Abstract
Background:
Impetigo can result from Staphylococcus aureus (S. aureus). Wolf’s isotopic response is the occurrence of a new cutaneous disorder at the site of a previously healed disease. A cutaneous immunocompromised district is an area of skin that is more vulnerable than the rest of the individual’s body.
Purpose:
To describe a man with impetigo localized to a unilateral dermatome and review the clinical features of other patients with zosteriform Staphylococcus aureus cutaneous infection.
Methods:
PubMed was used to search the following terms, separately and in combination: cutaneous, dermatome, dermatomal, district, herpes, immunocompromised, impetigo, infection, isotopic, response, skin, staphylococcal, Staphylococcus aureus, Wolf, zoster, zosteriform. All papers were reviewed and relevant manuscripts, along with their reference citations, were evaluated.
Results:
Crusted, eroded and intact, erythematous papules and nodules acutely presented localized to the mandibular branch of the left trigeminal nerve on the face of a 66-year-old man; he did not recall a prior episode of varicella-zoster virus infection in that area. A bacterial culture isolated methicillin-susceptible S. aureus. Viral cultures and direct fluorescent absorption studies were negative for herpes simplex and herpes zoster virus. All of the lesions resolved after oral treatment with cefdinir. Impetigo and/or furunculosis in a zosteriform distribution have also been described in 3 additional patients. The bacterial culture showed either methicillin-susceptible or methicillin-resistant S. aureus; the skin infection resolved after treatment with oral antibiotics; however one man experienced 2 recurrences in the same area.
Conclusions:
Zosteriform cutaneous staphylococcal impetigo may be an example of Wolf’s isotopic response in a cutaneous immunocompromised district
Keywords: cutaneous, dermatome, dermatomal, district, herpes, immunocompromised, impetigo, infection, isotopic, response, skin, staphylococcal, Staphylococcus aureus, Wolf, zoster, zosteriform
Introduction
Impetigo is a bacterial infection that can be caused by Staphylococcus aureus (S. aureus) [1]. Herpes zoster is a viral infection caused by varicella-zoster virus [2]. Occasionally, non-viral conditions—including bacterial infections—can morphologically mimic herpes zoster by presenting with skin lesions in a dermatomal distribution [3]. A man who presented with zosteriform facial skin lesions of impetigo is described and the clinical features of previously reported patients with dermatomal S. aureus infection are summarized.
Case report
A 66-year-old healthy afebrile man presented for evaluation of skin lesions that had developed on the left side of his face. His cutaneous past medical history was remarkable for cryotherapy-treated actinic keratoses, excision of two basal cell carcinomas (on the left chest in 2004 and the right cheek in 2009), and genital herpes simplex virus type 2 infection. He had chicken pox as a child and no episode of herpes zoster; he had received the varicella vaccine 2 years ago.
A week earlier, he noticed that facial skin lesions began to appear. Initially the site was pruritic and then a crust would develop; there were no blisters. The lesions were first noted on the left side of his chin; subsequently, they developed on his left cheek and left neck, near his jaw. Based on the distribution and morphology of his skin lesions, his wife diagnosed him to have a shingles.
Cutaneous examination showed crusted, eroded and intact, erythematous papules and nodules—ranging in size from 3 to 5 mm in diameter—restricted to only the left side of his face. There were about 12 lesions. They were located on his chin, cheek, and neck in a distribution that corresponded to the third division of the fifth cranial nerve (Figure 1).
Figure 1. (A, B, and C).
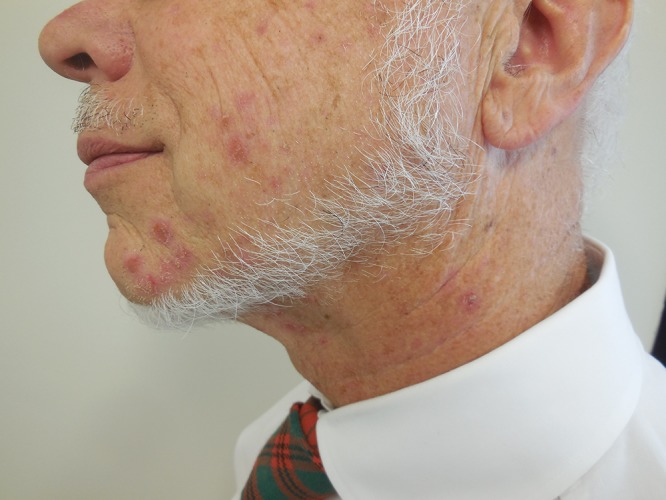
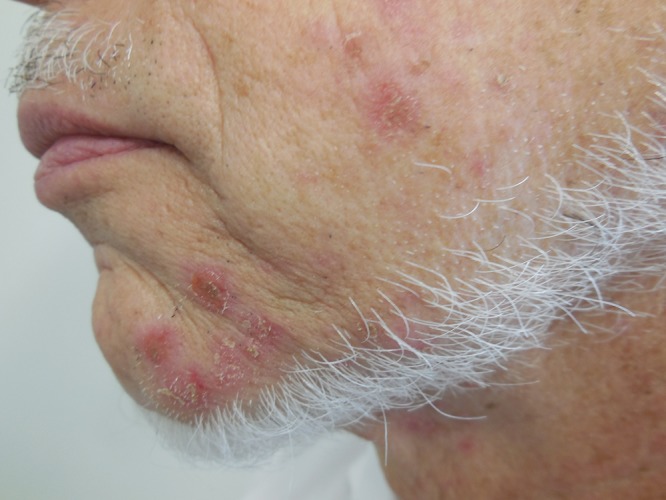
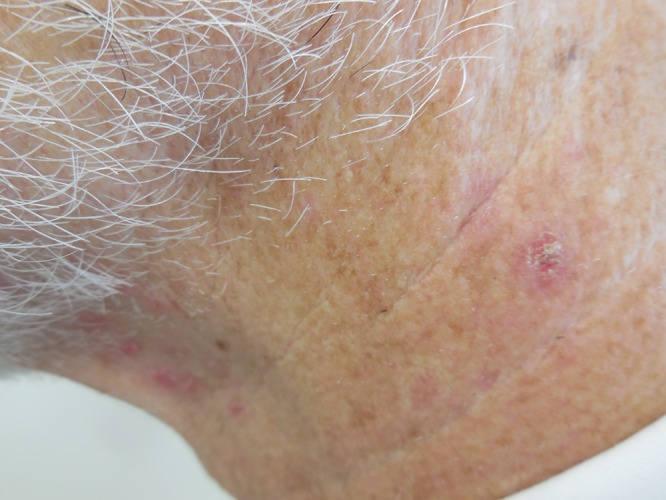
Distant (A) and closer (B and C) views of zosteriform impetigo located on facial skin innervated by the mandibular branch of the left trigeminal nerve; the lesion appear as crusted, eroded and intact, erythematous papules and nodules on the left side of the chin (B) and neck (C). [Copyright: ©2015 Cohen.
The unilateral and dermatome location of the skin lesions raised the possibility of herpes zoster. However, the appearance of the individual crusted erosions was consistent with impetigo. Additional laboratory studies were performed and he was empirically treated for both herpes zoster (with 1 gram of oral valacyclovir three times daily for 10 days) and impetigo (with 300 mg of oral cefdinir twice daily for 10 days and topical mupirocin 2% ointment three times daily for 10 days).
All of the facial skin lesions resolved within 1 week; no residual crusts were present at his 1-month follow up examination. A Tzanck smear taken from one of the lesions at presentation was negative for multinucleated epidermal giant cells. In addition, not only the direct fluorescent absorption studies, but also the viral cultures were negative for both herpes zoster virus and herpes simplex virus. The bacterial culture from a crusted erosion was positive for methicillin susceptible S. aureus; the bacteria was also susceptible to cefazolin.
Discussion
Non-bullous impetigo is caused by either S. aureus or Streptococcus pyogenes. It typically presents as a superficial ulcer covered by a purulent exudate that dries into an adherent yellow to honey-colored crust. Lesions are frequently found on the face and extremities. Satellite lesions are common, resulting from self-inoculation [1,4–6].
Herpes zoster occurs when there is reactivation of a latent varicella-zoster infection. Skin lesions typically present as unilateral, erythematous-based vesicles in a dermatomal distribution. Less commonly, disseminated herpes zoster can present as multiple, widely distributed, individual vesicles—with or without—associated dermatomal skin involvement. In addition to a positive viral culture for varicella-zoster virus, bacterial culture can concurrently be positive for S. aureus when the vesicular, ulcerated or crusted lesions become colonized or secondarily infected by the bacterial organism [2,7–9].
Zosteriform impetigo represents the development of nonbullous impetigo, to date caused by S. aureus infection, in which the cutaneous lesions appear in a dermatomal distribution. Varicella-zoster virus is absent from the bacterial skin lesions. In addition to the reported patient, zosteriform S. aureus cutaneous infection has been described in 3 other individuals (Table 1) [3,10–13]; however, one might speculate that zosteriform cutaneous staphylococcal infection is probably more common than the paucity of reported individuals would suggest.
TABLE 1.
| C |
A Ra S |
Location | Morphology | CP |
Bact cult |
VC |
Prior VZV |
Other conditions |
Tx | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 W F |
Breast, upper flank, & back R T4 |
Multiple erythematous pustules, papules, & nodules | I & F | MRSA | - | - | MEN 2B Metastatic medullary thyroid cancer |
[c] | 13C2 |
| 2 | 63 W M |
Abdomen R T11 |
Tender red nodules × 3 with intact, ulcerated, and pustular centers | F | MSSA | - | - | SCC: L temple | [d] | 13C1 |
| 3 | 66 W M |
Face L CNV-3 |
12 crusted, eroded & intact, red papules & nodules | I | MSSA | - | - | AK BCC × 2: R cheek & L chest HSV 2: genital |
[e] | CR |
| 4 | 76 ND M |
Face L CNV-3 |
Furuncles [f] | F | ND | ND | + [f] | Colon carcinoma [f] | [f] | 10 |
Abbreviations: A, age (in years); AK, actinic keratosis; Bact cult, bacterial culture; BCC, basal cell carcinoma; C, case; CNV-3, third division (mandibular branch) of fifth cranial (trigeminal) nerve dermatome; CP, clinical presentation; CR, current report; F, furunculosis; HSV 2, herpes simplex virus, type 2 infection; I, impetigo; L, left; MEN 2B, multiple endocrine neoplasia, type 2B (also known as either mucosal neuroma syndrome or multiple endocrine adenomatosis, type 2B; an autosomal dominant syndrome characterized by medullary thyroid carcinoma, pheochromocytoma, multiple mucosal neuromas and intestinal ganglioneuromas, and often a marfanoid habitus and other skeletal abnormalities); MRSA; methicillin-resistant Staphylococcus aureus; MSSA, methicillin susceptible Staphylococcus aureus; ND, not done; R, right; Ra, race; Ref, references; S, sex; SCC, squamous cell carcinoma; T, thoracic dermatome; TMP/SMX DS, trimethoprim/sulfamethoxazole double strength; Tx, treatment; VC, viral culture (for VZV); VZV, varicella-zoster virus infection (herpes zoster); &, and; −, negative or none, + positive, %, percent.
Furunculosis has been reported as a postherpetic isotopic response in 3 individuals; however, for 2 of the people, it is not definitively defined whether the herpetic infection was caused by herpes simplex virus or varicella-zoster virus in each of these individuals [5,10,11,12].
Treatment included oral TMP/SMX DS twice daily for 15 days; topical therapy included cleaning with povodine iodine (betadine) 10% cleanser and mupirocin 2% ointment 3 times daily to lesions, nostrils and umbilicus.
Treatment included oral TMP/SMX DS twice daily for 4 weeks; topical therapy included cleaning with chlorhexidine (hibiclens) 4% solution and mupirocin 2% ointment 3 times daily to lesions and nostrils.
Treatment included oral cefdinir 300 mg twice daily for 10 days; topical therapy included mupirocin 2% ointment 3 times daily to lesions.
The diagnosis of colon cancer occurred at the same time as his initial episode of herpes zoster. The herpes zoster virus infection occurred 1 month prior to his zosteriform furunculosis and presented as numerous small, solitary and grouped vesicles that were successfully treated with oral acyclovir 800 mg 5 times daily. He had 3 episodes of zosteriform furunculosis; the second and third episodes occurred 2 months and 5 months after the initial episode. Each episode was confined to the same area of his face and the “furuncles were not arranged in groups, thus a recurrent herpes simplex could easily be excluded clinically.” The initial and second episodes were each treated with oral cefaclor 500 mg twice daily for 5 days.
There were 3 men and 1 girl; the latter was 17 years old. The men ranged in age from 63 to 76 years; the median age was 66 years. Dermatomes were affected equally on the right and left side of the body; two of the men had involvement of the same area: the facial skin innervated by the mandibular branch of the trigeminal nerve. The infection presented with clinical features of furunculosis (2 patients), impetigo (1 patient) or both (1 patient). Bacterial cultures of the skin lesion were positive for either methicillin-susceptible (2 patients) or methicillin-resistant (1 patient) S. aureus [14]. Viral culture, at this time of S. aureus infection, was negative for varicella-zoster virus; in addition, 3 of the 4 patients did not have a history of clinically significant herpes zoster (either in the affected area or elsewhere on their body). The bacterial infection was successfully treated with oral antibiotic in all patients; however one man experienced 2 recurrences in the same area.
Wolf’s isotopic response designates the occurrence of a new cutaneous disorder at the location of a previously healed skin disease [15]. It was originally termed “isoloci response” by Wolf and Wolf in 1985 [16]. A decade later, Ruocco suggested changing the name to “isotopic response” [10,17,18]. However, since the latter designation generated hundreds of references linked with radioactive isotopes during computer-obtained literature searches, it was subsequently changed to “Wolf’s isotopic response” [19].
Several types of postherpetic isotopic responses have been observed following either herpes simplex virus or varicella-zoster virus infections. These not only include fungal and other viral infections, but also acneiform lesions, dysimmune reactions, granulomatous reactions, leukemia and lymphomatous infiltrates, malignant tumors and other conditions [3,11,12]. Bacterial infections, such as furunculosis and S. aureus-associated nonbullous impetigo have each only been observed in 4 individuals (Table 1) [3,10–13].
A cutaneous immunocompromised district is an area of skin that—for either genetic or acquired reasons—is more vulnerable than the rest of the individual’s body. This vulnerable site often facilitates the onset of skin disorders or immunity-related eruptions at that area because of the local dysregulation of immune control. The immunocompromised district in dermatology is a designation that is intended to include all possible etiologies potentially involved in a local immune destabilization of the affected area of mucosa or skin [20,21].
Zosteriform impetigo is favored to represent Wolf’s postherpetic isotopic response in a cutaneous immunocompromised district [3,8,11,12]. The absence of a definitive history of preceding varicella-zoster virus infection in 75 percent of the individuals (3 of the 4 patients) may reflect a prior subclinical varicella-zoster virus infection, such as an episode of asymptomatic zoster sine herpete [7,8]. Alternatively, and often similar in presentation with a dermatomal distribution, is the possibility of a Blascho linear arrangement due to skin mosaicism [22].
Conclusion
Impetigo and herpes zoster are common bacterial and viral infections, respectively. Concurrent S. aureus infection or impetinization of varicella-zoster virus infectious skin lesions may occur; however, cultures of the skin lesions are typically positive for both bacteria and virus. However, similar to the described patient, the skin lesions of zosteriform impetigo are only culture positive for S. aureus; viral studies—including Tzanck smear, culture, direct fluorescent absorption, and polymerase chain reaction—are negative. Wolf’s isotopic postherpetic response in a cutaneous immunocompromised district is not uncommon; however, bacterial infection is seldom reported in this setting. Some of individuals with zosteriform cutaneous staphylococcal infections did not recall a prior herpes zoster infection at that site raising the possibility that they had previously experienced an episode of asymptomatic zoster sine herpete. Similar to non-dermatomal S. aureus infection, zosteriform impetigo promptly responded to antibiotics to which the bacteria are susceptible.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Darmstadt GL, Lane AT. Impetigo: an overview. Pediatr Dermatol. 1994;11:293–303. doi: 10.1111/j.1525-1470.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 2.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidience and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruocco V, Ruocco E, Ghersetich I, Bianchi B, Lotti T. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90–94. doi: 10.1067/mjd.2002.118362. [DOI] [PubMed] [Google Scholar]
- 4.Hartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. Am Fam Physician. 2014;90:229–235. [PubMed] [Google Scholar]
- 5.Pereira LB. Impetigo-review. An Bras Dermatol. 2014;89:293–299. doi: 10.1590/abd1806-4841.20142283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangert S, Levy M, Hebert AA. Bacterial resistance and impetigo treatment trends: a review. Pediatr Dermatol. 2012;29:243–248. doi: 10.1111/j.1525-1470.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- 7.Gilden D, Nagel MA, Cohrs RJ. Varicella-zoster. Handb Clin Neurol. 2014;123:265–283. doi: 10.1016/B978-0-444-53488-0.00012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staikov I, Neykov N, Marinovic B, Lipozencic J, Tsankov N. Herpes zoster as a systemic disease. Clin Dermatol. 2014;32:424–429. doi: 10.1016/j.clindermatol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Kim SR, Khan F, Ramirez-Fort MK, Downing C, Tyring SK. Varicella zoster: an update on current treatment options and future perspectives. Expert Opin Pharmacother. 2014;15:61–71. doi: 10.1517/14656566.2014.860443. [DOI] [PubMed] [Google Scholar]
- 10.Wolf R, Brenner S, Ruocco V, Filioli FG. Isotopic response. Int J Dermatol. 1995;34:341–348. doi: 10.1111/j.1365-4362.1995.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruocco V, Brunetti G, Puca RV, Ruocco E. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2008;23:1364–1373. doi: 10.1111/j.1468-3083.2009.03345.x. [DOI] [PubMed] [Google Scholar]
- 12.Ruocco V, Ruocco E, Brunetti G, et al. Wolf’s post-herpetic isotopic response: infections, tumors, and immune disorders arising on the site of healed herpetic infection. Clin Dermatol. 2014;32:561–568. doi: 10.1016/j.clindermatol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Schepp ED, Cohen PR. Zosteriform Staphylococcus aureus cutaneous infection: report of two patients with dermatomal bacterial infection. SkinMed. 2015;13:275–81. [PubMed] [Google Scholar]
- 14.Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: a review of epidemiology, clinical features, management, and prevention. Int J Dermatol. 2007;46:1–11. doi: 10.1111/j.1365-4632.2007.03215.x. [DOI] [PubMed] [Google Scholar]
- 15.Wolf R, Wolf D, Ruocco V, Ruocco E. Wolf’s isotopic response: the first attempt to introduce the concept of vulnerable areas in dermatology. Clin Dermatol. 2014;12:557–560. doi: 10.1016/j.clindermatol.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Wolf R, Wolf D. Tinea in a site of healed herpes zoster (isoloci response?) [Letter] Int J Dermatol. 1985;24:539. doi: 10.1111/j.1365-4362.1985.tb05844.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolf R, Ruocco V, Filioli FG. Isotopic response [letter] Br J Dermatol. 1997;136:466–467. doi: 10.1111/j.1365-2133.1997.tb14967.x. [DOI] [PubMed] [Google Scholar]
- 18.Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123–125. doi: 10.1046/j.1468-3083.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 19.Wolf R, Wolf D, Ruocco E, Brunetti G, Ruocco V. Wolf’s isotopic response. Clin Dermatol. 2011;29:237–240. doi: 10.1016/j.clindermatol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Ruocco V. The immunocompromised district: how the pieces of the puzzle gradually fell into place [commentary] Clin Dermatol. 2014;32:549–552. doi: 10.1016/j.clindermatol.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Ruocco V, Ruocco E, Piccolo V, et al. The immunocomporomised district in dermatology: a unifying pathogenic view of the regional immune dysregulation. Clin Dermatol. 2014;32:569–576. doi: 10.1016/j.clindermatol.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Ruocco V, Ruocco E, Brunetti G, Saniuliano S, Wolf R. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483–488. doi: 10.1016/j.clindermatol.2011.03.003. [DOI] [PubMed] [Google Scholar]


