Abstract
OBJECTIVES
The purpose of this study was to determine the predictors of mortality in patients with pulmonary hypertension (PH) associated with heart failure with preserved ejection fraction (HFpEF).
BACKGROUND
PH is commonly associated with HFpEF. The predictors of mortality for patients with these conditions are not well characterized.
METHODS
In a prospective cohort of patients with right heart catheterization, we identified 73 adult patients who had pulmonary hypertension due to left heart disease (PH-LHD) associated with HFpEF (left ventricular ejection fraction ≥50% by echocardiography); hemodynamically defined as a mean pulmonary artery pressure ≥25 mm Hg and pulmonary artery wedge pressure >15 mm Hg. PH severity was classified according to the diastolic pressure gradient (DPG). Cox proportional hazards ratios were used to estimate the associations between clinical variables and mortality. Receiver-operating characteristic curves were used to evaluate the ability of hemodynamic measurements to predict mortality.
RESULTS
The mean age for study subjects was 69 ± 12 years and 74% were female. Patients classified as having combined post-capillary PH and pre-capillary PH (DPG ≥7) were not at increased risk of death as compared to patients with isolated post-capillary PH (DPG <7). A baseline pulmonary arterial capacitance (PAC) of <1.1 ml/mm Hg was 91% sensitive in predicting mortality, with better discriminatory ability than DPG, transpulmonary gradient, or pulmonary vascular resistance (area under the curve of 0.73, 0.50, 0.45, and 0.37, respectively). Fifty-seven subjects underwent acute vasoreactivity testing with inhaled nitric oxide. Acute vasodilator response by the Rich or Sitbon criteria was not associated with improved survival.
CONCLUSIONS
PAC is the best predictor of mortality in our cohort and may be useful in describing phenotypic subgroups among those with PH-LHD associated with HFpEF. Acute vasodilator testing did not predict outcome in our cohort but needs to be further investigated.
Keywords: heart failure with preserved ejection fraction, pulmonary heart disease, survival, vasodilation
Pulmonary hypertension (PH) due to left heart disease (LHD) (1,2) is the most common form of PH and is associated with increased hospitalization and mortality (3–5). PH-LHD, also termed Group 2 PH, is defined by a mean pulmonary artery pressure (mPAP) ≥25 mm Hg and a pulmonary artery wedge pressure (PAWP) or left ventricular end-diastolic pressure >15 mm Hg (1,6). Patients with PH-LHD exhibit variable pulmonary vascular phenotypes with some individuals manifesting a “disproportionate” increase in mPAP relative to PAWP and others demonstrating a “proportionate” pressure rise, a distinction previously defined by a transpulmonary gradient (TPG) of >12 mm Hg or ≤12 mm Hg, respectively (6,7). According to experts at the Fifth World PH Symposium, these 2 groups are better discriminated by the diastolic pressure gradient (DPG) with DPG ≥7 mm Hg and <7 mm Hg to refer to those with combined post-capillary and pre-capillary PH (Comb-PH) and isolated post-capillary PH (Iso-PH), respectively (8).
Among patients with left ventricular (LV) systolic dysfunction, also referred to as heart failure with reduced ejection fraction (HFrEF), the presence of PH portends a worse prognosis due to right ventricular (RV) failure in the setting of a progressive increase in RV afterload as the LV fails (9,10). Recent studies of patients with HFrEF demonstrate that pulmonary arterial capacitance (PAC), the ratio of stroke volume (SV) to pulmonary pulse pressure (PP), is a better determinant of RV afterload and survival than pulmonary vascular resistance (PVR) (11,12). Furthermore, in HFrEF, the pulmonary vascular response to acute vasodilators has prognostic significance; the failure of PH to improve and PAWP to normalize during administration of nitroprusside and/or diuretics is associated with a high risk of right heart failure and mortality after cardiac transplantation (13,14). The incidence of LV diastolic dysfunction, or heart failure with preserved ejection fraction (HFpEF), is rising and now accounts for one-half of the patients with heart failure (15). In contrast to those with HFrEF, there is far less information on the role of PH in HFpEF outcomes. Also, the relative utility of the various hemodynamic metrics (TPG, DPG, PVR, and PAC) and that of vasodilator testing, to predict outcomes in HFpEF with PH have not been previously assessed.
Thus, in our cohort of HFpEF patients with PH, we aimed to: 1) determine the prognostic implications of PAC compared to TPG, DPG, PVR, and other hemodynamic parameters in patients with PH-LHD and HFpEF; 2) determine whether the current definition for Comb-PH is associated with a worse survival than PH-LHD with Iso-PH; and 3) evaluate the safety and prognostic value of acute vasodilator testing in PHLHD and HFpEF.
METHODS
STUDY DESIGN
This is a prospective cohort study of consecutive patients undergoing diagnostic right heart catheterization for suspected PH in the Pulmonary Hypertension Center at Tufts Medical Center from January 2004 to December 2012. Patients were included if they: 1) were adults ≥18 years of age; 2) had mPAP ≥25 mm Hg; 3) had PAWP >15 mm Hg; 4) had LV ejection fraction ≥50% by echocardiogram; and 5) had no evidence of moderate to severe left-sided valvular disease or known causes for elevated left-sided filling pressure such as hypertrophic cardiomyopathy, pericardial disease, or amyloidosis. Patients were excluded if they met diagnostic criteria (clinical, radiological imaging, pulmonary function tests, and laboratory testing) for PH Groups 1, 3, 4, or 5 (4). Comb-PH and Iso-PH were defined as meeting eligibility criteria plus a DPG ≥7 mm Hg or <7 mm Hg, respectively. All patients were followed until death or October 31, 2013. None of the patients underwent cardiac transplantation during the study period. The study was approved by the Tufts Medical Center and Tufts University Health Sciences Campus Institutional Review Board (#7347) and all subjects provided written informed consent.
MEASUREMENTS
Demographic data including age, sex, race, height, and weight were recorded. All subjects underwent routine laboratory testing, echocardiography and right heart catheterization. Right heart catheterization was performed in the cardiac catheterization laboratory on patients in the fasting state after minimal sedation at rest and in the supine position. The system was zeroed and referenced at the level of the patient’s heart (fourth intercostal space midway between anterior and posterior chest wall). A balloon-tipped thermodilution catheter (Edwards Lifesciences, Irvine, California) was inserted through the internal jugular vein as previously described (16). All hemodynamic tracings were reviewed by the operator (N.S.H., I.R.P., or K.E.R.) and pressures were recorded at end-expiration. Cardiac output (CO) was determined in triplicate by the thermodilution technique. PVR was calculated as: (mPAP − PAWP)/CO. Systemic blood pressure was measured using a digital sphygmomanometer at the time of the right heart catheterization. PAC (ml/mm Hg) and systemic arterial capacitance (SAC) (ml/mm Hg) were calculated using the following equations: PAC = SV/pulmonary PP and SAC = SV/systemic PP.
VASODILATOR TESTING
Vasodilator testing was performed at the discretion of the operator (N.S.H., I.R.P., or K.E.R.). Inhaled nitric oxide (iNO) (Ikaria, Hampton, New Jersey) was administered via an inline system at 20 parts per million for 10 min as previously described (17,18). Measurements of PAP, PAWP, and CO were performed after 10 min on iNO. Acute vasodilator response was defined according to the criteria of Rich and Sitbon. These definitions (respectively) are: 1) a decrease in mPAP and PVR by ≥20% (19); or 2) a decrease in mPAP of ≥10 mm Hg (or at least 20%) to a value below 40 mm Hg with unchanged or increased CO (20).
STATISTICAL ANALYSIS
Continuous variables were summarized using means and standard deviations (7) or medians and interquartile range. Categorical variables were summarized using frequencies and proportions. Cox proportional hazards models were used to estimate hazard ratios and their 95% confidence intervals for the associations between clinical and hemodynamic factors and mortality. Based on our clinical expertise and previous literature (6,21,22), age, sex, race/ethnicity, body mass index, and hemodynamic parameters were selected as possible confounders of the PAC-mortality association, and evaluated in univariate models. Variables that were predictors of mortality in the univariate analysis with a p value <0.20 were considered for inclusion in the multivariate model. A relatively relaxed significance threshold was chosen to prioritize validity concerns. We used a backward stepwise elimination based on the likelihood-ratio test statistics with 0.05 for entry criterion and 0.10 for removal criterion for selection of the final multivariate model.
Receiver-operating characteristic (ROC) curves were used to evaluate the ability of PAC, TPG, DPG, and PVR to discriminate between patients who had died by the end of follow-up and those still alive. Area under the curve and associated 95% confidence interval (CI) were determined for PAC, PVR, TPG, and DPG individually, and compared using the DeLong method (23). The optimal cut point for prediction of mortality was chosen to have a sensitivity in excess of 90% with maximal achievable specificity, given the clinical significance of this outcome and potential for it to inform treatment decisions. Sensitivity, specificity, and predictive values were calculated to assess the accuracy of the cut point to predict mortality at the end of follow-up.
Kaplan-Meier curves and the log-rank test were used to compare survival among acute vasodilator responders versus nonresponders. All analyses were performed using SPSS (version 12.0; IBM, Chicago, Illinois).
RESULTS
BASELINE CHARACTERISTICS
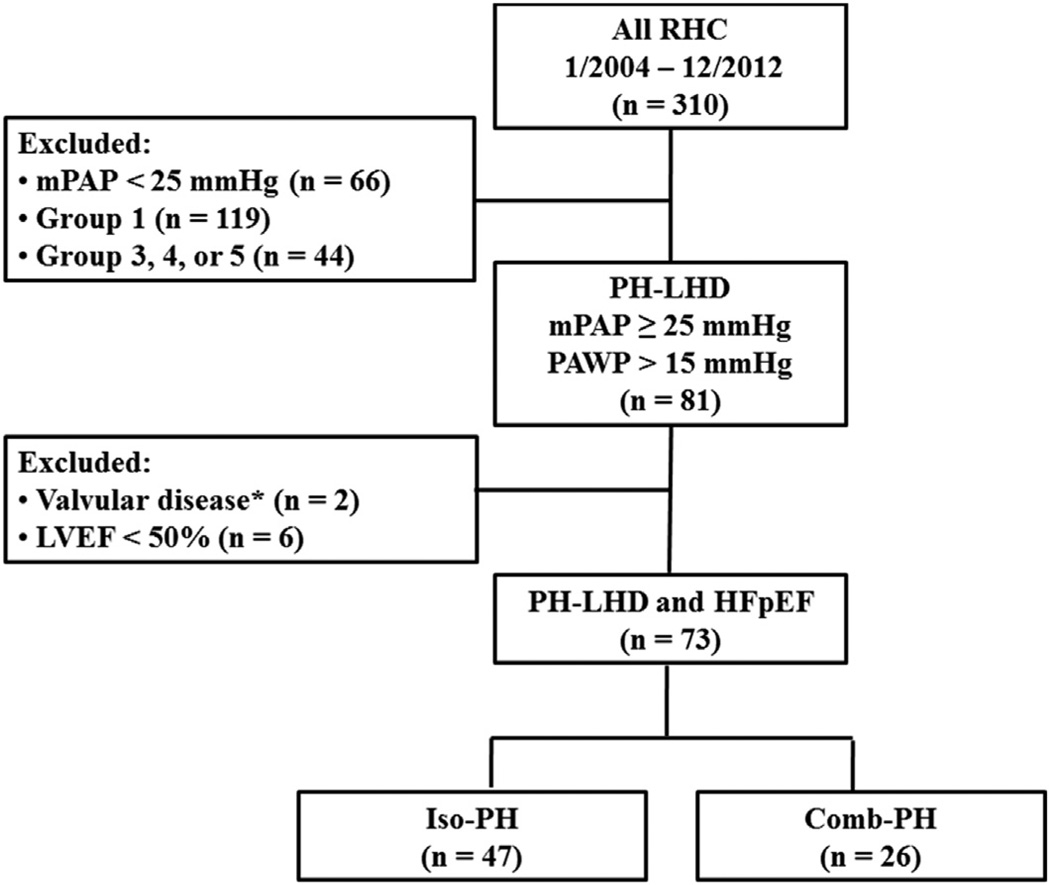
We identified 310 subjects who had undergone right heart catheterization between January 2004 and December 2012. Seventy-three subjects met the inclusion criteria and were classified as having Group 2 PH associated with HFpEF. Twenty-six subjects (36%) met the definition of Comb-PH (Figure 1). The mean age was 69 ± 12 years and three-quarters were women (Table 1). About one-half of the cohort had New York Heart Association functional class II symptoms at the time of catheterization. Patients in both diagnostic groups were similar with respect to their baseline demographic and clinical characteristics, including age, sex, race, and body mass index (Table 1). As expected, hemodynamic parameters differed between the groups, with Comb-PH patients having higher right atrial pressure, PAWP, mPAP, TPG, and PVR and lower PAC than those with Iso-PH (Table 2). The median follow-up time was 3.6 years (interquartile range: 4.5 years). The cumulative incidence of death during the study period was 34%.
FIGURE 1. Flow Chart for Patients Included in Study.
This figure illustrates the selection process of our study cohort. *Patients with moderate-severe aortic or mitral valve disease were excluded. Comb-PH = combined post-capillary pulmonary hypertension and pre-capillary pulmonary hypertension; HFpEF = heart failure with preserved ejection fraction; Iso-PH = isolated post-capillary pulmonary hypertension; LVEF = left ventricular ejection fraction; mPAP = mean pulmonary artery pressure; PAWP = pulmonary artery wedge pressure; PH-LHD = pulmonary hypertension due to left heart disease; RHC = right heart catheterization.
TABLE 1.
Baseline Demographic and Clinical Characteristics of Patients With PH-LHD and HFpEF
| Total Population (n = 73) |
Iso-PH (n = 47) |
Comb-PH (n = 26) |
|
|---|---|---|---|
| Age, yrs | 69 ± 12 | 70 ± 12 | 67 ± 11 |
| Female | 54 (74) | 33 (70) | 21 (81) |
| Body mass index, kg/m2 | 33 ± 10 | 32 ± 10 | 34 ± 10 |
| Non-Hispanic White | 68 (93) | 44 (94) | 24 (92) |
| NYHA functional class (n = 69) | |||
| I | 2 (3) | 2 (4) | 0 (0) |
| II | 33 (48) | 22 (49) | 11 (46) |
| III | 28 (40) | 17 (38) | 11 (46) |
| IV | 6 (9) | 4 (9) | 2 (8) |
| Comorbidities | |||
| Atrial fibrillation | 32 (44) | 21 (45) | 11 (42) |
| Coronary artery disease | 23 (32) | 14 (30) | 9 (35) |
| Diabetes mellitus | 22 (30) | 14 (30) | 8 (31) |
| Hyperlipidemia | 37 (51) | 23 (49) | 14 (54) |
| Hypertension | 55 (75) | 38 (81) | 17 (65) |
| Obstructive sleep apnea | 28 (38) | 18 (38) | 10 (38) |
| Follow-up, yrs* | 3.6 (4.5) | 3.7 (2.3) | 3.5 (2.1) |
| Death | 25 (34) | 16 (34) | 9 (35) |
Values are mean ± SD or n (%).
Values in parentheses are the difference between the 25th and 75th percentiles.
Comb-PH = combined post-capillary pulmonary hypertension and pre-capillary pulmonary hypertension; HFpEF = heart failure with preserved ejection fraction; Iso-PH = isolated post-capillary pulmonary hypertension; NYHA = New York Heart Association; PH-LHD = pulmonary hypertension due to left heart disease.
TABLE 2.
Baseline Hemodynamics of Patients With PH-LHD and HFpEF
| Total Population (n = 73) |
Iso-PH (n = 47) |
Comb-PH (n = 26) |
|
|---|---|---|---|
| Heart rate, beats/min | 71 ± 14 | 71 ± 14 | 75 ± 15 |
| Mean arterial pressure, mm Hg | 97 ± 14 | 96 ± 13 | 98 ± 15 |
| Mean RA pressure, mm Hg | 13 ± 5 | 12 ± 5 | 15 ± 4 |
| Mean PA pressure, mm Hg | 41 ± 11 | 35 ± 8 | 49 ± 11 |
| PAWP, mm Hg | 21 ± 4 | 21 ± 5 | 21 ± 4 |
| PA pulse pressure, mm Hg | 38 ± 16 | 35 ± 15 | 43 ± 16 |
| TPG, mm Hg | 19 ± 10 | 15 ± 6 | 29 ± 9 |
| DPG, mm Hg | 5 ± 7 | 1 ± 3 | 13 ± 5 |
| CO (thermodilution), l/min1 | 4.7 ± 1.5 | 4.9 ± 1.5 | 4.4 ± 1.5 |
| SV, ml | 69 ± 29 | 74 ± 32 | 61 ± 21 |
| PA oxygen saturation (n = 68) | 63 ± 9 | 63 ± 8 | 64 ± 10 |
| PAC, ml/mm Hg | 2.1 ± 1.2 | 2.3 ± 1.2 | 1.6 ± 1.0 |
| SAC (n = 70), ml/mm Hg | 1.1 ± 0.5 | 1.2 ± 0.5 | 1.0 ± 0.5 |
| PVR, WU | 4.9 ± 3.7 | 3.4 ± 2.1 | 7.5 ± 4.5 |
| SVR, WU | 19.8 ± 8.6 | 19.3 ± 8.2 | 20.8 ± 9.2 |
| BNP (n = 56), pg/ml | 313 ± 357 | 275 ± 328 | 390 ± 406 |
Values are mean ± SD.
BNP = brain natriuretic peptide; CO = cardiac output; DPG = diastolic pulmonary gradient; PA = pulmonary artery; PAC = pulmonary arterial capacitance; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance; RA = right atrium; SAC = systemic arterial capacitance; SV = stroke volume; SVR = systemic vascular resistance; TPG = transpulmonary gradient; WU = Wood units; other abbreviations as in Table 1.
UNIVARIATE AND MULTIVARIATE ANALYSES
Univariate analysis revealed that older age, lower PAC, SAC, body mass index, CO, and pulmonary artery (PA) oxygen saturation, and higher PVR and brain natriuretic peptide, were statistically significantly associated with mortality (Table 3). Comb-PH was not significantly associated with greater mortality (hazard ratio: 1.15; 95% CI: 0.51 to 2.62; p = 0.73). After multivariate adjustment in the entire study population, older age and lower PAC were the only independent predictors of mortality (Table 4). A statistically significant inverse association was observed between PAC and DPG (r = −0.28; p = 0.02), TPG (r = −0.59; p < 0.001), PVR (r = −0.63; p < 0.001), and age (r = −0.41; p = 0.001). PAC was positively associated with SAC (r = 0.54; p < 0.001).
TABLE 3.
Univariate Risk Factors Associated With Mortality in Patients With PH-LHD and HFpEF (n = 73)
| HR (95% CI) | p Value | |
|---|---|---|
| Age per 10-yr increase | 2.05 (1.31–3.20) | 0.002 |
| Female | 0.77 (0.33–1.81) | 0.55 |
| Non-Hispanic White | 0.90 (0.21–3.85) | 0.89 |
| Body mass index per 5 kg/m2 increase | 0.71 (0.53–0.95) | 0.02 |
| Mean RA pressure per 1 mm Hg increase | 1.05 (0.97–1.13) | 0.26 |
| Mean PA pressure per 1 mm Hg increase | 1.02 (0.99–1.06) | 0.16 |
| PAWP per 1 mm Hg increase | 1.05 (0.96–1.15) | 0.32 |
| TPG per 1 mm Hg increase | 1.02 (0.98–1.06) | 0.22 |
| DPG per 1 mm Hg increase | 1.00 (0.96–1.06) | 0.77 |
| CO per 1 l/min increase | 0.72 (0.53–0.96) | 0.03 |
| PA oxygen saturation per 1% increase | 0.95 (0.91–0.99) | 0.02 |
| PAC per 1 ml/mm Hg increase | 0.47 (0.27–0.83) | 0.009 |
| SAC per 1 ml/mm Hg increase | 0.26 (0.08–0.89) | 0.03 |
| PVR per 1 WU increase | 1.21 (1.10–1.34) | <0.001 |
| BNP per 50 pg/ml increase | 1.05 (1.01–1.09) | 0.02 |
| Comb-PH | 1.15 (0.51–2.62) | 0.73 |
TABLE 4.
Multivariate Adjusted Risk Factors for Mortality in Patients With PH-LHD and HFpEF (n = 73)
| HR (95% CI) | p Value | |
|---|---|---|
| Age per 10-yr increase | 1.78 (1.14–2.79) | 0.01 |
| PAC per 1 ml/mm Hg increase | 0.48 (0.26–0.89) | 0.02 |
CI = confidence interval; HR = hazard ratio; HFpEF = heart failure with preserved ejection fraction; PAC = pulmonary arterial capacitance; PH-LHD = pulmonary hypertension due to left heart disease.
RECEIVER-OPERATING CHARACTERISTIC CURVES
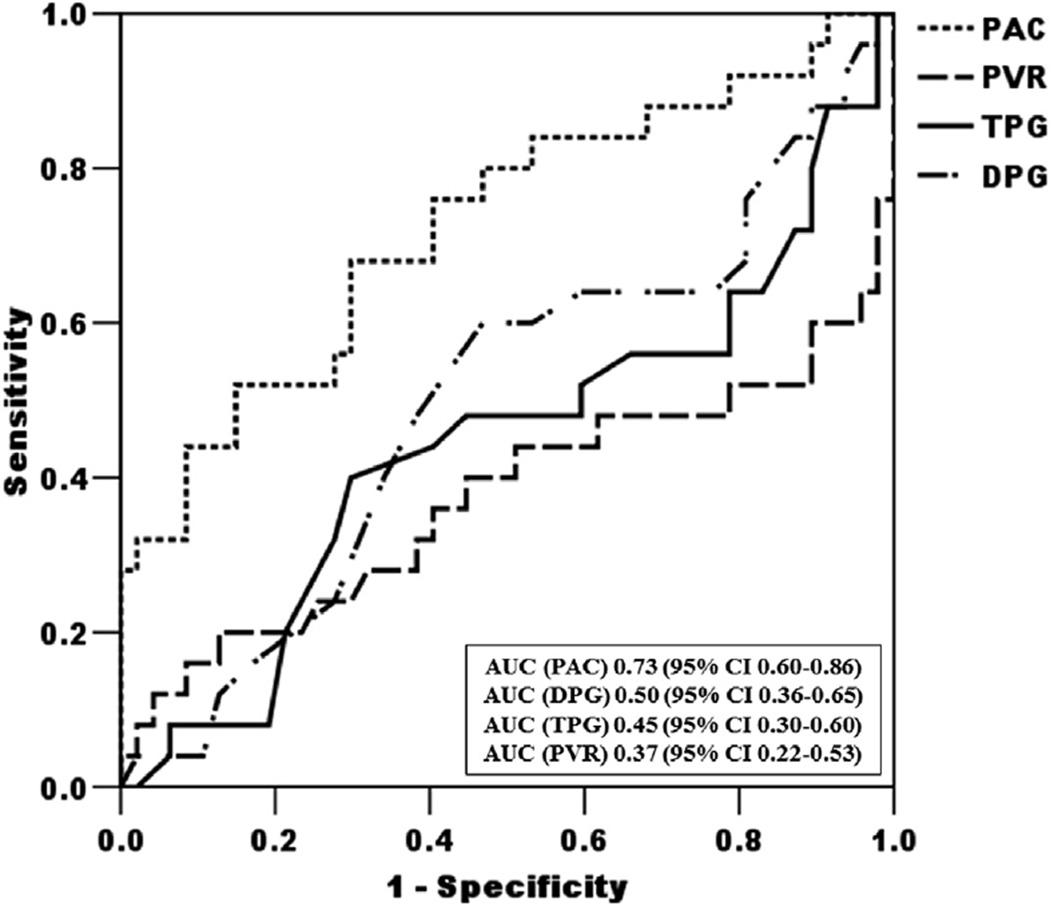
PAC was significantly more discriminating than DPG, TPG or PVR for the prediction of survival, with an area under the curve of 0.73 for PAC (95%CI: 0.60 to 0.86), 0.50 for DPG (95% CI: 0.36 to 0.65), 0.45 for TPG (95% CI: 0.30 to 0.60), and 0.37 for PVR (95% CI: 0.22 to 0.53; p = 0.004, 0.001, and 0.001, respectively, for the comparison with the PAC ROC curve) (Figure 2). There was no difference in the area under the curve between TPG and PVR (p = 0.30) (Figure 2). Stratification of our cohort according to DPG, TPG, or PVR criteria yielded no difference in survival (p =0.73, 0.96, and 0.64 respectively) (Online Figures 1 to 3).
FIGURE 2. Receiver-Operating Characteristic Curves for Prediction of Mortality.
Receiver-operating characteristic curves for each of the hemodynamic parameters (pulmonary arterial capacitance [PAC], diastolic pressure gradient [DPG], transpulmonary gradient [TPG], and pulmonary vascular resistance [PVR]) and prediction of mortality during the study follow-up period (n = 73). AUC = area under the curve; CI = confidence interval.
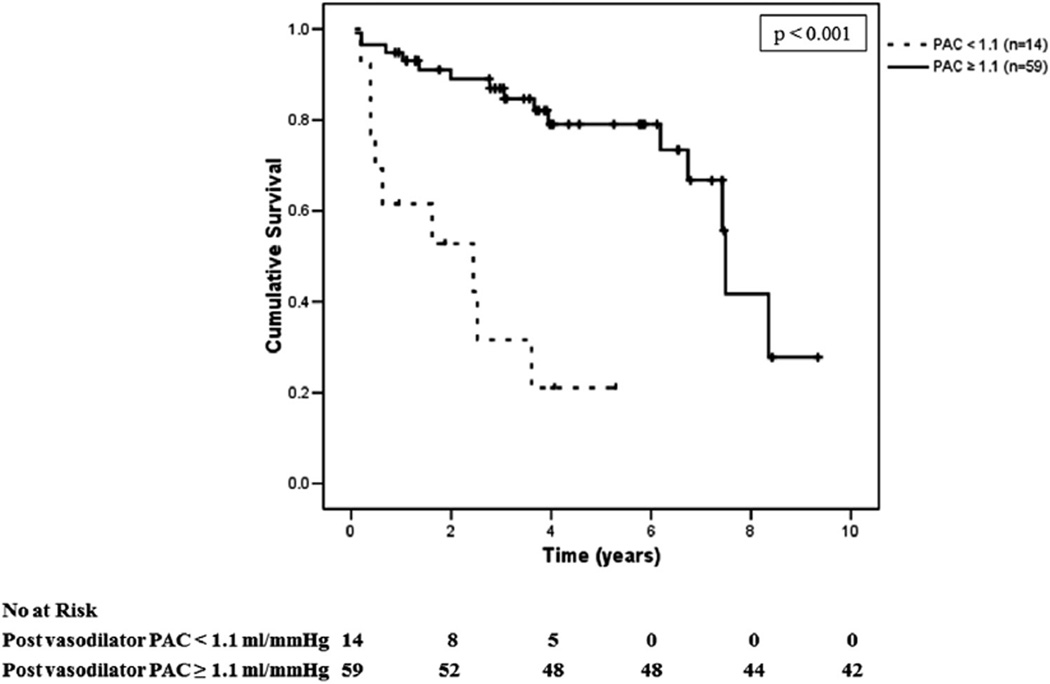
Based on clinical expertise and to optimize the potential use of PAC as a screening measure, PAC <1.1 ml/mm Hg was selected to be the optimal cutoff as it was associated with sensitivity >90% and the highest achievable specificity as determined by ROC curves. Patients with PH-LHD associated with HFpEF and a PAC <1.1 ml/mm Hg had a significantly worse survival than those with PAC ≥1.1 ml/mm Hg (hazard ratio: 4.9; 95% CI: 1.9 to 12.4; p < 0.001) (Figure 3). A PAC of <1.1 ml/mm Hg was associated with a sensitivity of 91% (95% CI: 84% to 98%), specificity 38% (95% CI: 27% to 49%), positive predictive value 74% (95% CI: 64% to 84%), and negative predictive value 69% (95% CI: 58% to 80%) in predicting mortality among our patients with HFpEF.
FIGURE 3. Survival of Patients With PH-LHD and HFpEF.
Kaplan-Meier estimates of survival in patients with pulmonary hypertension due to left heart disease (PH-LHD) and heart failure with preserved ejection fraction (HFpEF) relative to pulmonary hypertension severity as defined by pulmonary arterial capacitance (PAC) <1.1 ml/mm Hg or ≥1.1 ml/mm Hg (n = 73).
ACUTE VASODILATOR TESTING
A total of 57 subjects (78%) underwent acute vasodilator testing with iNO. No adverse side effects or events occurred during iNO testing despite 30 patients (55%) having an increase in PAWP ranging between 1 and 16 mm Hg (Online Figure 4). Ten subjects (18%) met the Rich definition of acute vasodilator response, of whom 2 (4%) also met the more stringent Sitbon definition (Table 5). As compared with nonresponders, responders were more likely to be female and more obese (Table 5). Responders, regardless of the criteria, had no difference in survival when compared to nonresponders (p = 0.60 and 0.95 by Sitbon and Rich definitions, respectively) (Online Figure 5). Patients in whom PAC increased to ≥1.1 ml/mm Hg after iNO testing (n = 6) had a trend toward improved survival as compared to those who did not (median survival 29 months vs. 7 months; p = 0.26) (Online Figure 6).
TABLE 5.
Baseline Demographic and Hemodynamic Characteristics of Patients by Acute Vasodilator Testing Status (n = 73)
| Acute Vasodilator Responders* (n = 10) |
Nonresponders (n = 47) |
Not Tested (n = 16) |
|
|---|---|---|---|
| Baseline clinical data | |||
| Age, yrs | 66 ± 15 | 69 ± 11 | 73 ± 10 |
| Female | 10 (100) | 33 (70) | 11 (69) |
| Body mass index, kg/m2 | 40 ± 17 | 32 ± 9 | 29 ± 6 |
| Non-Hispanic White | 10 (100) | 42 (89) | 16 (100) |
| NYHA functional class (n = 69) | |||
| I | 0 (0) | 1 (2) | 1 (7) |
| II | 4 (40) | 23 (52) | 6 (40) |
| III | 4 (40) | 17 (39) | 7 (46) |
| IV | 2 (20) | 3 (7) | 1 (7) |
| Hemodynamic data | |||
| Heart rate, beats/min | 79 ± 13 | 73 ± 15 | 64 ± 6 |
| Mean arterial pressure, mm Hg | 96 ± 10 | 97 ± 15 | 97 ± 14 |
| Mean RA pressure, mm Hg | 13 ± 5 | 13 ± 4 | 14 ± 7 |
| Mean PA pressure, mm Hg | 41 ± 12 | 41 ± 11 | 41 ± 12 |
| PAWP, mm Hg | 20 ± 3 | 21 ± 4 | 23 ± 6 |
| Pulse pressure, mm Hg | 36 ± 19 | 36 ± 14 | 43 ± 17 |
| TPG, mm Hg | 21 ± 12 | 20 ± 10 | 18 ± 8 |
| Thermodilution CO, l/min1 | 4.8 ± 1.6 | 4.9 ± 1.5 | 4.2 ± 1.3 |
| PAC, ml/mm Hg | 2.3 ± 1.8 | 2.2 ± 1.1 | 1.7 ± 0.7 |
| SAC (n = 70), ml/mm Hg | 1.1 ± 0.5 | 1.2 ± 0.5 | 0.9 ± 0.3 |
| PVR, WU | 5.1 ± 3.4 | 4.9 ± 4.1 | 4.8 ± 2.6 |
| SVR, WU | 19.4 ± 8.8 | 19.1 ± 8.0 | 22.1 ± 10. |
| Laboratory data | |||
| BNP (n = 63), pg/ml | 164 ± 140 | 308 ± 388 | 432 ± 316 |
| Outcome | |||
| Death | 10 (30) | 17 (36) | 5 (31) |
Values are mean ± SD or n (%).
Using Rich criteria (decrease in mPAP and PVR by ≥20%).
Abbreviations as in Table 2.
DISCUSSION
In this referral-based cohort of patients with HFpEF and PH-LHD, age and PAC were strong independent predictors of mortality. Mortality was directly related to age, PVR, and brain natriuretic peptide and inversely related to CO, PA saturation, PAC, and SAC. PAC was the strongest predictor of mortality, with a PAC <1.1 ml/mm Hg associated with nearly a 5-fold increased risk of death in our cohort. Acute vasodilator testing in patients with PH-LHD and HFpEF was safe and well tolerated although the presence of vasoreactivity as currently defined for Group 1 pulmonary artery hypertension (PAH) was not associated with a survival advantage.
Similar to HFrEF, RV performance in the face of increased afterload is one of the most significant prognostic factors in HFpEF (9–12). PVR reflects RV afterload but does not account for the pulsatility of CO and is not a sufficient indicator of RV afterload under all conditions (24). The 3-element Windkessel model provides a more comprehensive representation of RV afterload, by including PVR, total arterial compliance and characteristic impedance (25).
The product of vascular resistance and compliance in the pulmonary circuit (PVR × PAC: the pulmonary arterial time constant) is constant over a wide range of etiologies and severities of PH (24,26,27). This implies that PVR and PAC perform similarly as indices of RV afterload in most forms of PH—as one rises, the other falls proportionately. However, as demonstrated by Tedford et al. (27), the presence of an elevated left-sided filling pressure shifts this relationship to the left resulting in a less compliant pulmonary circuit (lower PAC) for any given resistance (PVR). This means that the RV ejects into a stiff pulmonary vasculature, greatly increasing its afterload. Consequently PAC provides a better assessment of RV afterload than either PVR or DPG when PAWP is elevated (27–29) and has proved to be a reliable predictor of outcome in patients with HFrEF (11,12,30). Our data now extend this observation to HFpEF, demonstrating that PAC is a significantly better predictor of mortality than TPG, DPG, or PVR.
In our study, PAC was correlated with SAC, an observation that may have prognostic as well as pathophysiologic significance. Specifically, increased systemic arterial stiffness (i.e., lower SAC) is associated with increased mortality in patients with HFrEF (31). In our HFpEF cohort, univariate analysis demonstrated that SAC and mortality were inversely correlated, though SAC was not an independent predictor by multivariate analysis. Theoretically, systemic vascular stiffening could contribute to PA stiffening via promotion of LV hypertrophy with resultant LV diastolic dysfunction and elevated PAWP. This mechanism of PA stiffening may contribute to remodeling of the distal pulmonary arterial bed. A recent study demonstrated that lower PAC of the proximal large PAs led to high pulsatility flow with the subsequent induction of endothelial cell dysfunction and smooth muscle cell hypertrophy in distal PAs (32).
Recognizing that pulmonary vasoreactivity predicts outcomes in Group 1 PAH (19,20), as well as of heart transplantation in HFrEF (13), we examined the prognostic significance of the pulmonary vasodilator response to inhaled nitric oxide in our cohort of HFpEF patients. As expected, some patients with PHLHD and HFpEF experienced increased PAWP, likely due in part to increased pulmonary arterial blood flow to the left side of the heart. Interestingly, this hemodynamic profile occurred in only one-half of patients tested and importantly, patients tolerated it well, without detectible changes in oxygenation, systemic blood pressure, or symptoms.
Acute vasodilator responses were uncommon in our cohort; 18% by the Rich criteria and 4% by the Sitbon criteria. These compare to rates of 28% and 13%, respectively, in Group 1 PAH patients (33). In contrast to the PAH population (20), acute vasodilator responders in our PH-LHD and HFpEF cohort manifested no difference in survival when compared to nonresponders. Interestingly, we found that an increase in PAC to ≥1.1 ml/mm Hg post-vasodilator had a trend toward better survival than a PAC <1.1 ml/mm Hg. This observation favors the hypothesis that in HFpEF the reactivity of the pulmonary arterial bed, as manifested by amelioration of PA stiffness, does carry a favorable prognosis, although this needs to be validated.
STUDY LIMITATIONS
The main limitation of our study is our small sample size, which limits our ability to detect possible significant associations between DPG and outcomes. We nonetheless successfully identified several factors that were significantly associated with mortality. We also acknowledge the risk of false positive results in our study given multiple testing and that generated hypotheses will need to be tested in other cohorts. In addition, we should emphasize that this cohort was limited to a referral-based population of HFpEF patients with PH and should not be generalized to those without PH. Thus we anticipated a high prevalence of Comb-PH as well as greater disease severity. Despite these limitations, the prospective and consecutive enrollment is a strength of our study and may minimize the potential for selection bias. We recognize that our observations need to be replicated and then potentially extended to a more diverse HFpEF population.
CONCLUSIONS
PA capacitance, a major determinant of RV afterload, is the best predictor of mortality in our cohort of patients with PH-LHD and HFpEF. Interestingly, Comb-PH, defined with DPG criteria, did not have a worse prognosis than Iso-PH. We also found that while vasodilator testing was safe and well tolerated in our cohort, pulmonary vasoresponsiveness, as defined by criteria for Group 1 PAH, failed to impart a favorable prognosis. Our findings suggest that PAC is a more accurate indicator than PVR, TPG, or DPG of clinically important pulmonary vascular disease and RV dysfunction in PH-LHD and HFpEF and future studies should validate and extend the prognostic and pathophysiologic significance of PAC in this patient population.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Pulmonary hypertension due to left heart disease, in particular heart failure with preserved ejection fraction, is increasingly recognized and associated with poor outcomes. Pulmonary arterial capacitance, determined at the time of diagnosis, may be an important assessment that will enable enhanced risk stratification and treatment planning of this patient population.
TRANSLATIONAL OUTLOOK
Additional clinical studies are needed to validate the prognostic implications of pulmonary arterial capacitance. Future researchers should evaluate whether improvements in pulmonary arterial capacitance with treatment confer a survival benefit.
ACKNOWLEDGEMENTS
The authors thank Stefan Richter and Anthony Faugno for their contributions to data collection, cleaning and entry.
Dr. Preston has received grant support from Actelion, Bayer, Gilead, and United Therapeutics; and has served as a consultant for Actelion, Bayer, Gilead, United Therapeutics, and Pfizer. Dr. Hill has received research grant support from Actelion, Gilead, Bayer, United Therapeutics, and Reata; has served on the medical advisory board for Gilead and Bayer; and has served on the data monitoring committee for Lung LLC (Limited Liability Company). Dr Al-Naamani is supported by the National Center for Advancing Translational Science (NCATS), National Institutes of Health (NIH), grant Number UL1 TR001064 and TL1 TR001062. Dr Paulus is supported by a grant from NCATS, grant number UL1 TR001064.
ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- CO
cardiac output
- Comb-PH
combined post-capillary pulmonary hypertension and pre-capillary pulmonary hypertension
- DPG
diastolic pressure gradient
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- iNO
inhaled nitric oxide
- Iso-PH
isolated post-capillary pulmonary hypertension
- LHD
left heart disease
- LV
left ventricular
- mPAP
mean pulmonary artery pressure
- PA
pulmonary artery
- PAC
pulmonary arterial capacitance
- PAH
pulmonary arterial hypertension
- PAWP
pulmonary artery wedge pressure
- PH
pulmonary hypertension
- PP
pulse pressure
- PVR
pulmonary vascular resistance
- ROC
receiver-operating characteristic
- RV
right ventricular
- SAC
systemic arterial capacitance
- SV
stroke volume
- TPG
transpulmonary gradient
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For supplemental figures, please see the online version of this article.
REFERENCES
- 1.Hill NS, Preston I, Roberts K. Defining the phenotypes for pulmonary hypertension associated with diastolic heart failure. Circ Heart Fail. 2011;4:238–240. doi: 10.1161/CIRCHEARTFAILURE.111.962217. [DOI] [PubMed] [Google Scholar]
- 2.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 3.Damy T, Goode KM, Kallvikbacka-Bennett A, et al. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J. 2010;31:2280–2290. doi: 10.1093/eurheartj/ehq245. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–265. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 7.Hansdottir S, Groskreutz DJ, Gehlbach BK. WHO’s in second?: A practical review of World Health Organization group 2 pulmonary hypertension. Chest. 2013;144:638–650. doi: 10.1378/chest.12-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vachiery JL, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Cappola TP, Felker GM, Kao WH, Hare JM, Baughman KL, Kasper EK. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation. 2002;105:1663–1668. doi: 10.1161/01.cir.0000013771.30198.82. [DOI] [PubMed] [Google Scholar]
- 10.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 11.Dupont M, Mullens W, Skouri HN, et al. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ Heart Fail. 2012;5:778–785. doi: 10.1161/CIRCHEARTFAILURE.112.968511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegrini P, Rossi A, Pasotti M, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 2014;145:1064–1070. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 13.Costard-Jackle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54. doi: 10.1016/0735-1097(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 15.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 16.Preston IR, Klinger JR, Houtches J, Nelson D, Farber HW, Hill NS. Acute and chronic effects of sildenafil in patients with pulmonary arterial hypertension. Respir Med. 2005;99:1501–1510. doi: 10.1016/j.rmed.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Preston IR, Sagliani KD, Roberts KE, et al. Comparison of acute hemodynamic effects of inhaled nitric oxide and inhaled epoprostenol in patients with pulmonary hypertension. Pulm Circ. 2013;3:68–73. doi: 10.4103/2045-8932.109916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sitbon O, Brenot F, Denjean A, et al. Inhaled nitric oxide as a screening vasodilator agent in primary pulmonary hypertension. A dose-response study and comparison with prostacyclin. Am J Respir Crit Care Med. 1995;151:384–389. doi: 10.1164/ajrccm.151.2.7842196. [DOI] [PubMed] [Google Scholar]
- 19.Rich S, Kaufmann E. High dose titration of calcium channel blocking agents for primary pulmonary hypertension: guidelines for short-term drug testing. J Am Coll Cardiol. 1991;18:1323–1327. doi: 10.1016/0735-1097(91)90556-o. [DOI] [PubMed] [Google Scholar]
- 20.Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Shah SJ, Foreman AJ, et al. Risk assessment in pulmonary hypertension associated with heart failure and preserved ejection fraction. J Heart Lung Transplant. 2012;31:467–477. doi: 10.1016/j.healun.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 24.Lankhaar JW, Westerhof N, Faes TJ, et al. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2006;291:H1731–H1737. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 25.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 26.Lankhaar JW, Westerhof N, Faes TJ, et al. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 27.Tedford RJ, Hassoun PM, Mathai SC, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 30.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. J Am Coll Cardiol HF. 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33:951–958. doi: 10.1016/s0735-1097(98)00679-2. [DOI] [PubMed] [Google Scholar]
- 32.Scott D, Tan Y, Shandas R, Stenmark KR, Tan W. High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am J Physiol Lung Cell Mol Physiol. 2013;304:L70–L81. doi: 10.1152/ajplung.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Douwes JM, van Loon RL, Hoendermis ES, et al. Acute pulmonary vasodilator response in paediatric and adult pulmonary arterial hypertension: occurrence and prognostic value when comparing three response criteria. Eur Heart J. 2011;32:3137–3146. doi: 10.1093/eurheartj/ehr282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.