Abstract
Arterial aging may link cardiovascular risk to white coat hypertension (WCH). The aims of the present study were to investigate the role of arterial aging in the white coat effect, defined as the difference between office and 24-hour ambulatory systolic blood pressures, and to compare WCH with pre-hypertension (PH) with respect to target organ damage and long-term cardiovascular mortality. A total of 1257 never-been-treated volunteer subjects from a community-based survey were studied. WCH and PH were defined by office and 24-hour ambulatory blood pressures. Left ventricular mass index, carotid intima-media thickness (IMT), estimated glomerular filtration rate (eGFR), carotid-femoral pulse wave velocity (cf-PWV), carotid augmentation index (AIx), amplitude of the reflection pressure wave (Pb), and 15-year cardiovascular mortality were determined. Subjects with WCH were significantly older and had greater body mass index, blood pressure values, IMT, cf-PWV, AIx, and Pb, and a lower eGFR than PH. Pb was the most important independent correlate of the white coat effect in multi-variate analysis (model r2 = 0.451; partial r2/model r2 = 90.5%). WCH had significantly greater cardiovascular mortality than PH (hazard ratio and 95% confidence interval, 2.94, 1.09–7.91), after accounting for age, gender, body mass index, smoking, fasting plasma glucose, and total cholesterol/high density lipoprotein cholesterol ratio. Further adjustment of the model for Pb eliminated the statistical significance of the WCH effect. In conclusion, the white coat effect is mainly due to arterial aging. WCH carries higher risk for cardiovascular mortality than PH, probably via enhanced wave reflections that accompany arterial aging.
Keywords: white coat hypertension, pre-hypertension, cardiovascular mortality, arterial aging, arterial wave reflections
Introduction
Hypertension is the most important cardiovascular disease (CVD) risk factor and it’s control remains unsuccessful in almost all countries of the world.1 Early detection of subjects with high risk for sustained hypertension (SH) may facilitate the implementation of a high risk strategy for the prevention of CVD.2 Based on office blood pressure measurements, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) has introduced a category, pre-hypertension (PH), for those with blood pressures ranging from 120–139 mmHg systolic and/or 80–89 mmHg diastolic.3 PH is considered as an early sign of developing SH and has been correlated with a more than 2-fold increase in the risk of developing CVD compared with blood pressure levels below 120/80 mmHg.3–6 On the other hand, discrepancies between office and out-of-office blood pressure measurements have been recognized by home or ambulatory blood pressure monitoring (ABPM) and new blood pressure categories, such as masked hypertension and white coat hypertension (WCH) are identified from the out-of-office blood pressure measurements.7 Masked hypertension, characterized by a normal office blood pressure but high out-of-office blood pressure, is an adverse condition that should be treated as SH.8, 9 In contrast, WCH, defined by a high office blood pressure but normal out-of-office blood pressure,7 is generally recognized as a benign entity as compared to SH.8, 10–13 Current guidelines therefore emphasize the importance of identifying WCH to reduce the risk of inappropriate pharmacological treatment for hypertension in normotensive subjects. 3, 14 Several studies, however, have reported the association of WCH with target-organ damage or dysfunction and have suggested that it may not be prognostically innocent.15–20
Arterial aging increases arterial stiffness and wave reflections and is the major independent risk factor for the development of SH21 and CVD.22, 23 Since WCH becomes more common with increasing age,14 arterial aging may play a role in the pathogenesis of WCH and its associated CVD risk. In this regard, WCH may also be justified as a form of “pre-hypertension”. We hypothesized that WCH is more important and useful than PH in identifying subjects with high risk of developing SH and CVD in an aging society. The purposes of the present study, therefore, were to investigate, in a community-based population, the relationships of arterial aging to the white coat effect, and PH and WCH to target organ indices and 15-year CVD mortality.
Methods
Study cohort
In 1992–1993, 2230 Chinese residents in Pu-Li town and Kinmen county, Taiwan, were invited to participate in a comprehensive cardiovascular survey, including medical history and physical examination, arterial tonometry and ultrasonography, echocardiography, 24 hour ABPM, and biochemical examinations in the fasting state.24 Among 2039 participants with ABPM, a total of 1257 never-been-treated subjects (47% women, aged 53 ± 13 years) were included in the present study cohort (Figure 1). All participants gave informed consent and the study was approved by the institutional review board at the Johns Hopkins University.
Figure 1.
Selection of study population. ABPM = ambulatory blood pressure monitoring.
Office blood pressure measurements
After > 5 minutes of full rest, 2–3 measurements of brachial systolic (SBP) and diastolic (DBP) blood pressures separated by > 5 minutes were taken from the right arm in the seated position with a mercury sphygmomanometer and a standard-sized cuff (13 cm × 50 cm) by one of 4 experienced cardiologists who had been informed of the standard procedures. Pulse pressure (PP) was the difference between SBP and DBP. Averaged blood pressure values were used for all statistical analysis.25, 26
ABPM
Average 24-hour, daytime, and nighttime SBP (SBP-24h, SBP-D, and SBP-N, respectively) and DBP (DBP-24h, DBP-D, and DBP-N, respectively), and heart rate (HR-24h, HR-D, and HR-N, respectively) were derived from the oscillometric monitors (Model 90207, SpaceLabs Inc., Redmond, WA, USA). SBP variability (SBPV-24h, SBPV-D, SBPV-N, respectively) and heart rate variability (HRV-24h, HRV-D, HRV-N, respectively) were the standard deviations of SBP-24h, SBP-D, SBP-N, HR-24h, HR-D, and HR-N, correspondingly. Well-trained staffs set up the ABPM devices one day before the office visit on weekday mornings. Participants were instructed to maintain their daily activities. Automated blood pressure measurements were taken at 20-min and 60-min intervals during the daytime (0700–2200) and nighttime (2200–0700), respectively.24, 27 Blood pressure readings were not edited manually.24, 27
Arterial stiffness and wave reflections
Carotid-femoral pulse wave velocity (PWV) was calculated from the surface distance and the foot-to-foot pulse transit time between the right common carotid and right femoral arteries, using a tape measure and ECG-gated Doppler flow velocity signals (Parks model 802, Parks Medical Electronics, Inc).25 The magnitude of the wave reflections was estimated by calculating augmentation index (AIx) and amplitude of the backward pressure wave (Pb) from a calibrated ensemble averaged carotid artery pressure waveform registered with a tonometer (model SPC-350, Millar Instruments, Inc.).25 The inflection point on the carotid pressure waveform for the calculation of AIx was identified using the zero-crossing timings of the fourth derivative of the pressure wave.25 The carotid pressure waveform was separated into its forward and reflected components using the triangulation method28 according to the following equations:
| (1) |
| (2) |
where Pm(t) is the carotid pressure wave, F(t) is the approximated triangular-shaped flow wave, Zc is the characteristic impedance, Pf(t) is the decomposed forward pressure component, and Pb(t) is the decomposed backward pressure component.25
Variables of target organ indices
All echocardiographic examinations were performed by the same sonographer on a Hewlett-Packard SONOS 500 unit (Hewlett-Packard, Andover, MA, USA) incorporated with a 2.5 MHz transthoracic probe and a 7 MHz vascular probe.29 Images during sinus rhythm were measured for at least 3 cardiac cycles and all measurements were averaged for data analysis. Left ventricular mass was calculated from 2-dimensional-guided M-mode echocardiography and indexed to body surface area (LVMI). Intima-media thickness (IMT) of the posterior wall of the right common carotid artery was measured at end-diastole. Estimated glomerular filtration rate (eGFR) was calculated with previously published formula for Chinese.26
Classification
Participants were classified into 4 groups on the basis of office blood pressure and daytime ABPM levels: a. normotension (NT) (n = 250, 19.9%), office blood pressure < 120/80 mmHg and daytime ABPM blood pressure < 135/85 mmHg; b. PH (n = 318, 25.3%), office blood pressure ≥ 120/80 mmHg and < 140/90 mmHg, and daytime ABPM blood pressure < 135/85 mmHg; c. WCH (n = 153, 12.2%), office blood pressure ≥ 140/90 mmHg and daytime ABPM blood pressure < 135/85 mmHg; and d. SH (n = 536, 42.6%), daytime ABPM blood pressure > 135/85 mmHg.3 White coat effect was defined as the difference between office SBP and ABPM SBP-24h.
Follow-up
Mortality statistics for the 1257 participants were obtained by linking our database with the National Death Registry. The National Death Registry database registers valid information based on the certified death certificates coded according to the International Classification of Disease, Ninth Revision (ICD-9). The ICD-9 codes for cardiovascular death were 390–459. Subjects not appearing on the National Death Registry on December 31, 2007 were considered as survivors.25
Statistical Analysis
Characteristics of subjects are presented as mean ± standard deviation or number (%). The 4 groups were compared via a one-way analysis of variance and post hoc Bonferroni test for continuous variables. Categorical variables were compared with χ2 test. Because of significant age differences among the groups, the estimated marginal mean values were used for adjusting LVMI, IMT, eGFR, PWV, AIx, and Pb for age. Determinants of the white coat effect were examined by simple correlation and stepwise multiple linear regression analysis. The incidence of total and CVD mortality were calculated by dividing the number of total or CVD deaths at the end of follow-up by the total number of person-years of follow-up. CVD survival curves among groups were estimated using Kaplan-Meier product-limit method and compared a Log Rank test with pairwise comparisons. A Cox proportional hazards model was used to estimate the relative risks of all-cause and CVD mortality between groups, adjusting for conventional risk factors. Effects of arterial stiffness and wave reflections on the relative risks of CVD mortality were examined by sequentially entering individual parameter into the Cox model. Statistical significance was defined as two-tailed P < 0.05. All statistical analyses were performed using the statistical package SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline Characteristics
Table 1 presents the characteristics at the baseline survey. PH was 3 years older than NT, SH was 4 years older than PH, and WCH was 3 years older than SH. SH had more men than NT. BMI significantly increased in the order of NT, PH, WCH, and SH. WCH and SH had significantly higher total cholesterol/high density lipoprotein cholesterol and fasting plasma glucose levels than NT or PH. PH had significantly higher total cholesterol/high density lipoprotein cholesterol than NT.
Table 1.
Characteristics of the study population by blood pressure categories
| NT (n= 250) |
PH (n= 318) |
WCH (n= 153) |
SH (n= 536) |
|
|---|---|---|---|---|
| Age, years | 48 ± 13 | 51 ± 13* | 58 ± 13*† | 55 ± 12*† |
| Male gender, n (%) | 119 (48) | 164 (52) | 78 (51) | 308 (58)* |
| Current smoking, n (%) | 68 (27) | 72 (23) | 34 (22) | 143 (27) |
| Body mass index, kg/m2 | 23 ± 3 | 24 ± 3* | 25 ± 4*† | 26 ± 4*†‡ |
| Total cholesterol/HDL | 3.5 ± 0.9 | 4.0 ± 1.3* | 4.3 ± 1.1* | 4.4 ± 1.3*† |
| Fasting plasma glucose, mmol/L | 5.3 ± 1.2 | 5.4 ± 0.6 | 5.8 ± 2.3*† | 5.8 ± 1.7*† |
| Office blood pressure measures | ||||
| SBP-b, mmHg | 107 ± 7 | 124 ± 8* | 145 ± 13*† | 155 ± 20*†‡ |
| DBP-b, mmHg | 68 ± 6 | 77 ± 7* | 86 ± 9*† | 94 ± 12*†‡ |
| PP-b, mmHg | 39 ± 6 | 46 ± 11* | 59 ± 18*† | 61 ± 18*† |
| Heart rate, beats/min | 72 ± 9 | 75 ± 11* | 72 ± 10 | 75 ± 10* |
| Ambulatory blood pressure | ||||
| SBP-24h, mmHg | 110 ± 8 | 116 ± 8* | 122 ± 7*† | 143 ± 14*†‡ |
| DBP-24h, mmHg | 70 ± 6 | 74 ± 5* | 76 ± 5*† | 91 ± 9*†‡ |
| PP-24h, mmHg | 40 ± 4 | 43 ± 6* | 45 ± 7*† | 51 ± 11*†‡ |
| SBPV-24h, mmHg | 9.9 ± 2.3 | 10.7 ± 2.5* | 12.4 ± 3.1*† | 14.6 ±3.9*†‡ |
| HR-24h, beats/min | 77 ± 8 | 77 ± 8 | 75 ± 8 | 79 ±9*†‡ |
| HRV-24h, beats/min | 14.8 ± 6.4 | 13.2 ±5.0* | 12.7 ± 5.1* | 12.1 ± 4.8*† |
| SBP-D, mmHg | 111 ± 8 | 118 ± 8* | 123 ± 7*† | 145 ± 14*†‡ |
| DBP-D, mmHg | 71 ± 6 | 75 ± 6* | 78 ± 5*† | 93 ± 9*†‡ |
| PP-D, mmHg | 40 ± 5 | 43 ± 6* | 45 ± 7*† | 52 ± 11*†‡ |
| SBPV-D, mmHg | 9.3 ± 2.5 | 10.1 ±2.7* | 12.0 ± 3.3*† | 13.6 ±4.0*†‡ |
| HR-D, beats/min | 80 ± 9 | 80 ± 8 | 77 ± 9† | 82 ± 10‡ |
| HRV-D, beats/min | 13.7 ± 6.9 | 12.2 ± 5.4 | 12.0 ± 5.7 | 11.1 ± 5.2* |
| SBP-N, mmHg | 104 ± 9 | 110 ± 10* | 117 ± 11*† | 134 ± 16*†‡ |
| DBP-N, mmHg | 65 ± 7 | 68 ± 7* | 72 ± 7*† | 84 ± 10*†‡ |
| PP-N, mmHg | 39 ± 5 | 42 ± 7* | 45 ± 9*† | 50 ± 11*†‡ |
| SBPV-N, mmHg | 8.6 ± 3.4 | 8.9 ± 3.6* | 10.5 ± 4.5*† | 12.2 ± 4.6*†‡ |
| HR-N, beats/min | 66 ± 8 | 66 ± 8 | 66 ± 8 | 68 ± 9*†‡ |
| HRV-N, beats/min | 10.2 ± 8.4 | 8.7 ± 5.8 | 8.1 ± 5.4* | 7.6 ± 4.4* |
| White coat effect, mmHg | −2.7 ± 7.7 | 7.1 ± 8.8* | 23.4 ± 14.2*† | 11.9 ± 16.0*†‡ |
| Target organ indices and arterial aging | ||||
| LVMI, g/m2 | 89 ± 21 | 93 ± 25 | 98 ± 24* | 111 ± 28*†‡ |
| IMT, mm | 0.9 ± 0.3 | 1.0 ± 0.2 | 1.1 ± 0.3*† | 1.1 ± 0.3*† |
| eGFR, ml/min/1.73 m2 | 98 ± 22 | 93 ± 22 | 89 ± 24* | 90 ± 24* |
| PWV, m/sec | 8.1 ± 1.6 | 8.9 ± 2.1* | 10.0 ± 2.4*† | 10.3 ± 2.4*† |
| AIx, % | 6.1 ± 12.4 | 9.1 ± 13.5 | 17.7 ± 15.1*† | 17.2 ± 15.0*† |
| Pb, mmHg | 10.4 ± 2.4 | 12.8 ± 3.6* | 18.4 ± 6.7*† | 18.3 ± 6.9*† |
P < 0.05 vs. NT
P < 0.05 WCH or SH vs. PH
P < 0.05 SH vs. WCH.
-24h = average 24-hour measurements; -b = brachial; -D = average daytime measurements; -N = average nighttime measurements; AIx = carotid augmentation index; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HDL = high density lipoprotein cholesterol; HR = heart rate; HRV = heart rate variability; IMT = intima-media thickness; LVMI = left ventricular mass index; NT = normotension; Pb = amplitude of the decomposed carotid backward pressure; PH = pre-hypertension; PP = pulse pressure; PWV = carotid-femoral pulse wave velocity; SBP = systolic blood pressure; SBPV = SBP variability; SH = sustained hypertension; WCH = white-coat hypertension.
All office and ambulatory blood pressure values (SBP, DBP, and PP), and SBP variability increased significantly in the order of NT, PH, WCH, and SH, except that brachial PP did not significantly differ between WCH and SH (Table 1). The office heart rate was significantly higher in PH and SH than in NT, whereas HR-24h and HR-N were only significantly higher in SH than the other 3 groups. In contrast, HR-D in WCH was significantly lower than that in PH and SH. According to JNC-7, 73.2% of WCH fell into stage 1 hypertension and 53.9% of SH had stage 2 hypertension. WCH and SH, respectively, had 47.1% and 17.5% isolated systolic hypertension (SBP ≥ 140 mmHg and DBP < 90 mmHg). Among the 4 groups, WCH had the highest white coat effect, followed by SH, PH, and NT.
Target organ indices among groups
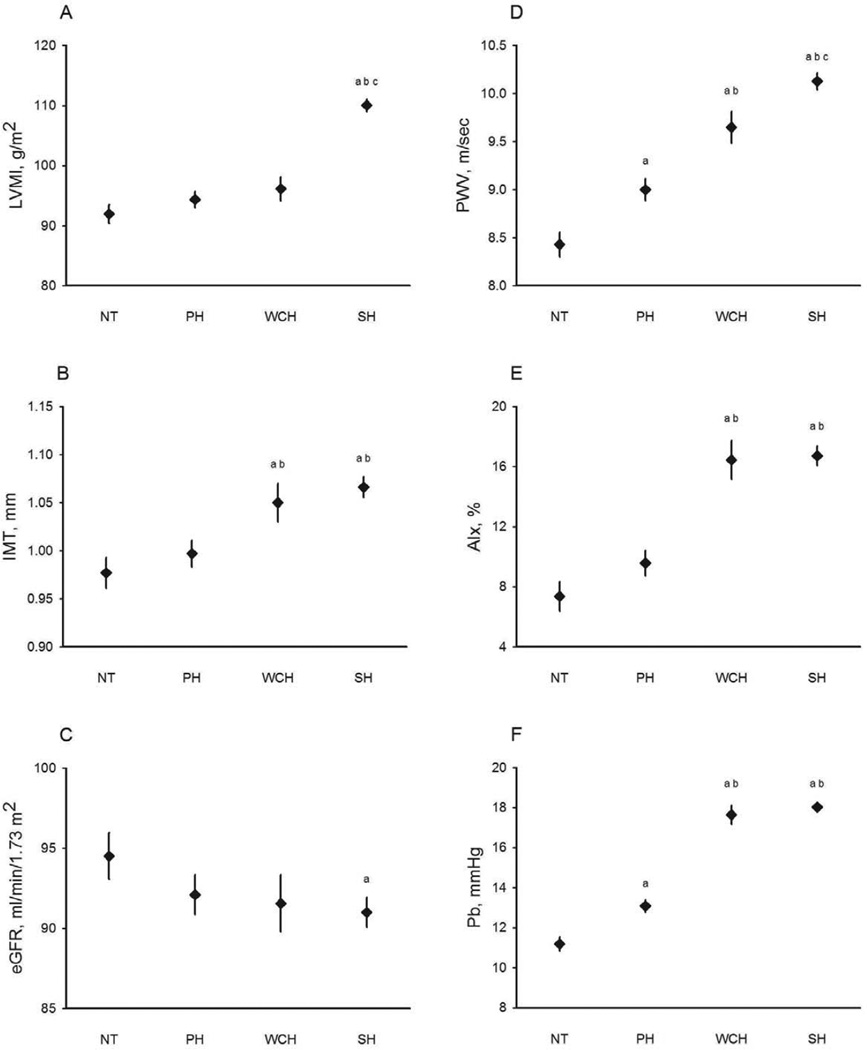
Compared with NT, PH had significantly higher PWV and Pb, but similar LVMI, IMT, eGFR, and AIx (Table 1). Compared with PH, WCH had significantly higher IMT, PWV, AIx, and Pb, but similar LVMI and eGFR. Compared with WCH, SH had significantly higher LVMI but similar IMT, eGFR, PWV, AIx, and Pb. Following adjustment for age, WCH still had significantly higher IMT, PWV, AIx, and Pb than PH (Figure 2).
Figure 2.
Means and standard errors of the age-adjusted target organ indices for normotension (NT), pre-hypertension (PH), white coat hypertension (WCH), and sustained hypertension (SH): (A) left ventricular mass index (LVMI); (B) carotid intima-media thickness (IMT); (C) estimated glomerular filtration rate (eGFR); (D) carotid-femoral pulse wave velocity (PWV); (E) carotid augmentation index (AIx); (F) amplitude of the backward pressure wave (Pb).
a : P < 0.05 in comparison with NT; b: P < 0.05 in comparison with PH; c: P < 0.05 in comparison with WCH.
Determinants of white coat effect
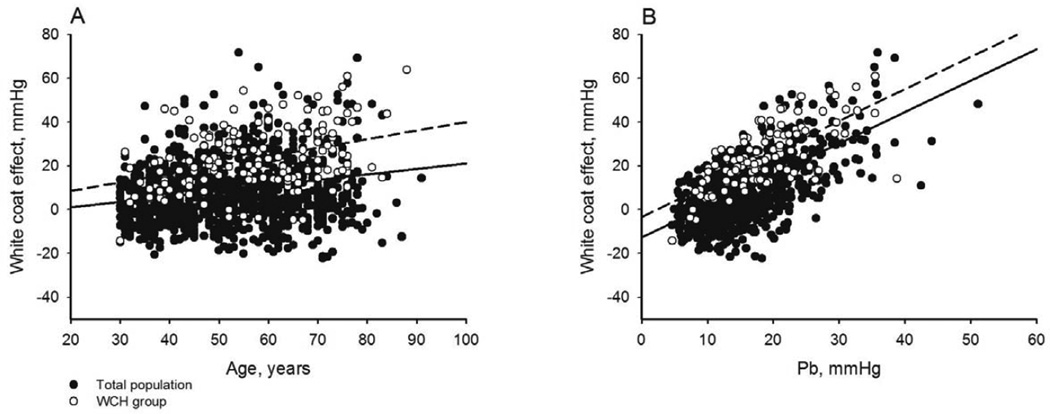
The white coat effect was significantly positively correlated with age (Figure 3A), body mass index, total cholesterol/high density lipoprotein cholesterol, fasting plasma glucose, SBPV-24h, SBPV-D, SBPV-N, LVMI, IMT, PWV, AIx, Pb (Figure 3B), and was significantly negatively correlated with male gender, eGFR, HR-24h, HR-D, HR-N, HRV-24h, HRV-D, and HRV-N (Table 2). HR-D, SBPV-D, and HRV-24h best correlated with the white coat effect among the variables of heart rate, SBP variability, and heart rate variability, respectively. By stepwise multiple linear regression analysis, Pb, LVMI, SBPV-D, fasting plasma glucose levels, male gender, HRV-24h, age and PWV were significantly independently associated with the white coat effect in descending order of importance (Table 2). Pb alone explained 90.5% (partial r2/model r2) of the total explainable variance of the white coat effect.
Figure 3.
Regressions of white coat effect on (A) age and (B) amplitude of the backward pressure wave, Pb, in total population (solid dots and solid lines) and subjects with white coat hypertension (WCH, hollow dots and dash lines). Model r2=0.043 and 0.137 for total and WCH cohorts, respectively in Panel A, and 0.411 and 0.504 for total and WCH cohorts, respectively in Panel B, all P<0.001.
Table 2.
Correlates of the white-coat effect: uni- and multi-variate analyses
| Variable | r | stepwise regression analysis (Model r2 = 0.451) |
||
|---|---|---|---|---|
| Partial r2 | Standardized coefficients |
P value | ||
| Age, years | 0.208* | 0.003 | −0.092 | 0.006 |
| Male gender | −0.154* | 0.005 | −0.069 | 0.013 |
| Body mass index, kg/m2 | 0.091* | -- | -- | -- |
| Total Cholesterol/HDL | 0.129* | -- | -- | -- |
| Fasting plasma glucose, mmol/L | 0.114* | 0.006 | 0.078 | 0.005 |
| eGFR, ml/min/1.73 m2 | −0.122* | -- | -- | -- |
| HR-D, beats/min | −0.126* | -- | -- | -- |
| SBPV-D, mmHg | 0.245* | 0.007 | −0.109 | 0.001 |
| HRV-24h, beats/min | −0.101† | 0.004 | 0.061 | 0.029 |
| LVMI, g/m2 | 0.119* | 0.013 | −0.095 | 0.002 |
| IMT, mm | 0.134* | -- | -- | -- |
| PWV, m/sec | 0.272* | 0.005 | 0.079 | 0.011 |
| AIx, % | 0.360* | -- | -- | -- |
| Pb, mmHg | 0.641* | 0.408 | 0.727 | <0.001 |
| Variables not selected for the stepwise regression analysis | ||||
| HR-24h, beats/min | −0.123* | |||
| HR-N, beats/min | −0.075† | |||
| SBPV-24h, mmHg | 0.245* | |||
| SBPV-N, mmHg | 0.095† | |||
| HRV-D, beats/min | −0.079† | |||
| HRV-N, beats/min | −0.073† | |||
P<0.001
P<0.01
AIx = carotid augmentation index; HDL = high density lipoprotein cholesterol; HR-24h = average 24-hour heart rate; HR-D = average daytime heart rate; HR-N = average nighttime heart rate; HRV-24h = average 24-hour heart rate variability; HRV-D = average daytime heart rate variability; HRV-N = average nighttime heart rate variability; eGFR = estimated glomerular filtration rate; LVMI = left ventricular mass index; IMT = intima-media thickness; PWV = carotid-femoral pulse wave velocity; Pb = amplitude of the backward pressure; SBPV-24h = average 24-hour systolic blood pressure variability; SBPV-D = daytime systolic blood pressure variability; SBPV-N = nighttime systolic blood pressure variability.
Mortality among the 4 blood pressure categories
During a median follow-up period of 15 years (17,128 person-years), 272 subjects died and 73 with a CVD cause (26.8%). The incidence of all-cause and CVD mortality was 9.7 and 0.6 in NT, 11.0 and 1.4 in PH, 24.6 and 6.5 in WCH, and 19.5 and 7.2 per 1,000 person years in SH, respectively.
Table 3 displays hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality by blood pressure categories. With NT as the reference (HR = 1), PH had a crude HR similar to NT, whereas WCH and SH had significantly higher HRs (Model 1). However, the higher HRs in WCH and SH became statistically insignificant after adjustment for age, gender, body mass index, smoking, fasting plasma glucose, and total cholesterol/high density lipoprotein cholesterol ratio (Model 2). On the other hand, with PH as the reference (HR = 1) and following adjustment for age, gender, body mass index, smoking, fasting plasma glucose, and total cholesterol/high density lipoprotein cholesterol ratio (Model 3), WCH had a non-significantly higher HR and SH had a significantly higher HR than PH.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality by blood pressure categories
| Model | NT (n= 250) |
PH (n= 318) |
WCH (n= 153) |
SH (n= 536) |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| 1 | 1 | Reference | 1.13 | 0.73–1.75 | 2.57 | 1.66–3.98 | 2.03 | 1.39–2.95 |
| 2 | 1 | Reference | 0.93 | 0.59–1.47 | 1.30 | 0.81–2.09 | 1.36 | 0.90–2.07 |
| 3 | - | - | 1 | Reference | 1.43 | 0.95–2.15 | 1.52 | 1.08–2.14 |
Events/1000 Person-Years for normotension (NT), pre-hypertension (PH), white-coat hypertension (WCH), and sustained hypertension were 9.7, 11.0, 24.6, and 19.5, respectively.
Model 1: crude hazard ratio
Moel 2 and Model 3: adjusted for age, gender, body mass index, smoking, fasting plasma glucose, and total cholesterol/high density lipoprotein cholesterol ratio.
Table 4 displays HR and 95% CI for CVD mortality by blood pressure categories. With NT as the reference (HR = 1), PH, WCH, and SH all had a higher crude HR but only the latter 2 groups reached statistical significance (Model 1). The higher HRs in WCH and SH remained statistically significant after adjustment for age, gender, body mass index, smoking, fasting plasma glucose, and total cholesterol/high density lipoprotein cholesterol ratio (Model 2). Furthermore, with PH as the reference (HR = 1) and following the same adjustment (Model 3), both WCH and SH had a significantly higher HR than PH. WCH still had a significantly higher HR than PH with further adjustment for cf-PWV (Model 4), but the significance disappeared when AIx (Model 5) or Pb (Model 6) was adjusted instead.
Table 4.
Hazard ratios (HR) and 95% confidence intervals (CI) for cardiovascular mortality by blood pressure categories
| Model | NT (n= 250) |
PH (n= 318) |
WCH (n= 153) |
SH (n= 536) |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| 1 | 1 | Reference | 2.36 | 0.48–11.67 | 11.56 | 2.61–51.24 | 12.69 | 3.09–52.11 |
| 2 | 1 | Reference | 1.96 | 0.39–9.79 | 5.59 | 1.22–25.55 | 8.54 | 2.02–36.03 |
| 3 | - | - | 1 | Reference | 2.94 | 1.09–7.91 | 4.36 | 1.84–10.31 |
| 4 | - | - | 1 | Reference | 2.75 | 1.02–7.40 | 3.86 | 1.62–9.17 |
| 5 | - | - | 1 | Reference | 2.46 | 0.78–7.73 | 4.16 | 1.59–10.89 |
| 6 | - | - | 1 | Reference | 2.40 | 0.76–7.63 | 3.87 | 1.42–10.54 |
Events/1000 Person-Years for the normotension (NT), pre-hypertension (PH), white-coat hypertension (WCH), and sustained hypertension were 0.6, 1.4, 6.5, and 7.2, respectively.
Model 1: crude hazard ratio
Model 2 and Model 3: adjusted for age, gender, body mass index, smoking, fasting plasma glucose, and total cholesterol/high density lipoprotein cholesterol ratio.
Model 4: additional adjustment for cf-PWV to Model 3.
Model 5: additional adjustment for AIx to Model 3.
Model 6: additional adjustment for Pb to Model 3.
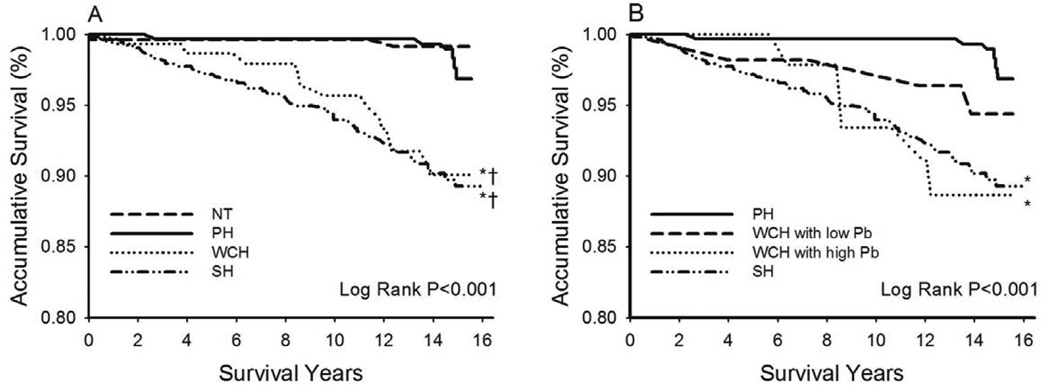
Figure 4A shows the Kaplan-Meier CVD survival curves for the 4 blood pressure groups. NT and PH had near overlapping survival curves without significant difference (P = 0.293). Both WCH and SH had significantly worse survival than PH (both P < 0.001). When WCH was stratified by Pb (median of the study population, 17.7 mmHg), WCH with high Pb but not with low Pb had significantly greater CVD mortality than PH (Figure 4B). In addition, the survival curve of WCH with high Pb overlapped with that of SH (P = 0.938).
Figure 4.
(A) Cardiovascular disease survival curves by blood pressure categories. *: Log Rank P < 0.05 for white coat hypertension (WCH) or sustained hypertension (SH) vs. normotension (NT); †: Log Rank P < 0.05 for WCH or SH vs. pre-hypertension (PH). (B) WCH was stratified by the amplitude of the carotid backward pressure (Pb, median value 17.7 mmHg). *: Log Rank P < 0.05 vs. PH.
Discussion
Clinically, patients with PH defined by office blood pressure criteria are considered at increased risk for progression to SH.3 In contrast, the clinical relevance of WCH is mainly to avoid unnecessary pharmacologic treatment.3, 14 To our knowledge, the present study is the first report on the comparison of these two important blood pressure categories. In the present study, subjects with PH defined by both office and out-of-office blood pressure measurements had significantly lower blood pressure values (central and peripheral, office and ABPM) than WCH, implying less adverse effects on target organs and future CVD risk.30 Indeed, both functional and structural vascular changes were more evident in WCH than in PH. Subjects with WCH experienced more CVD mortality than PH. The white coat effect was mainly determined by arterial aging. In addition, WCH with high wave reflections presented with substantial target organ damages (Supplementary Figure 1S) and carried risk of CVD mortality equivalent to that of SH. This may imply that pharmacological therapy may be indicated in patients with WCH and high wave reflections, as in patients with SH.
Compared to NT, PH has long been associated with increased CVD events both in women and men.4, 31 However, conflicting results indeed existed and few studies had excluded the potential confounders of masked hypertension and comorbidities.32, 33 Masked hypertension can reliably be diagnosed by self-measured blood pressure or ABPM and should be treated as SH.8, 9 In the present study, the finding of a low CVD mortality in subjects with PH but without masked hypertension may suggest the need to search for the latter in the clinical evaluation of the former detected by office blood pressure measurements alone. The absence of masked hypertension in subjects with PH is reassuring with respect to continuation of the non-pharmocological treatment for the prevention of SH.
WCH is a CVD risk factor when compared with normotensive controls19, 20 but has a relatively benign outcome when compared with mild SH.34 However, other studies have reported a cardiovascular risk in WCH that is not significantly different from that in NT.8, 12, 35 Apparently, substantial prognostic heterogeneity exists in WCH. The present study indicates that WCH may also be a marker of early arterial aging.36 Measures of arterial aging, the magnitude of wave reflections in particular, may be useful in identifying a high-risk subgroup in subjects with WCH who may benefit from the pharmacological treatment as in patients with SH.
Compared to measures of ABPM, the transient blood pressure rise during clinical visits has been referred as the white coat effect and has been attributed to the alerting reactions.37–39 Determinants of the white coat effect have been identified, including age, gender, body mass index, smoking, office blood pressure, and SBPV-D.37–39 The present study confirmed that advancing age, female gender, and increased body mass index and SBPV-D were positively associated with the white coat effect. Moreover, we demonstrated for the first time that arterial aging is the dominant determinant of the white coat effect. Although the white coat effect is triggered by a stress-related sympathetic activation, the blood pressure response due to the enhanced cardiac contraction and peripheral vasoconstriction may be markedly magnified in the presence of arterial aging, manifested as increased arterial stiffness and wave reflections measured in the office setting. Since arterial aging is a recognized major CVD risk factor, our study further suggests that arterial aging is the major pathophysiology that links WCH to increased CVD risk.
Study limitations
The present study has several limitations. First, the study was relatively small for the 4 blood pressure categories and the reported CVD event rate was relatively low in this homogenous Chinese population. Therefore, we may not have had sufficient power to demonstrate a significantly higher HR than PH for all-cause mortality (Table 3, Model 3) and CVD mortality (Table 4, Models 5 and 6) in some of the multi-variate Cox proportional hazards analyses. However, the overall results should be sufficient to demonstrate that WCH is more risky than PH. Second, the age difference between PH and WCH was large and the aging effect may not be fully accounted for by age adjustment in statistical models. Third, the pulsatile indices (PWV, AIx, and Pb) had been taken during the office procedures and those values were probably not relevant to the daily lives of the individuals. Although home and ABPM blood pressures are superior to the office blood pressure in the association of target organ damages and future cardiovascular events,7, 27 it remains to be established if the clinical values of the out-of-office measures of the pulsatile indices are also superior to the office measures. On the other hand, the present study clearly demonstrated the clinical value of high office blood pressure in the presence of normal out-of-office blood pressure (i.e., the identification of WCH category), and the add-on value of high office Pb in WCH (Figure 4B, Supplementary Table S1). Fourth, Pb was possibly mathematically related to the white coat effect, since the former was derived from a carotid pressure waveform calibrated to office mean blood pressure (=DBP + 1/3PP) and DBP and the latter was calculated directly from office SBP. However, when Pb was replaced by AIx, a calibration independent wave reflection index, AIx alone explained 55.9% of the total explainable variance of the white coat effect in the stepwise regression analysis (Supplementary Table S2). Therefore, the observed strong relationship between Pb and the white coat effect was most likely physiological. Furthermore, information regarding comorbidities and medical treatments during the follow-up period was not available. Therefore, our findings may not be generalizable to other populations with older PH, younger WCH, or those with comorbidities, that is, high cardiovascular risks cohort.
Perspectives
Effective hypertension control programs involve early detection of the high risk subjects for SH or its related CVD events. To this end, JNC-7 designated a new blood pressure category of PH. Our results suggest that the clinical management of PH can be refined by the application of ABPM or home blood pressure monitoring because PH without masked hypertension may carry only minimal CVD risk. On the other hand, arterial aging may be responsible for the prevalent WCH and its associated CVD risk. Measurement of arterial aging in subjects with WCH may be relevant to the identification of a high risk subgroup within WCH that may require pharmacological treatment. Future studies are required to demonstrate whether treatments targeted to arterial aging are effective in reducing the risks for the development of SH and/or CVD events in WCH with increased wave reflections.
Supplementary Material
What Is New?
White coat effect is mainly due to arterial aging.
WCH is more risky than PH, probably via enhanced wave reflections that accompany arterial aging.
What Is Relevant?
WCH is more important than PH in early detection of subjects with high risk for SH and the related CVD.
WCH with enhanced wave reflections may carry a cardiovascular risk equivalent to that of SH.
Summary - Arterial aging may link cardiovascular risk to WCH. WCH with evidence of arterial aging should be more aggressively managed.
Acknowledgments
Source(s) of Funding
This work was supported in part by the intramural grants from the Taipei Veterans General Hospital (Grant No. V95C1-052), grants in aid from the Research Foundation of Cardiovascular Medicine, (91-02-032, 93-02-014), Taipei, Taiwan, ROC, and Research and Development contract NO1-AG-1-2118 and the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Conflict(s) of Interest/Disclosure(s)
None
References
- 1.Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among united states adults, 1999 to 2010. J Am Coll Cardiol. 2012;60:599–606. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Manuel DG, Lim J, Tanuseputro P, Anderson GM, Alter DA, Laupacis A, Mustard CA. Revisiting rose: Strategies for reducing coronary heart disease. BMJ. 2006;332:659–662. doi: 10.1136/bmj.332.7542.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Suri MF, Kirmani JF, Divani AA, Mohammad Y. Is prehypertension a risk factor for cardiovascular diseases? Stroke. 2005;36:1859–1863. doi: 10.1161/01.STR.0000177495.45580.f1. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Lee ET, Devereux RB, Yeh J, Best LG, Fabsitz RR, Howard BV. Prehypertension, diabetes, and cardiovascular disease risk in a population-based sample: The strong heart study. Hypertension. 2006;47:410–414. doi: 10.1161/01.HYP.0000205119.19804.08. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Manolis A, Nilsson PM, Redon J, Struijker-Boudier HA, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, O’Brien E, Ponikowski P, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. 2007 guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the european society of hypertension (esh) and of the european society of cardiology (esc) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 8.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the ohasama study. J Am Coll Cardiol. 2005;46:508–515. doi: 10.1016/j.jacc.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 9.Angeli F, Reboldi G, Verdecchia P. Masked hypertension: Evaluation, prognosis, and treatment. Am J Hypertens. 2010;23:941–948. doi: 10.1038/ajh.2010.112. [DOI] [PubMed] [Google Scholar]
- 10.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: A population based study. Am J Hypertens. 2006;19:243–250. doi: 10.1016/j.amjhyper.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Kotsis V, Stabouli S, Toumanidis S, Papamichael C, Lekakis J, Germanidis G, Hatzitolios A, Rizos Z, Sion M, Zakopoulos N. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens. 2008;21:393–399. doi: 10.1038/ajh.2008.15. [DOI] [PubMed] [Google Scholar]
- 12.Pierdomenico SD, Lapenna D, Di Mascio R, Cuccurullo F. Short- and long-term risk of cardiovascular events in white-coat hypertension. J Hum Hypertens. 2008;22:408–414. doi: 10.1038/jhh.2008.6. [DOI] [PubMed] [Google Scholar]
- 13.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: A meta-analysis. J Hypertens. 2007;25:2193–2198. doi: 10.1097/HJH.0b013e3282ef6185. [DOI] [PubMed] [Google Scholar]
- 14.Hypertension: The clinical management of primary hypertension in adults: Update of clinical guidelines 18 and 34. London: 2011. [PubMed] [Google Scholar]
- 15.Gomez-Cerezo J, Rios Blanco JJ, Suarez GI, Moreno AP, Garcia RP, Vazquez-Munoz E, Barbado Hernandez FJ. Noninvasive study of endothelial function in white coat hypertension. Hypertension. 2002;40:304–309. doi: 10.1161/01.hyp.0000030198.48441.65. [DOI] [PubMed] [Google Scholar]
- 16.Pose-Reino A, Rodriguez-Fernandez M, Lopez-Barreiro L, Coleman IC, Estevez-Nunez JC, Mendez-Naya I. Diagnostic criteria of white coat hypertension (wch): Consequences for the implications of wch for target organs. Blood Press. 2002;11:144–150. doi: 10.1080/080370502760050377. [DOI] [PubMed] [Google Scholar]
- 17.Puato M, Palatini P, Zanardo M, Dorigatti F, Tirrito C, Rattazzi M, Pauletto P. Increase in carotid intima-media thickness in grade i hypertensive subjects: White-coat versus sustained hypertension. Hypertension. 2008;51:1300–1305. doi: 10.1161/HYPERTENSIONAHA.107.106773. [DOI] [PubMed] [Google Scholar]
- 18.Strandberg TE, Salomaa V. White coat effect, blood pressure and mortality in men: Prospective cohort study. Eur Heart J. 2000;21:1714–1718. doi: 10.1053/euhj.1999.2042. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853. doi: 10.1161/01.HYP.0000215363.69793.bb. [DOI] [PubMed] [Google Scholar]
- 20.Gustavsen PH, Hoegholm A, Bang LE, Kristensen KS. White coat hypertension is a cardiovascular risk factor: A 10-year follow-up study. J Hum Hypertens. 2003;17:811–817. doi: 10.1038/sj.jhh.1001643. [DOI] [PubMed] [Google Scholar]
- 21.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension. 2012;60:534–541. doi: 10.1161/HYPERTENSIONAHA.112.194571. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Ting CT, Lin SJ, Hsu TL, Chou P, Kuo HS, Wang SP, Yin FCP, Chang MS. Relation between diurnal variation of blood pressure and left ventricular mass in a chinese population. Am J Cardiol. 1995;75:1239–1243. [PubMed] [Google Scholar]
- 25.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: A community-based study. Hypertension. 2010;55:799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FCP, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: Which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang CM, Wang KL, Cheng HM, Chuang SY, Sung SH, Yu WC, Ting CT, Lakatta EG, Yin FCP, Chou P, Chen CH. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens. 2011;29:454–459. doi: 10.1097/HJH.0b013e3283424b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: A proof of principle. Hypertension. 2006;48:595–601. doi: 10.1161/01.HYP.0000238330.08894.17. [DOI] [PubMed] [Google Scholar]
- 29.Chen CH, Ting CT, Lin SJ, Hsu TL, Ho SJ, Chou P, Chang MS, O’Connor F, Spurgeon H, Lakatta E, Yin FCP. Which arterial and cardiac parameters best predict left ventricular mass? Circulation. 1998;98:422–428. doi: 10.1161/01.cir.98.5.422. [DOI] [PubMed] [Google Scholar]
- 30.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 31.Hsia J, Margolis KL, Eaton CB, Wenger NK, Allison M, Wu L, LaCroix AZ, Black HR. Prehypertension and cardiovascular disease risk in the women’s health initiative. Circulation. 2007;115:855–860. doi: 10.1161/CIRCULATIONAHA.106.656850. [DOI] [PubMed] [Google Scholar]
- 32.Mainous AG, III, Everett CJ, Liszka H, King DE, Egan BM. Prehypertension and mortality in a nationally representative cohort. Am J Cardiol. 2004;94:1496–1500. doi: 10.1016/j.amjcard.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Hozawa A, Kuriyama S, Kakizaki M, Ohmori-Matsuda K, Ohkubo T, Tsuji I. Attributable risk fraction of prehypertension on cardiovascular disease mortality in the japanese population: The ohsaki study. Am J Hypertens. 2009;22:267–272. doi: 10.1038/ajh.2008.335. [DOI] [PubMed] [Google Scholar]
- 34.Khattar RS, Senior R, Lahiri A. Cardiovascular outcome in white-coat versus sustained mild hypertension: A 10-year follow-up study [see comments] Circulation. 1998;98:1892–1897. doi: 10.1161/01.cir.98.18.1892. [DOI] [PubMed] [Google Scholar]
- 35.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: A tale of eva and adam in cardiovascular risk assessment and prevention. Hypertension. 2009;54:3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114. [DOI] [PubMed] [Google Scholar]
- 37.Lindbaek M, Sandvik E, Liodden K, Mjell J, Ravnsborg-Gjertsen K. Predictors for the white coat effect in general practice patients with suspected and treated hypertension. BrJ GenPract. 2003;53:790–793. [PMC free article] [PubMed] [Google Scholar]
- 38.Streitel KL, Graham JE, Pickering TG, Gerin W. Explaining gender differences in the white coat effect. Blood Press Monit. 2011;16:1–6. doi: 10.1097/MBP.0b013e32833f56c2. [DOI] [PubMed] [Google Scholar]
- 39.Manios ED, Koroboki EA, Tsivgoulis GK, Spengos KM, Spiliopoulou IK, Brodie FG, Vemmos KN, Zakopoulos NA. Factors influencing white-coat effect. Am J Hypertens. 2008;21:153–158. doi: 10.1038/ajh.2007.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.