250–280 g) were collectively housed in the experimental room for at least 7 days before the experiments were started. All protocols were approved by the Ethics Committee on the Use of Animals in Research – CEUA School of Pharmaceutical Sciences of Araraquara – UNESP (protocol number 34/2013). The rat model of vaginal infection was established based on Araújo et al2 for obtaining a chronic and homogeneous infection. Animals were immunosuppressed by administration of one dose of cyclophosphamide (Sigma®, 20 mg/kg bw), and estrus was induced by subcutaneous administration of estradiol (Sigma®) at a dose of 0.2 mg/mL once daily for 4 days before infection. Rats were inoculated intravaginally (day 6) with 0.1 mL of C. albicans (ATCC 18804) (5.0×107 cells/mL)34 using a micropipette with disposable tips. On days 2, 6, and 10 after the infection, the vaginal load of C. albicans was evaluated through vaginal lavage with 0.1 mL of PBS, and the fungal count was determined by the CFU assay on sabouraud dextrose agar (SDA).35 Eight days after the treatment period, the animals were subjected to recurrent control of the infection to further identify and to diagnose the infectious states that may eventually emerge after the previously performed treatment. At day 24, the animals were euthanatized by CO2 chamber intoxication.
For the evaluation of the biological effects of extract from A. urundeuva leaves on C. albicans vaginal infections, the formulation containing extract alone, the nanostructured deliver system, or the control treatment tetracycline hydrochloride amphotericin B cream (EMS® São Paulo, Brazil) was administered topically to the infected animals. Female Wistar rats (n=35) were randomized equally into the following seven groups (Table 1): non-infected controls (Group 1), infected controls (Group 2), infected and treated with vaginal cream containing tetracycline hydrochloride amphotericin B – 25 mg/g +12.5 mg/g (Group 3), infected and treated with 0.1 mL of a solution containing 20% DMSO (Group 4), infected and treated with 0.1 mL of pure extract (the MIC value obtained in in vitro screening [250 µg/mL]) (Group 5), infected and treated with the nanostructured lipid system (Group 6), and infected and treated with A. urundeuva extract-loaded nanostructured lipid system (Group 7).
Table 1.
Experimental groups and treatments used in the study
| Experimental groups | Specification | Treatment |
|---|---|---|
| 1 | Non-infected control | – |
| 2 | Infected control | – |
| 3 | Positive control (antimicrobial treatment) | 0.1 mL of antifungal cream (tetracycline + amphotericin B) |
| 4 | Solvent control | 0.1 mL of a solution (20% DMSO) |
| 5 | Treatment 1 | 0.1 mL of pure extract (250 µg/mL) |
| 6 | Vehicle control for microemulsion | 0.1 mL of microemulsion |
| 7 | Treatment 2 | 0.1 mL of extract-loaded nanostructured lipid system (250 µg/mL) |
Abbreviation: DMSO, dimethyl sulfoxide.
Results and discussion
In vitro studies of Astronium extracts
The MIC values for the hydroethanolic extracts of the stems and leaves of A. fraxinifolium, A. graveolens, and A. urundeuva alone or incorporated into the nanostructured system are shown in Table 2.
Table 2.
Results of biological assessments (MIC and IC50) and determination of the SI of extracts of Astronium sp. alone (unincorporated) or incorporated into the nanostructured lipid system for Candida albicans
| Extracts | Parameters
|
|||||
|---|---|---|---|---|---|---|
| Unincorporated
|
Incorporated
|
|||||
| IC50(µg/mL) | MIC (µg/mL) | SI | IC50(µg/mL) | MIC (µg/mL) | SI | |
| AFs | 767.20 | >1,000.00 | <0.76 | >1,000.00 | 62.50 | >16.00 |
| AFl | 950.00 | >1,000.00 | <0.95 | >1,000.00 | 250.00 | >4.00 |
| AGs | >1,000.00 | >1,000.00 | <1.00 | >1,000.00 | 62.50 | >16.00 |
| AGl | 833.00 | >1,000.00 | <0.83 | >1,000.00 | 125.00 | >8.00 |
| AUs | 900.00 | >1,000.00 | <0.90 | >1,000.00 | 62.50 | >16.00 |
| AUl | 536.10 | 125.00 | 4.28 | >1,000.00 | 15.62 | >64.02 |
| Positive controls | MIC (µg/mL) | |||||
| Amphotericin B | 0.06 | |||||
| Fluconazole | 8.00 | |||||
Abbreviations: MIC, minimal inhibitory concentration; IC50, half maximal inhibitory concentration; AFs, Astronium fraxinifolium stem; AFl, A. fraxinifolium leaves; AGs, A. graveolens stem; AGl, A. graveolens leaves; AUs, A. urundeuva stem; AUl, A. urundeuva leaves; SI, selectivity index.
The unincorporated hydroethanolic extracts from stems and leaves of A. fraxinifolium and A. graveolens and stems of A. urundeuva showed no antifungal activity. The hydroethanolic extract from leaves of A. urundeuva showed activity against C. albicans with an MIC of 125 µg/mL.
After incorporation into the nanostructured system, the results showed that C. albicans stem and leaf extracts from all species of Astronium sp. had their activity enhanced: the stems of all three species showed MICs of 62.5 µg/mL; the leaves of A. fraxinifolium and A. graveolens, respectively, showed MICs of 250 µg/mL and 125 µg/mL; and the leaves of A. urundeuva demonstrated even more enhanced activity, presenting an MIC of 15.62 µg/mL.
Pseudoternary phase diagram and characterization
From our initial studies with ME formulations, we found that the clearest system containing CHO, SPC/Brij® 58, and aqueous buffer was obtained from a surfactant mixture with an HLB value of 12.47, which corresponds to an SPC/Brij® 58 ratio of approximately 1:2. Thus, this surfactant composition with an HLB value of 12.47 was used throughout the remainder of the study.
To suitably characterize the ME systems, it is necessary to determine the pseudoternary phase diagram that describes the ideal experimental conditions and ratios for combining the components to obtain a clear preparation. For SPC/Brij® 58-stabilized ME, the pseudoternary phase diagram (Figure 1) demonstrates that a broad range of possibilities can successfully obtain clear systems in the phase diagram regions in which the oil-in-water ME prevails.
Figure 1.
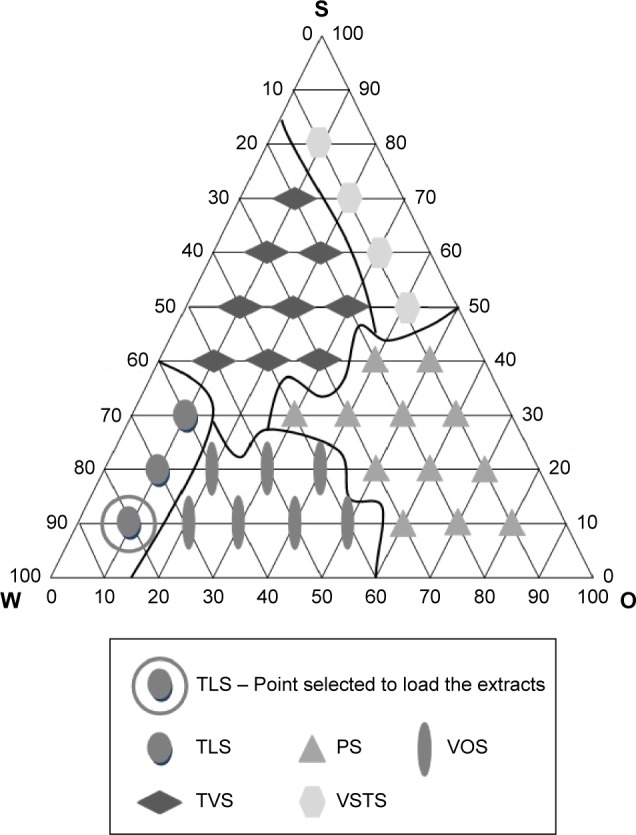
Pseudoternary phase diagram for the system containing SPC/Brij® 58 (S), CHO (O), and aqueous buffer (W).
Abbreviations: TLS, transparent liquid system; VOS, viscous and opaque system; PS, phase separation; TVS, transparent viscous system; VSTS, viscous semitransparent system.
The ME formulation was selected to obtain an oil-in-water system when diluted with aqueous buffer. The circled point in the phase diagram of Figure 1 represents the composition of the formulation used to incorporate the vegetable extracts in the present study.
The points were obtained with different structural characteristics according to the variation of the oil and water ratios.
The phase diagram shows the characteristics of points classified as transparent liquid system (TLS), viscous and opaque system (VOS), phase separation (PS), transparent viscous system (TVS), and viscous semi-transparent system (VSTS). The regions known as TLS were observed where there was low oil concentration (10%) and surfactant concentration (10%–30%). VOS was found in the interior region and center of the diagram with a low oil concentration (20%–50%) and surfactant concentration (10%–20%). PS occurred with a low water concentration (10%–40%) and surfactant concentration (10%–40%). TVS had an oil phase concentration between 20% and 50% and a surfactant concentration between 40% and 70%, and VSTS (viscous semi transparent system) had a low water concentration (10%) and high surfactant concentration (50%–80%).
Diameter mean and PDI
Light scattering is a routine technique that is used for determining the diameter of the internal phase of MEs. Light scattering assays have been developed for liquid MEs that have been diluted with deionized water to detect experimental errors.36
Table 3 shows the mean values and standard deviation of the particle size and PDI for the nanostructured lipid system (ME) and the extracts incorporated in the ME.
Table 3.
Determination of the droplet size and polydispersity of the ME by light scattering
| Formulation | Mean diameter ± SD (nm)* | Mean PDI ± SD* |
|---|---|---|
| ME | 117.200±1.966 | 0.245±0.006 |
| AFs | 123.600±0.252 | 0.222±0.003 |
| AFl | 133.100±0.551 | 0.276±0.008 |
| AGs | 126.700±0.702 | 0.249±0.007 |
| AGl | 135.400±0.551 | 0.237±0.002 |
| AUs | 141.900±1.137 | 0.268±0.000 |
| AUl | 146.700±1.825 | 0.255±0.010 |
Note:
SD, PDI.
Abbreviations: ME, microemulsion; SD, standard deviation; PDI, polydispersity index; AFs, Astronium fraxinifolium stem; AFl, A. fraxinifolium leaves; AGs, A. graveolens stem; AGl, A. graveolens leaves; AUs, A. urundeuva stem; AUl, A. urundeuva leaves.
As per Table 3, the diameter of the particles of the ME was 117.200±1.966 nm. The incorporation of the extracts caused a small variation in the particle diameter size, without exception, varying between 123.600±0.252 nm and 146.700±1.825 nm. All values are in the range of 10–200 nm (100–2,000 Å), which is the optimal range for MEs according to Formariz et al.37 When comparing the ME and the formulations containing the plant extracts, there was a small increase in the size of the particle diameter, a strong indication that the incorporation of the extracts in the nanostructured lipid system occurred. The PDI was calculated by dividing the mean size of the droplets by the mean number of the measured droplets. For both the MEs and the plant extract-loaded MEs, the light scattering analysis showed PDI values of 0.222–0.276 indicating a good size distribution of the droplets in the ME system. This parameter directly reflects the size homogeneity of the droplets in the total ME.
Transmission electron microscopy
Figure 2 shows TEM photographs of the nanostructured lipid system, where isolated nanoparticles can be seen at a panoramic vision (Figure 2A – 400,000× magnification) and as expected, the spherical shape of a particle (Figure 2B – 800,000× magnification).
Figure 2.
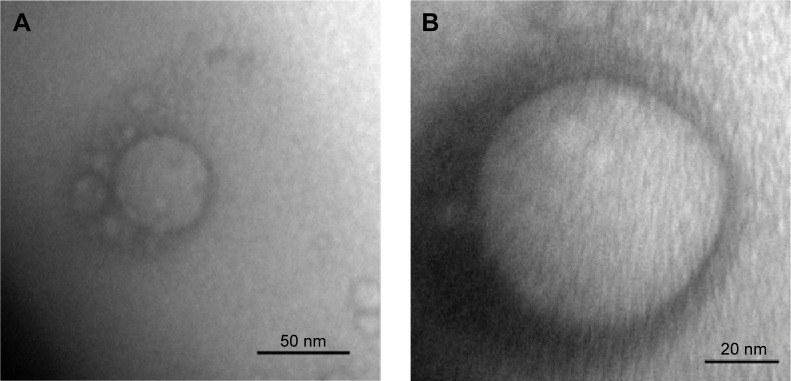
Transmission electron micrographs of ME at a magnification of 400,000× (A) and 800,000× (B), respectively.
Abbreviation: ME, microemulsion.
X-ray diffraction
Figure 3 observed a very sharp scattering peak, which is followed by another less pronounced one that was observed in formulations ME and ME + AUl. The periodic interlayer spacing (d) was calculated by the Braggs’s equation nλ = 2dsinθ.38,39 These two peaks are equidistant and indicate lamellar structure.
Figure 3.
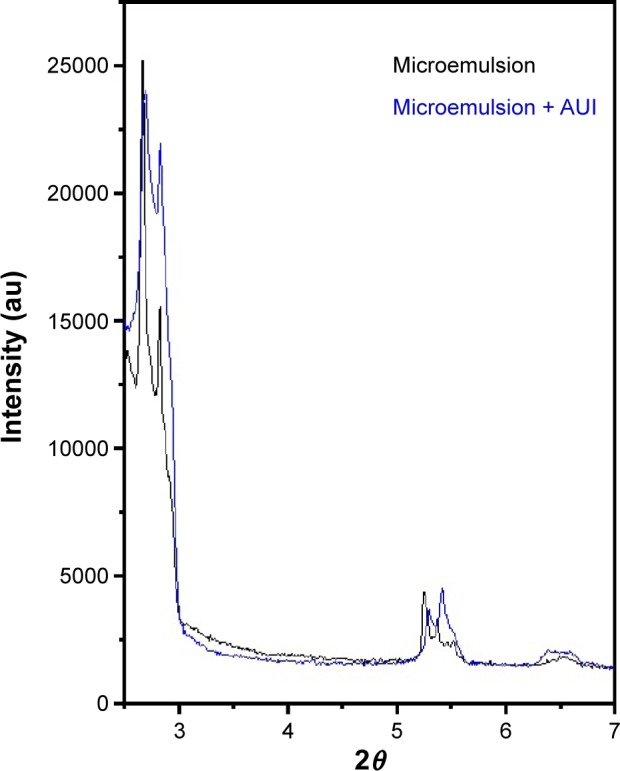
X-ray scattering curves for ME and ME + AUl.
Abbreviations: ME, microemulsion; AUl, A. urundeuva leaves.
Bioadhesion assay
A bioadhesion assay is used to describe the adhesion of synthetic or biological macromolecules to biological tissue, and Figure 4 shows the peak force corresponding to the maximum force between the probe and the tissue as a function of time. The results showed that only the ME had already presented a bioadhesive force, and after loading the extract from leaves of A. urundeuva into the nanosystem, no statistically significant difference was detected.
Figure 4.
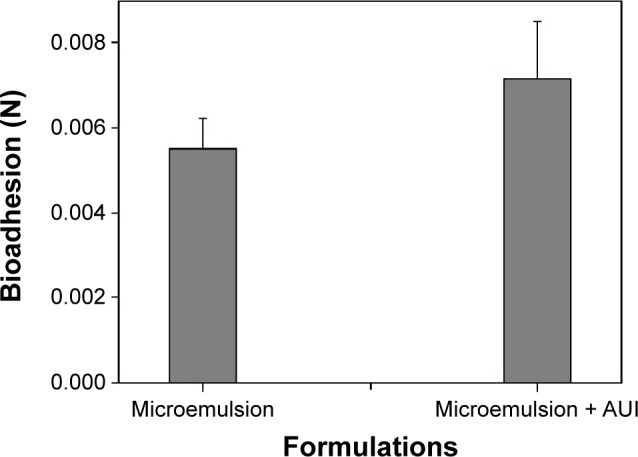
Peak of bioadhesion (N) of AUl-unloaded microemulsion and AUl-loaded microemulsion.
Note: Each value represents the mean (± standard deviation) of at least seven replicates.
Abbreviation: AUl, A. urundeuva leaves.
Cytotoxic activity
The results found in the cytotoxicity assay are shown in Table 2.
Before evaluating any substance in in vivo models, it is widely recommended that an alternative method, such as cell culture, be used. Based on this recommendation, all extracts, alone or incorporated into the nanostructured system, were submitted to cytotoxicity testing in Vero cells, which is a model system of normal eukaryotic cells. The unincorporated extracts tested showed low selectivity (SI <10) compared to the incorporated extracts. Four of the incorporated extracts tested showed SI >10; of these, AUl was considered the best with an SI >64.02. These results showed that this nanostructured system was able to improve the activity of these extracts against the yeast while reducing their cytotoxicity. Thus, the best incorporated extract (AUl) was selected for further testing via in vivo assays performed in an animal model.
In vivo experiments of A. urundeuva leaves
The results obtained from the cultures of the vaginal fluids collected from all of the animals used in the study during the treatment period are shown in Table 4.
Table 4.
Fungal loads (CFUs) obtained from the culture of vaginal fluid collected during the treatment period
| Groups | Treatment
|
||
|---|---|---|---|
| Day 2 | Day 4 | Day 6 | |
| Positive control (infection) | 1,585.0±13.0 | 1,692.0±37.0 | 986.8±11.0 |
| Positive control (tetracycline + amphotericin B) | 1,600.0±9.0 | 836.0±33.0 | 264.6±23.0 |
| Solvent control (DMSO) | 1,608.0±8.0 | 1,641.0±65.0 | 982.8±18.0 |
| Treatment 1 | 1,553.0±27.0a,* | 776.0±73.0b,* | 195.6±50.0b,* |
| Microemulsion | 1,590.0±13.0 | 1,657.0±17.0 | 932.8±21.0 |
| Treatment 2 | 1,589.0±47.0a | 349.0±31.0b,* | 0.0±27.0b,* |
Notes:
Non-significant and
significant differences between treatments 1 and 2 according to parametric post-hoc test (P<0.05 – Tukey’s test).
Significant difference (P<0.05) when compared with the positive control group (tetracycline + amphotericin B – Dunnett’s test).
Abbreviations: CFU, colony-forming unit; DMSO, dimethyl sulfoxide.
All of the groups from the present study behaved in accordance with the antifungal profile expected. The negative and positive controls of infection (Groups 1 and 2) remained constant throughout the experiment, as did the positive anti-fungal control (tetracycline + amphotericin B) (Group 3), which was effective against the fungal strain used. Vehicle control groups (Groups 4 and 6) consistently did not interfere with the elimination of the pathogen in question. The treatment groups (Groups 5 and 6) yielded promising results, as the complete analysis on the eighth day of treatment showed that the incorporation of the A. urundeuva extract in the nanostructured lipid system increased its ability to eliminate the infectious agent and improved its activity compared to the activity of the unincorporated extract.
Regarding the analysis of recurrent infections, all groups exhibited a stable vaginal fungal burden, and new infectious events were not detected after the period of treatment examined in this study.
The biological activity of the plant extracts can be greatly influenced by the presence and amount of active components, which may or may not demonstrate the physicochemical and biological properties responsible for the antimicrobial activity. According to Silva et al40 the main components of the species in the Astronium sp. genus include flavonoids; proanthocyanidins, such as profisetinidine and prorobinetinidine; and a high content of polyphenols, such as tannins and lignins.
From the chemical point of view, tannins are highly reactive compounds, possessing a variety of behaviors, including antimicrobial activity. In addition, protein-binding by adhesins and inhibition of enzymes are able to suppress microbial substrates. These compounds are easily oxidizable, capable of complexing with the cell wall and provoking rupture of the plasmatic membrane, as well as forming complexes with metal ions.41,42
Isolating a biologically active compound from a plant material depends on the polarity of the solvent chosen as a solvent with similar polarity will be able to extract a greater amount of the compound. A suitable solvent for antimicrobial drugs must possess certain characteristics, including low toxicity, ease of evaporation at low temperatures, rapid physiological absorption of the extract, preservative action, and the inability to cause the extract to become disconnected or to form a complex.43
The literature does not present a consensus on evaluation of antimicrobial treatments in relation to MICs. Aligiannis et al44 consider MIC values equal to or lower than 500 µg/mL as potent inhibitors, MICs between 600 µg/mL and 1,500 µg/mL as moderate inhibitors, and MICs above 1,600 µg/mL as weak inhibitors. However, Webster et al45 established MIC values equal to or less than 1,000 µg/mL as satisfactory.
By following the first classification, the extracts of the leaves and stems of A. fraxinifolium and A. graveolens and the stems of A. urundeuva were considered weak inhibitors (MIC greater than 1,000 µg/mL). Only the leaf extract of A. urundeuva showed an MIC value (125 µg/mL) that corresponds to a strong degree of inhibition against C. albicans.
The literature reports the presence of key components in the extract of Astronium sp., such as chalcones, flavonoid precursors, and essential oils and tannins, which are usually related to antimicrobial activity.43 Preliminary tests showed that a 70% water–alcohol solution can extract most of the Astronium sp. components. Nevertheless, it is difficult to solubilize some of the compounds that can be precipitated from the extract. According to the literature, the most biologically active constituents in the extract are essential oils and tannins, which have different degrees of hydrophilicity/lipophilicity, further justifying the use of nanotechnology directed to the incorporation of plant extracts.
According to Overtone’s concept of cell membrane permeability, the lipophilicity of oil-soluble substances facilitates their passage across the cell membrane. Therefore, the lipophilicity of a compound is considered an important factor in the research and development of new drugs, including antimicrobial agents, as it facilitates the interaction of the hydrophobic chain with the lipids of the cell membrane and thus promotes penetration of the active compound into the cytoplasm.46
The reduced MIC of hydroethanolic extracts from A. urundeuva leaves incorporated into lipid ME systems might be explained by the presence of cholesterol in the composition of the ME system, which could promote interaction with the ergosterol that is present in the fungal cell membrane, thus inducing release of the active compound directly onto the target. Moreover, the composition of this system may have helped the solubilization of substances with different degrees of hydrophilicity/lipophilicity, such as essential oils and tannins, significantly increasing the antifungal activity. One of the reasons that can explain why the other extracts showed antifungal activity only after their incorporation into this lipid system is the easier solubilization of some of the constituents that are most likely responsible for the activity. With the exception of the A. urundeuva leaves’ extracts (MIC 15.62 µg/mL), which were responsible for the most promising result, the stems of all studied species of Astronium sp. (MIC 62.5 µg/mL) most likely have a larger amount of antifungal constituents, which can be proved only after their incorporation into the nanostructured system. According to Cunha Júnior et al47 surfactants can increase cell membrane permeability, which may help the absorption of active ingredients, enhancing bioavailability.
It is worth mentioning that the lipid ME synthesized (base), when subjected to the test against all microorganisms (solvent control), was not active. In other words, it did not inhibit fungal and bacterial growth, ensuring that the activity was presented solely by the plant extract and not by any other disturbance such as the components that form the nanostructured system.
These data allow us to infer that the lipid system developed provided a better permeability through the membrane extracts of the yeast, thus favoring contact with the most effective targets and acting as a highly effective carrier for the leaf extract of A. urundeuva. The composition of the ME prepared in this work can be safely applied to other systems in the future to provide better biological characterization.
The use of natural products for topical administration has been extensively studied in recent years. Recently, our research group2 developed a model of infection of VVC caused by C. albicans with the purpose of studying the therapeutic use of methanolic extracts of the scapes of Syngonanthus nitens (Bong) Ruhland (Eriocaulaceae) as an antifungal compound. In this study, the authors demonstrated a significant inhibitory profile for these extracts against C. albicans.
The incorporated extracts were prepared in accordance with the methods of D’Cruz et al19 and D’Cruz and Uckun48 for the purpose of improving the pharmacological properties of the extracts. Thus, the results found in this study confirm the assertion that the incorporation of bioactive compounds into nanostructured lipid systems for drug delivery enhances the action in comparison to the free form of the same bioactive compounds. This is evidenced by the observation that the extract alone did not yield satisfactory results in reducing the vaginal fungal burden (CFU/mL) compared to that present in the ME.
The vehicles used in this study for the administration of the plant compounds (DMSO and ME) showed no interference, as they had no detectable antifungal activity. Therefore, the inhibitory potential of the nanostructured lipid system was assigned exclusively to the leaf extracts of A. urundeuva.
Conclusion
Based on these results, we conclude that the hydroethanolic extract of A. urundeuva leaves has in vitro and in vivo antifungal activity and that the incorporation of this extract into the nanostructured system, developed herein, enhances this activity. Our results suggest that nanostructured lipid systems can be used as part of a strategy to improve the in vitro anti-C. albicans activity of hydroethanolic extract of Astronium sp.
In our future work, we will investigate the influence of the lipid type used in the nanostructured lipid system on the antifungal activity of the hydroethanolic extract from the leaves of A. urundeuva, according to Angelova et al.49
Acknowledgments
We thank Professor Laudemir Carlos Varanda (University of São Paulo, São Carlos Institute of Chemistry, Brazil) for his help in performing the XRD and TEM techniques. We also thank grant #2009/52237-9, grant #2013/25432-0 and grant #2013/25121-5 São Paulo Research Foundation (FAPESP), Brazilian National Council for Scientific and Technological Development – CNPq (process number 133916/2012-0), Programa de Apoio ao Desenvolvimento Científico (PADC) and School of Pharmaceutical Sciences/UNESP, São Paulo, Brazil for financial support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Barbedo LS, Sgarbi DBC. Candidiasis. J Bras Doenças Sex Transm. 2010;22(1):22–38. [Google Scholar]
- 2.Araújo MGF, Pacífico M, Vilegas W, et al. Evaluation of Syngonanthus nitens (Bong.) Ruhl. extract as antifungal and in treatment of vulvovaginal candidiasis. Med Mycol. 2013;51(7):673–682. doi: 10.3109/13693786.2013.795294. [DOI] [PubMed] [Google Scholar]
- 3.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23(2):253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales GI, Yaneth MC. Candidiasis in reproductive age women attending the Eduardo Arredondo Daza hospital in the city of Valledupar. RCMT. 2012;22(2):13–21. [Google Scholar]
- 5.Repentigny L. Animal models in the analysis of Candida host – pathogen interactions. Curr Opin Microbiol. 2004;7(4):324–329. doi: 10.1016/j.mib.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Barbosa C, Fernandes G, Quintas C, Ester Teixeira M, Pedro Neves J. Anti-fungal treatment with azole compounds for uncomplicated vulvovaginal candidiasis. Acta Obstet Ginecol Port. 2012;6(3):118–123. [Google Scholar]
- 7.Barai L, Fatema K, Haq JA, et al. Bacterial profile and their antimicrobial resistance pattern in an intensive care unit of a tertiary care hospital in Dhaka. Ibrahim Med Coll. 2010;4(2):66–69. [Google Scholar]
- 8.Aguiar AV, Bortolozo FR, Moraes MLT, Andrade JAC. Genetic variation in Astronium fraxinifolium populations in consortium. Crop Breed Appl Biotechnol. 2003;3(2):95–106. [Google Scholar]
- 9.Luna CV. Distribution and importance of the family Anacardiaceae timber in the gran chaco of Argentina. Ra Ximhai. 2012;8(3):83–95. [Google Scholar]
- 10.Cunha FP, Costa LJL, Fernandes AJD, Souza TP, Soares LAL. Development and optimization of extractives from Astronium urundeuva (allemão) Engl. by factorial design. Braz Arch Biol Technol. 2009;52(3):647–652. [Google Scholar]
- 11.Carlini EA, Duarte-Almeida JM, Rodrigues E, Tabach R. Antiulcer effect of the pepper trees Schinus terebinthifolius Raddi (aroeira-da-praia) and Myracrodruon urundeuva Allemão, Anacardiaceae (aroeira-do-sertão) Rev Bras Farmacogn. 2010;20(2):140–146. [Google Scholar]
- 12.Silva PB, Ramos MAS, Bonifácio BV, et al. Nanotechnological strategies for vaginal administration of drugs – a review. J Biomed Nanotechnol. 2014;10(9):2218–2243. doi: 10.1166/jbn.2014.1890. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhang L, Wang Q, Zhang D. Recent advances in the nanotechnology-based drug delivery of silybin. J Biomed Nanotechnol. 2014;10(4):543–558. doi: 10.1166/jbn.2014.1798. [DOI] [PubMed] [Google Scholar]
- 14.Bonifácio BV, Silva PB, Ramos MAS, Negri KMS, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines – a review. Int J Nanomedicine. 2014;9:1–15. doi: 10.2147/IJN.S52634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezrahi S, Aserin A, Garti N, Berkovic G. An excursion into phase tetrahedra – where physical chemistry and geometry meet. J Chem Educ. 1998;75(12):1648–1652. [Google Scholar]
- 16.Kovarik JM, Mueller EA, Van Bree JB, Tetzloff W, Kutz K. Reduced inter and intra intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation. J Pharm Sci. 1994;83(3):444–446. doi: 10.1002/jps.2600830336. [DOI] [PubMed] [Google Scholar]
- 17.Damasceno BP, Dominici VA, Urbano IA, et al. Amphotericin B micro-emulsion reduces toxicity and maintains the efficacy as an antifungal product. J Biomed Nanotechnol. 2012;8(2):290–300. doi: 10.1166/jbn.2012.1374. [DOI] [PubMed] [Google Scholar]
- 18.Franzini CM, Pestana KC, Molina EF, Scarpa MV, Egito ES, Oliveira AG. Structural properties induced by the composition of biocompatible phospholipid-based microemulsion and amphotericin B association. J Biomed Nanotechnol. 2012;8(2):350–359. doi: 10.1166/jbn.2012.1435. [DOI] [PubMed] [Google Scholar]
- 19.D’Cruz OJ, Yiv H, Waurzyniak B, Uckun FM. Contraceptive efficacy and safety studies of a novel microemulsion-based lipophilic vaginal spermicide. Fertil Steril. 2001;75(1):115–124. doi: 10.1016/s0015-0282(00)01636-8. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira MB, Calixto G, Graminha M, Cerecetto H, Gonzáles M, Chorilli M. Development, characterization, and in vitro biological performance of fluconazole-loaded microemulsions for the topical treatment of cutaneous leishmaniasis. Biomed Res Int. 2015;2015:396894. doi: 10.1155/2015/396894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silveira WLL, Damasceno BPGL, Silva KGH, Oliveira AG, Egito EST. Aspectos fundamentais no desenvolvimento de sistemas microemul-sionados contendo anfotericina B para uso oftálmico [Fundamental aspects in the development of microemulsion systems containing amphotericin B for ophthalmic use] Rev Ciênc Farm Básica Apl. 2013;34(1):9–15. [Google Scholar]
- 22.Silva GBRF, Scarpa MV, Rossanezi G, Egito EST, Oliveira AG. Development and characterization of biocompatible isotropic and anisotropic oil-in-water colloidal dispersions as a new delivery system for methyl dihydrojasmonate antitumor drug. Int J Nanomedicine. 2014;9:867–876. doi: 10.2147/IJN.S46055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assumpção JUC, Campos ML, Filho MAFN, et al. Biocompatible microemulsion modifies the pharmacokinetic profile and cardiotoxicity of doxorubicin. J Pharm Sci. 2013;102(1):289–296. doi: 10.1002/jps.23368. [DOI] [PubMed] [Google Scholar]
- 24.Sato MR, Silva PB, Souza RA, Santos KC, Chorilli M. Recent advances in nanoparticle carriers for coordination complexes. Curr Top Med Chem. 2015;15(4):287–297. doi: 10.2174/1568026615666150108145614. [DOI] [PubMed] [Google Scholar]
- 25.Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Pedrovick PR. Farmacognosia: da planta ao medicamento [Pharmacognosy: from the Plant to the Drug] Porto Alegre: UFRGS; 2010. Portuguese. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute . Reference Methods for Broth Dilution Antifungal Susceptibility Tests for Yeasts; Approved Standards. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. CLSI document (M27-A3) [Google Scholar]
- 27.Duarte MCT, Figueira GM, Sartoratto A, Rehder VLG, Delarmelina C. Anti-Candida activity of Brazilian medicinal plants. J Ethnofarmacol. 2005;97(2):305–311. doi: 10.1016/j.jep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Zanin SMW, Miguel MD, Chimelli MC, Oliveira AB. Hydrophile-lipophile balance (HLB) determination of vegetable oil family. Vis Acad. 2002;3(1):13–18. [Google Scholar]
- 29.Calixto G, Yoshii AC, Rocha eSilva H, Cury BSF, Chorilli M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: preparation and characterization. Pharm Dev Technol. 2014;19:1–7. doi: 10.3109/10837450.2014.882941. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho FC, Calixto G, Hatakeyama IN, Luz GM, Gremião MP, Chorilli M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev Ind Pharm. 2013;39(11):1750–1757. doi: 10.3109/03639045.2012.734510. [DOI] [PubMed] [Google Scholar]
- 31.Santos FK, Oyafuso MH, Kiill CP, Gremião MPD, Chorilli M. Nanotechnology-based drug delivery systems for treatment of hyperproliferative skin diseases - a review. Curr Nanosci. 2013;9(1):159–167. [Google Scholar]
- 32.Pavan FR, Maia PIS, Leite SRA, et al. Thiosemicarbazones, semi-carbazones, dithiocarbazates and hydrazide/hydrazones: anti – Mycobacterium tuberculosis activity and cytotoxicity. Eur J Med Chem. 2010;45(5):1898–1905. doi: 10.1016/j.ejmech.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Orme I. Search for new drugs for treatment of tuberculosis. Antimicrob Agents Chemother. 2001;45(7):1943–1946. doi: 10.1128/AAC.45.7.1943-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yano J, Kolls JK, Happel KI, Wormley F, Wozniak KL, Fidel PLJ. The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS One. 2012;7(9):e46311. doi: 10.1371/journal.pone.0046311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yano J, Jr, Fidel PL. Protocols for vaginal inoculation and sample collection in the experimental mouse model of Candida vaginitis. J Vis Exp. 2011;8(58):3382. doi: 10.3791/3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orthaber D, Glatter O. Synthetic phospholipid analogs: a structural investigation with scattering methods. Phys Lipids. 2000;107(2):179–189. doi: 10.1016/s0009-3084(00)00171-7. [DOI] [PubMed] [Google Scholar]
- 37.Formariz TP, Urban CC, Silva Júnior AA, Gremião MPD, Oliveira AG. Microemulsion and liquid crystals as drug delivery systems. Rev Bras Cien Farm. 2005;41(3):301–331. [Google Scholar]
- 38.Tomsic M, Podlogar F, Gasperlin M, Bester-Rogac M, Jamnik A. Water-Tween 40®/Imwistor 308® – isopropyl myristate microemulsions as delivery systems for ketoprofen: small-angle X-ray scattering study. Int J Pharm. 2006;327:170–177. doi: 10.1016/j.ijpharm.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Moghimipour E, Salimi A, Karami M, Isazadeh S. Preparation and characterization of dexamethasone microemulsion based on pseudoternary phase diagram. Jundishapur J Nat Pharm Prod. 2013;8(3):105–112. doi: 10.17795/jjnpp-9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva RMG, Saraiva TS, Silva RB, Gonçalves LA, Silva LP. Allelopathy potential of etanolic extract of Anadenanthera macrocarpa and Astronium graveolens. J Biosci. 2010;26(4):632–637. [Google Scholar]
- 41.Newman DJ, Gragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi T, Brito JO, Bittencourt E, Almeida RSR, Faria PN, Dias CTS. Eucalyptus waste effluent as a natural dyestuff for dyeing cotton fabrics. Redige. 2012;3(1):1–12. [Google Scholar]
- 43.Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. J Med Plants Res. 2010;4(2):104–111. [Google Scholar]
- 44.Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB. Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem. 2001;49(9):4168–4170. doi: 10.1021/jf001494m. [DOI] [PubMed] [Google Scholar]
- 45.Webster D, Taschereau P, Belland RJ, Sand C, Rennie RP. Antifungal activity of medicinal plant extracts preliminary screening studies. J Ethnopharmacol. 2008;115(1):140–146. doi: 10.1016/j.jep.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Missner A, Pohl P. 110 years of the Meyer-Overton rule: predicting membrane permeability of gases and other small compounds. Chemphyschem. 2009;10(9–10):1405–1414. doi: 10.1002/cphc.200900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha Júnior ASC, Fialho SL, Carneiro LB, Orefice F. Microemulsions as drug delivery systems for topical ocular administration. Arq Bras Oftalmol. 2003;66(3):385–391. [Google Scholar]
- 48.D’Cruz OJ, Uckun FM. Gel-microemulsions as vaginal spermicides and intravaginal drug delivery vehicles. Contraception. 2001;64(2):113–123. doi: 10.1016/s0010-7824(01)00233-5. [DOI] [PubMed] [Google Scholar]
- 49.Angelova A, Ionev R, Koch MHJ, Rapp G. Interaction of the peptide antibiotic alamethicin with bilayer and non-bilayer forming lipids: influence of increasing alamethicin concentration on the lipids supra-molecular structures. Arch Biochem Biophys. 2000;378(1):93–106. doi: 10.1006/abbi.2000.1696. [DOI] [PubMed] [Google Scholar]


